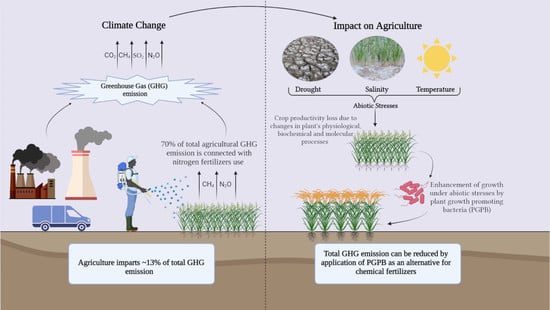
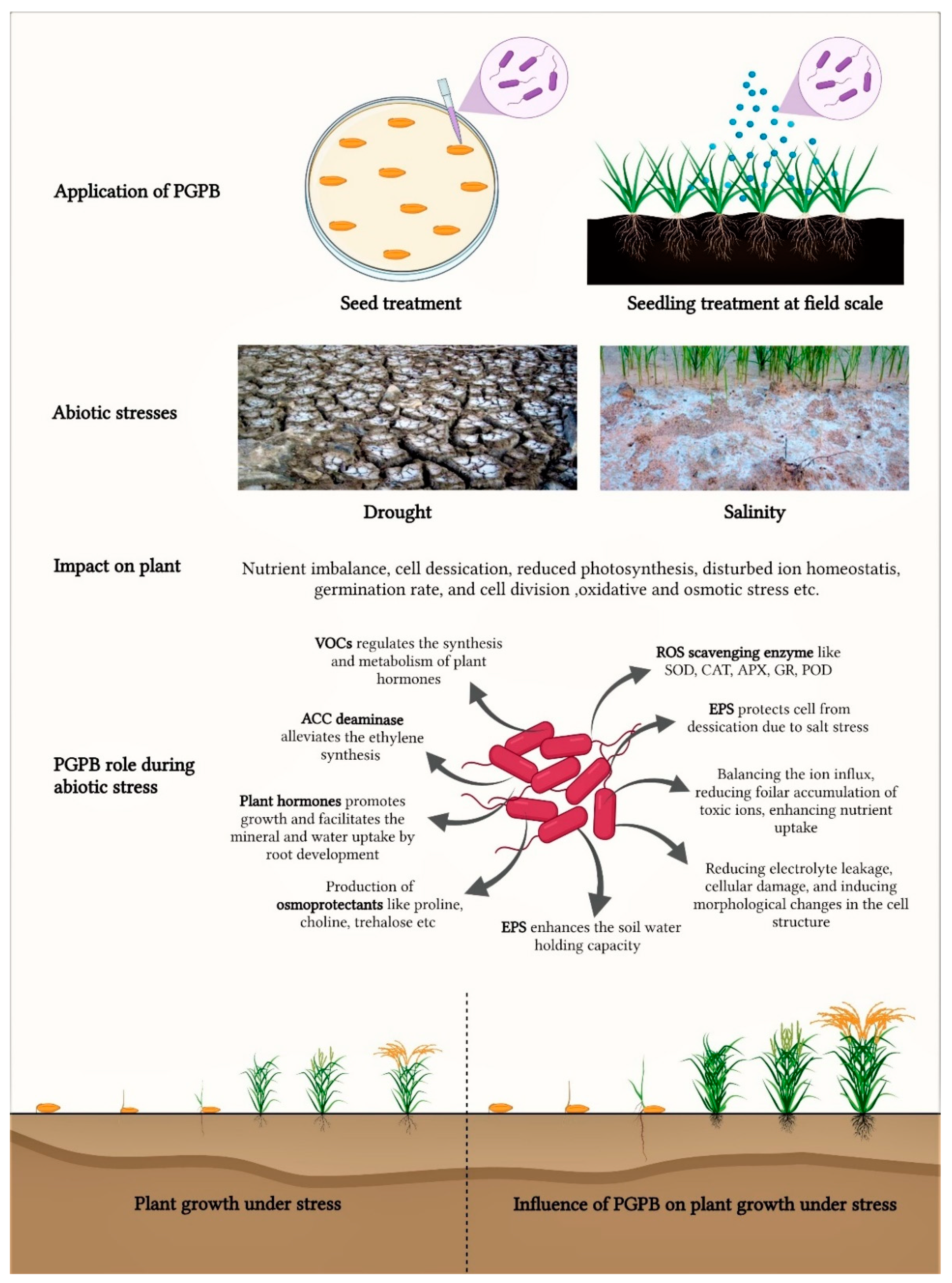
The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture
Abstract
:1. Introduction
2. Climate Change and Its Impact on Agriculture
2.1. Global Warming
2.2. Greenhouse Gas Emission
2.3. Abiotic Stresses
2.3.1. Salinization
2.3.2. Alkalinity and Acidity
2.3.3. Drought
3. Plant Growth Promoting Bacteria (PGPB)
3.1. About PGPB
3.2. Abiotic Stress Tolerant PGPB
4. PGPB and Its Role in Inducing Different Abiotic Stress Tolerance in Plants
4.1. PGPB Induced Salinity Tolerance
4.2. PGPB Induced Drought Tolerance
4.3. Challenges and Limitations Associated with PGPB Application
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; Enshasy, H.E. Plant growth promoting rhizobacteria (pgpr) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Naik, K.; Mishra, S.; Srichandan, H.; Singh, P.K.; Sarangi, P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol. 2019, 21, 101326. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Q.; Ervin, E.H.; Yang, Z.; Zhang, X. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front. Plant Sci. 2017, 8, 1017. [Google Scholar] [CrossRef] [Green Version]
- Delgado, C.; Mora-poblete, F.; Ahmar, S.; Chen, J.-T.; Figueroa, C.R. Jasmonates and plant salt stress: Molecular players, physiological effects, and improving tolerance by using genome-associated tools. Int. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer Nature International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef] [Green Version]
- Rizza, A.; Tang, B.; Stanley, C.E.; Grossmann, G.; Owen, M.R.; Band, L.R.; Jones, A.M. Differential biosynthesis and cellular permeability explain longitudinal gibberellin gradients in growing roots. Proc. Natl. Acad. Sci. USA 2021, 118, e1921960118. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [Green Version]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Arneth, A.; Denton, F.; Agus, F.; Elbehri, A.; Erb, K.; Osman Elasha, B.; Rahimi, M.; Rounsevell, M.; Spence, A.; Valentini, R. Framing and Context. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; The Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019; pp. 77–128. [Google Scholar]
- Reid, T.E.; Kavamura, V.N.; Abadie, M.; Torres-Ballesteros, A.; Pawlett, M.; Clark, I.M.; Harris, J.; Mauchline, T.H. Inorganic chemical fertilizer application to wheat reduces the abundance of putative plant growth-promoting rhizobacteria. Front. Microbiol. 2021, 12, 642587. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic climate change has slowed global agricultural productivity growth. Nat. Clim. Chang. 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Jansson, J.K.; Remais, J.V.; Rich, V.I.; Singh, B.K.; Trivedi, P. Climate change microbiology—Problems and perspectives. Nat. Rev. Microbiol. 2019, 17, 391–396. [Google Scholar] [CrossRef]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Barnard, P.; Moomaw, W.R. World scientists’ warning of a climate emergency. BioScience 2020, 70, 8–12. [Google Scholar] [CrossRef]
- Rising, J.; Devineni, N. Crop switching reduces agricultural losses from climate change in the united states by half under RCP 8.5. Nat. Commun. 2020, 11, 4991. [Google Scholar] [CrossRef] [PubMed]
- Camaille, M.; Fabre, N.; Clément, C.; Barka, E.A. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Diaz, P.A.E.; Lobo, L.L.B.; Rigobelo, E.C. Use of plant growth-promoting rhizobacteria in maize and sugarcane: Characteristics and applications. Front. Sustain. Food Syst. 2020, 4, 136. [Google Scholar] [CrossRef]
- Jobe, T.O.; Karvansara, P.R.; Zenzen, I.; Kopriva, S. Ensuring nutritious food under elevated CO2 conditions: A case for improved C4 crops. Front. Plant Sci. 2020, 11, 1267. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- He, A.-L.; Niu, S.-Q.; Zhao, Q.; Li, Y.-S.; Gou, J.-Y.; Gao, H.-J.; Suo, S.-Z.; Zhang, J.-L. Induced salt tolerance of perennial ryegrass by a novel bacterium strain from the rhizosphere of a desert shrub Haloxylon ammodendron. Int. J. Mol. Sci. 2018, 19, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahkami, A.H.; White, R.A.; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 233–243. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Romero-Puertas, M.C.; Bellin, D.; Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- Kumar, N.; Khamzina, A.; Knöfel, P.; Lamers, J.P.A.; Tischbein, B. Afforestation of degraded croplands as a water-saving option in irrigated region of the Aral sea basin. Water 2021, 13, 1433. [Google Scholar] [CrossRef]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate change and global food systems: Potential impacts on food security and undernutrition. Annu. Rev. Public Heal. 2017, 38, 259–277. [Google Scholar] [CrossRef]
- Laanisto, L.; Niinemets, Ü. Polytolerance to abiotic stresses: How universal is the shade-drought tolerance trade-off in woody species? Glob. Ecol. Biogeogr. 2015, 24, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.R.; Dube, O.P.; Solecki, W.; Aragón-Durand, F.; Cramer, W.; Humphreys, S.; Kainuma, M.; Kala, J.; Mahowald, N.; Mulugetta, Y.; et al. Framing and Context. In Global Warming of 1.5 C: An IPCC Special Report on the Impacts of Global Warming of 1.5 C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Sustainable Development, and Efforts to Eradicate Poverty’; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; The Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2018; pp. 41–91. [Google Scholar]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [Green Version]
- Seguini, L.; Bussay, A.; Baruth, B. From extreme weather to impacts: The role of the areas of concern maps in the JRC MARS Bulletin. Agric. Syst. 2019, 168, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crafts-Brandner, S.J.; Salvucci, M.E.; Schultz, C.J.; Rumsewicz, M.P.; Johnson, K.L.; Jones, B.J.; Gaspar, Y.M.; Bacic, A. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Pan, C.; Gu, S.; Ma, Q.; Zhang, Y.; Li, X.; Shi, K. Stomatal movements are involved in elevated CO2 -mitigated high temperature stress in tomato. Physiol. Plant. 2019, 165, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Barradas, M.C.; Gallego-Fernández, J.B.; Zunzunegui, M. Plant response to water stress of native and non-native Oenothera drummondii populations. Plant Physiol. Biochem. 2020, 154, 219–228. [Google Scholar] [CrossRef]
- Russo, S.; Dosio, A.; Graversen, R.G.; Sillmann, J.; Carrao, H.; Dunbar, M.B.; Singleton, A.; Montagna, P.; Barbola, P.; Vogt, J.V. Magnitude of extreme heat waves in present climate and their projection in a warming world. J. Geophys. Res. Atmos. 2014, 119, 12500–12512. [Google Scholar] [CrossRef] [Green Version]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef]
- Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Cohen, S.P.; Leach, J.E. Abiotic and biotic stresses induce a core transcriptome response in rice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Hawkings, J.R.; Linhoff, B.S.; Wadham, J.L.; Stibal, M.; Lamborg, C.H.; Carling, G.T.; Lamarche-Gagnon, G.; Kohler, T.J.; Ward, R.; Hendry, K.R.; et al. Large subglacial source of mercury from the southwestern margin of the greenland ice sheet. Nat. Geosci. 2021. [Google Scholar] [CrossRef]
- Yue, X.-L.; Gao, Q.-X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim. Chang. Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Wu, B.; Mu, C. Effects on greenhouse gas (CH4, CO2, N2O) emissions of conversion from over-mature forest to secondary forest and Korean pine plantation in northeast China. Forests 2019, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, A.M.; Abubakar, A.B.; Mamman, S.O. Relationship between greenhouse gas emission, energy consumption, and economic growth: Evidence from some selected oil-producing African countries. Environ. Sci. Pollut. Res. Int. 2020, 27, 15815–15823. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Younis, M.; Ullah, I. Impact of urbanization and economic growth on CO2 emission: A case of far East Asian countries. Int. J. Environ. Res. Public Health 2020, 17, 2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gołasa, P.; Wysokiński, M.; Bieńkowska-gołasa, W.; Gradziuk, P.; Golonko, M.; Gradziuk, B.; Siedlecka, A.; Gromada, A. Sources of greenhouse gas emissions in agriculture, with particular emphasis on emissions from energy used. Energies 2021, 14, 3784. [Google Scholar] [CrossRef]
- US EPA. Greenhouse Gas (GHG) Emissions. Sources of Greenhouse Gas Emissions. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions#agriculture (accessed on 8 June 2021).
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife 2014, 3, e02245. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Pramanik, K.; Mitra, S.; Soren, T.; Maiti, T.K. Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J. Plant Physiol. 2018, 231, 434–442. [Google Scholar] [CrossRef]
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 2016, 56, 1274–1288. [Google Scholar] [CrossRef]
- Vargas, R.; Pankova, E.I.; Balyuk, S.A.; Krasilnikov, P.V.; Khasankhanova, G.M. Handbook for SALINE Soil Management; FAO/LMSU: Rome, Italy, 2018. [Google Scholar]
- Suárez, R.; Wong, A.; Ramírez, M.; Barraza, A.; Orozco, M.D.C.; Cevallos, M.A.; Lara, M.; Hernández, G.; Iturriaga, G. Improvement of drought tolerance and grain yield in common bean by overexpressing Trehalose-6-Phosphate Synthase in rhizobia. Mol. Plant-Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; Naveed, M.; Zahir, Z.A.; Jacobsen, S.E. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632–642. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- FAO and ITPS. Status of the World’s Soil Resources (SWSR)—Main Report; FAO: Rome, Italy, 2017. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Cohan, F.M. Transmission in the origins of bacterial diversity, from ecotypes to phyla. Microb. Transm. 2019, 100, 311–343. [Google Scholar] [CrossRef] [Green Version]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, M.; Mishra, J.; Mishra, V. Environmental sustainability: Challenges and viable solutions. Environ. Sustain. 2018, 1, 309–340. [Google Scholar] [CrossRef]
- Aslam, F.; Ali, B. Halotolerant bacterial diversity associated with Suaeda fruticosa (L.) Forssk. Improved growth of maize under salinity stress. Agronomy 2018, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Alberton, D.; Valdameri, G.; Moure, V.R.; Monteiro, R.A.; de Oliveira Pedrosa, F.; Müller-Santos, M.; de Souza, E.M. What did we learn from plant growth-promoting rhizobacteria (pgpr)-grass associations studies through proteomic and metabolomic approaches? Front. Sustain. Food Syst. 2020, 4, 607343. [Google Scholar] [CrossRef]
- Sunita, K.; Mishra, I.; Mishra, J.; Prakash, J.; Arora, N.K. Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Front. Microbiol. 2020, 11, 567768. [Google Scholar] [CrossRef]
- Sunjeet, K.; Gaojie, L.; Jingjing, Y.; Xinfang, H.; Qun, J.; Zhengwei, L.; Weidong, K.; Hongwei, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant. Sci. 2021, 12, 1176. [Google Scholar] [CrossRef]
- Mahadik, S.; Kumudini, B.S. Enhancement of salinity stress tolerance and plant growth in finger millet using fluorescent Pseudomonads. Rhizosphere 2020, 15, 100226. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A review on potential plant-basedwater stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Demir, I.; Mavi, K. Effect of salt and osmotic stresses on the germination of pepper seeds of different maturation stages. Braz. Arch. Biol. Technol. 2008, 51, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.Q.; Jiao, Q.; Shui, Q.Z. Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower (Helianthus annuus L.). Plant Soil Environ. 2015, 61, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.; Luo, X.; Shuai, H.; Zhou, W.; Ding, J.; Du, J.; Liu, J.; et al. Salt stress represses soybean seed germination by negatively regulating ga biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghel, L.; Kataria, S.; Jain, M. Mitigation of adverse effects of salt stress on germination, growth, photosynthetic efficiency and yield in maize (Zea mays L.) through magnetopriming. Acta Agrobot. 2019, 72. [Google Scholar] [CrossRef] [Green Version]
- Neina, D. The Role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Naing, A.H.; Lee, D.B.; Ai, T.N.; Lim, K.B.; Kim, C.K. Enhancement of low ph stress tolerance in anthocyanin-enriched transgenic Petunia overexpressing Rsmyb1 Gene. Front. Plant Sci. 2018, 9, 1124. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.; Trenberth, K.E. Climate change and drought: A perspective on drought indices. Curr. Clim. Chang. Rep. 2018, 4, 145–163. [Google Scholar] [CrossRef]
- Golan, Y.; Shirron, N.; Avni, A.; Shmoish, M.; Gepstein, S. Cytokinins induce transcriptional reprograming and improve Arabidopsis plant performance under drought and salt stress conditions. Front. Environ. Sci. 2016, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional alterations reveal Bacillus amyloliquefaciens rice cooperation under salt stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.-L.; Zhang, R.-X.; Yuan, H.-Y.; Wang, M.-M.; Yang, H.-Y.; Ma, H.-Y.; Liu, D.; Jiang, C.-J.; Liang, Z.-W. Root Damage under Alkaline Stress Is Associated with Reactive Oxygen Species Accumulation in Rice (Oryza Sativa L.). Front. Plant Sci. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, C.; Li, G.; Khan, M.N.; Wu, H. Ros Homeostasis and Plant Salt Tolerance: Plant Nanobiotechnology Updates. Sustainability 2021, 13, 3552. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Alenezi, F.N.; Luptakova, L.; Bouremani, N.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Tailoring next generation plant growth promoting microorganisms as versatile tools beyond soil desalinization: A road map towards field application. Sustainability 2021, 13, 4422. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.-J.; Lee, C.H.; Kim, Y.-J.; Koh, S.C.; Yang, D.C. A growth-promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Ha-tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Moreno-Galván, A.; Romero-Perdomo, F.A.; Estrada-Bonilla, G.; Meneses, C.H.S.G.; Bonilla, R.R. Dry-Caribbean Bacillus spp. strains ameliorate drought stress in maize by a strain-specific antioxidant response modulation. Microorganisms 2020, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-tolerant Bacillus species as a promising strategy to mitigate the salinity stress in wheat (Triticum turgidum subsp. Durum). J. Arid. Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Wu, W.; Liu, H. Factors affecting variations of soil ph in different horizons in hilly regions. PLoS ONE 2019, 14, e0218563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-T.; Mu, C.-S. Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Sci. Plant Nutr. 2009, 55, 685–697. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Hirai, Y. Good and bad protons: Genetic aspects of acidity stress responses in plants. J. Exp. Bot. 2016, 67, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, A.F. Hydrological drought explained. Wiley Interdiscip. Rev. Water 2015, 2, 359–392. [Google Scholar] [CrossRef]
- Sharma, P.; Kumawat, K.C.; Kaur, S. Plant growth promoting rhizobacteria in nutrient enrichment: Current perspectives. Biofort. Food Crops 2016, 263–289. [Google Scholar] [CrossRef]
- Rodríguez-Salazar, J.; Suárez, R.; Caballero-Mellado, J.; Iturriaga, G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009, 296, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, P.; Das, B.S.; Sen, S.K. Soil water potential and recoverable water stress in drought tolerant and susceptible rice varieties. Agric. Water Manag. 2015, 152, 110–118. [Google Scholar] [CrossRef]
- Papastefanou, P.; Zang, C.S.; Pugh, T.A.M.; Liu, D.; Grams, T.E.E.; Hickler, T.; Rammig, A. A dynamic model for strategies and dynamics of plant water-potential regulation under drought conditions. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [Green Version]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ye, J.; Kong, Q. Potassium solubilizing activity of Bacillus aryabhattai SK1-7 and its growth-promoting effect on Populus alba L. Forests 2020, 11, 1348. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; Alkahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas Aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Cordovez, V.; Schop, S.; Hordijk, K.; de Boulois, H.D.; Coppens, F.; Hanssen, I.; Raaijmakers, J.M.; Carrióna, V.J. Priming of plant growth promotion by volatiles of root associated Microbacterium spp. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016, 7, 1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashfaq, M.; Hassan, H.M.; Ghazali, A.H.A.; Ahmad, M. Halotolerant Potassium solubilizing plant growth promoting rhizobacteria may improve potassium availability under saline conditions. Environ. Monitor. Assess. 2020, 192, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growthpromoting rhizobacteria containing 1-Aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR mediated alterations in root traits: Way toward sustainable crop production. Front. Sustain. Food Syst. 2021, 4, 287. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and above ground physiology than rhizosphere p solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef]
- Lee, T.; Park, D.; Kim, K.; Lim, S.M.; Yu, N.H.; Kim, S.; Kim, H.-Y.; Jung, K.S.; Jang, J.Y.; Park, J.-C.; et al. Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathol. J. 2017, 33, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Wu, X.; Wang, J.; Ding, X. Phosphate solubilization and gene expression of phosphate-solubilizing bacterium Burkholderia multivorans WS-FJ9 under different levels of soluble phosphate. J. Microbiol. Biotechnol. 2017, 27, 844–855. [Google Scholar] [CrossRef]
- Bhatt, K.; Maheshwari, D.K. Decoding Multifarious role of cow dung bacteria in mobilization of zinc fractions along with growth promotion of C. annuum L. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Jain, D.; Kour, R.; Bhojiya, A.A.; Meena, R.H.; Singh, A.; Mohanty, S.R.; Rajpurohit, D.; Ameta, K.D. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, P.; Zebelo, S.; McNear, D.; Kloepper, J.; Fadamiro, H. Plant growth-promoting rhizobacteria induce changes in Arabidopsis thaliana gene expression of nitrate and ammonium uptake genes. J. Plant Interact. 2019, 14, 224–231. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; De Salamone, I.E.G.; Nelson, L.M.; Novák, O.; Strnad, M.; Van Der Graaff, E.; Roitsch, T. Cytokinin production by Pseudomonas fluorescens G20-18 determines biocontrol activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef] [PubMed]
- Parmar, Y.S.; Sharma, S.; Sharma, A.; Kaur, M. Extraction and evaluation of gibberellic acid from Pseudomonas sp.: Plant growth promoting rhizobacteria. J. Pharmacogn. Phytochem. 2018, 7, 2790–2795. [Google Scholar]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- García-Fraile, P.; Menéndez, E.; Celador-Lera, L.; Díez-Méndez, A.; Jiménez-Gómez, A.; Marcos-García, M.; Cruz-González, X.A.; Martínez-Hidalgo, P.; Mateos, P.F.; Rivas, R. Bacterial probiotics: A truly green revolution. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 131–162. [Google Scholar] [CrossRef]
- Adeleke, R.A.; Raimi, A.R.; Roopnarain, A.; Mokubedi, S.M. Status and Prospects of Bacterial Inoculants for Sustainable Management of Agroecosystems. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Soil Biology; Springer: Cham, Switzerland, 2019; pp. 137–172. [Google Scholar] [CrossRef]
- Lobo, C.B.; Tomás, M.S.J.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- Pozo, M.J.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef]
- Sood, G.; Kaushal, R.; Chauhan, A.; Gupta, S. Indigenous plant-growth-promoting rhizobacteria and chemical fertilisers: Impact on wheat (Triticum aestivum) productivity and soil properties in north western himalayan region. Crop Pasture Sci. 2018, 69, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Godínez, L.J.; Fernandez-Valverde, S.L.; Romero, J.C.M.; Martínez-Romero, E. Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Syst. Appl. Microbiol. 2019, 42, 517–525. [Google Scholar] [CrossRef]
- Verma, D.K.; Pandey, A.K.; Mohapatra, B.; Srivastava, S.; Kumar, V.; Talukdar, D.; Yulianto, R.; Zuan, A.T.K.; Jobanputra, A.H.; Asthir, B. Plant growth-promoting rhizobacteria: An eco-friendly approach for sustainable agriculture and improved crop production. In Microbiology for Sustainable Agriculture, Soil Health, and Environmental Protection; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 3–80. [Google Scholar] [CrossRef]
- Pranaw, K.; Pidlisnyuk, V.; Trögl, J.; Malinská, H. Bioprospecting of a novel plant growth-promoting bacterium Bacillus altitudinis KP-14 for enhancing Miscanthus × giganteus growth in metals contaminated soil. Biology 2020, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, F.M.; Romero, D.; Pérez-García, A.; Lugtenberg, B.J.J.; De Vicente, A.; Bloemberg, G. Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J. Appl. Microbiol. 2007, 103, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Qessaoui, R.; Bouharroud, R.; Furze, J.N.; El Aalaoui, M.; Akroud, H.; Amarraque, A.; Van Vaerenbergh, J.; Tahzima, R.; Mayad, E.H.; Chebli, B. Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic effects of plant growth promoting rhizobacteria and chitosan on in vitro seeds germination, greenhouse growth, and nutrient uptake of maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Irizarry, I.; White, J.F. Application of bacteria from non-cultivated plants to promote growth, alter root architecture and alleviate salt stress of cotton. J. Appl. Microbiol. 2017, 122, 1110–1120. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant rhizobacterial strains mitigate the adverse effects of NaCl stress in soybean seedlings. Biomed Res. Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nagaraju, Y.; Gundappagol, R.C.; Swamy, M. Mining saline soils to manifest plant stress-alleviating halophilic bacteria. Curr. Microbiol. 2020, 77, 2265–2278. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Kotowska, U.; Wydrych, J.; Polińska, W.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Exopolysaccharide carbohydrate structure and biofilm formation by Rhizobium leguminosarum bv. trifolii strains inhabiting nodules of trifolium repens growing on an old Zn–Pb–Cd-polluted waste heap area. Int. J. Mol. Sci. 2021, 22, 2808. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K. Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr. Microbiol. 2014, 69, 484–494. [Google Scholar] [CrossRef]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef] [Green Version]
- Netzker, T.; Shepherdson, E.M.F.; Zambri, M.P.; Elliot, M.A. Bacterial volatile compounds: Functions in communication, cooperation, and competition. Annu. Rev. Microbiol. 2020, 74, 409–430. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Nazir, N.; Kamili, A.N.; Shah, D. Mechanism of plant growth promoting rhizobacteria (PGPR) in Enhancing plant growth—A review. Int. J. Manag. Technol. Eng. 2018, 8, 709–721. [Google Scholar]
- Wang, C.; Wang, H.; Li, Y.; Li, Q.; Yan, W.; Zhang, Y.; Wu, Z.; Zhou, Q. Plant growth-promoting rhizobacteria isolation from rhizosphere of submerged macrophytes and their growth-promoting effect on Vallisneria natans under high sediment organic matter load. Microb. Biotechnol. 2021, 14, 726–736. [Google Scholar] [CrossRef]
- Poulami, C.; Sandipan, S.; Rangasamy, A.; Yeongyeong, K.; Kiyoon, K.; Gopal, S.; Tongmin, S. Beneficial Soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P.; Saikia, A.R. Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr. Microbiol. 2009, 59, 489–496. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, C.; Wang, Y.; Xia, Y.; Xiao, W.; Cui, X. Salt-tolerant and plant-growth-promoting bacteria isolated from high-yield paddy soil. Can. J. Microbiol. 2018, 64, 968–978. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella Sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef]
- Cai, D.; Xu, Y.; Zhao, F.; Zhang, Y.; Duan, H.; Guo, X. Improved salt tolerance of Chenopodium quinoa willd. contributed by Pseudomonas sp. strain M30-35. PeerJ 2021, 9, e10702. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, J.; Thakur, D. Evaluation of multifarious plant growth promoting traits, antagonistic potential and phylogenetic affiliation of rhizobacteria associated with commercial tea plants grown in darjeeling, India. PLoS ONE 2017, 12, e0182302. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus Spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Sirajuddin; Zhao, X.Q.; Javed, M.T.; Khan, K.S.; Bano, A.; Shen, R.F.; Masood, S. Bacillus pumilus enhances tolerance in rice (Oryza sativa L.) to combined stresses of nacl and high boron due to limited uptake of Na+. Environ. Exp. Bot. 2016, 124, 120–129. [Google Scholar] [CrossRef]
- Shilev, S.; Sancho, E.D.; Benlloch-González, M. Rhizospheric bacteria alleviate salt-produced stress in sunflower. J. Environ. Manag. 2012, 95, S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wu, Z.; Zheng, Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Hahm, M.-S.; Son, J.-S.; Hwang, Y.-J.; Kwon, D.-K.; Ghim, S.-Y. Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. J. Microbiol. Biotechnol. 2017, 27, 1790–1797. [Google Scholar] [CrossRef] [Green Version]
- Tewari, S.; Sharma, S. Rhizobial exopolysaccharides as supplement for enhancing nodulation and growth attributes of Cajanus cajan under multi-stress conditions: A study from lab to field. Soil Tillage Res. 2020, 198, 104545. [Google Scholar] [CrossRef]
- Lee, B.; Farag, M.-A.; Park, H.-B.; Kloepper, J.-W.; Lee, S.-H.; Ryu, C.-M. Induced resistance by a long-chain bacterial volatile: Elicitation of plant systemic defense by a c13 volatile produced by Paenibacillus polymyxa. PLoS ONE 2012, 7, e48744. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ahmad, I. Fluorescent Pseudomonas-FAP2 and Bacillus licheniformis interact positively in biofilm mode enhancing plant growth and photosynthetic attributes. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. 3 Biotech 2020, 10, 1–12. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Ansari, M.W.; Pradhan, M.; Dangar, T.K.; Mohanty, S.; Tuteja, N. A novel Azotobacter vinellandii (SRIAz3) functions in salinity stress tolerance in rice. Plant Signal. Behav. 2014, 9, e29377. [Google Scholar] [CrossRef] [Green Version]
- Del Carmen Orozco-Mosqueda, M.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The production of ACC deaminase and trehalose by the plant growth promoting bacterium Pseudomonas Sp. UW4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar] [CrossRef] [Green Version]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ.-Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Saud, H.M.; Ismail, M.R.; Othman, R. Molecular characterization of stress tolerant plant growth promoting rhizobacteria (PGPR) for growth rnhancement of rice. Int. J. Agric. Biol. 2016, 18, 184–191. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Damodharan, K.; Yang, S.H.; Suh, J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of “Micro Tom” tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.A.; Akram, W.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A. Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ. Sci. Pollut. Res. 2018, 25, 23236–23250. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-Ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-Aminocyclopropane-1-carboxylate (ACC) in plant–bacterial interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef]
- Vimal, S.R.; Patel, V.K.; Singh, J.S. Plant growth promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Sales, C.R.G.; Da Silva, A.B.; Carmo-Silva, E. Measuring rubisco activity: Challenges and opportunities of NADH-Linked microtiter plate-based and 14 C-based assays. J. Exp. Bot. 2020, 71, 5302–5312. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM Fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Singh, V.K.; Mishra, A. halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 568289. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, J.P. Does plant—microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Cytokinin inhibition of leaf senescence. Plant Signal. Behav. 2013, 8, e24737. [Google Scholar] [CrossRef]
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.K. Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of Drought Stress Tolerance in Crops by Plant Growth Promoting Rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Creus, C.M.; Graziano, M.; Casanovas, E.M.; Pereyra, M.A.; Simontacchi, M.; Puntarulo, S.; Barassi, C.A.; Lamattina, L. Nitric oxide is involved in the Azospirillum brasilense induced lateral root formation in tomato. Planta 2005, 221, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Water Relations and yield in Azospirillum inoculated wheat exposed to drought in the field. Can. J. Bot. 2004, 82, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Sandhya, V.Z.A.S.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Armada, E.; Probanza, A.; Roldán, A.; Azcón, R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2016, 192, 1–12. [Google Scholar] [CrossRef]
- Shintu, P.V.; Jayaram, K.M. Phosphate solubilising bacteria (Bacillus polymyxa) -An Effective approach to mitigate drought in tomato (Lycopersicon esculentum mill.). Trop. Plant Res. 2015, 2, 17–22. [Google Scholar]
- Gou, W.; Zheng, P.; Chen, F.; Zhang, L.; Cui, Z.; Cao, M.; Zhang, L.; Hu, J. Accumulation of choline and glycinebetaine and drought stress tolerance induced in maize (Zea mays) by three plant growth promoting rhizobacteria (PGPR) strains. Pakistan J. Bot. 2015, 47, 581–586. [Google Scholar]
- Zhang, H.; Murzello, C.; Sun, Y.; Kim, M.S.; Xie, X.; Jeter, R.M.; Zak, J.C.; Dowd, S.E.; Paré, P.W. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). MPMI 2010, 23, 1097–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, B.; Chaudhary, A.; Singh, H.; Kumar, P.A. Prospective evaluation of individual and consortia plant growth promoting rhizobacteria for drought stress amelioration in rice (Oryza sativa L.). Plant Soil 2020, 457, 225–240. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.-J.; Park, J.-M.; Kim, B.-R.; Shin, D.-H.; Lee, I.-J. Gibberellin secreting rhizobacterium, Pseudomonas putida h-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kim, S.-D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R,3R-Butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, Is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; El-Daim, I.A.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant. Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Munir, A.; Asghar, H.N.; Shaharoona, B.; Arshad, M. Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J. Microbiol. Biotechnol. 2008, 18, 958–963. [Google Scholar]
- Vargas, L.; Brígida, A.B.S.; Filho, J.P.M.; De Carvalho, T.G.; Rojas, C.A.; Vaneechoutte, D.; Van Bel, M.; Farrinelli, L.; Ferreira, P.C.G.; Vandepoele, K.; et al. Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: A transcriptomic view of hormone pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Smith, M.D.; Glick, B.R.; Liang, Y. Effects of ACC deaminase containing rhizobacteria on plant growth and expression of TOC GTPases in tomato (Solanum lycopersicum) under salt stress. Botany 2014, 92, 775–781. [Google Scholar] [CrossRef]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Sen, S.; Chandrasekhar, C.N. Effect of PGPR on growth promotion of rice (Oryza sativa L.) under salt stress. Asian J. Plant. Sci. Res. 2014, 4, 62–67. [Google Scholar]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.V.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Bano, A. Isolation of PGPRS from rhizospheric soil of halophytes and its impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 2015, 61, 307–313. [Google Scholar] [CrossRef]
- Morcillo, R.J.L.; Manzanera, M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Chebotar, V.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. South Afr. J. Bot. 2017, 116, 91–102. [Google Scholar] [CrossRef]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; You, Y.-H.; Kim, J.-H.; Kim, J.-G.; Hamayun, M.; Lee, I.-J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant. Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.G.S. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 07, 096–102. [Google Scholar] [CrossRef]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Khan, M.S.I.; Shahid, N.; Aaliya, K. Bottlenecks in commercialisation and future prospects of PGPR. Appl. Soil Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
| Abiotic Stress | Effect on Plant | Alleviation Mechanism | PGPB | Crop | References |
|---|---|---|---|---|---|
| Drought | Increased plant growth | GA production by bacteria. Decreased level of ABA and salicylic acid, higher level of JA in plants. | Pseudomonas putida H-2-3 | Soybean (Glycine max L.) | [201] |
| Increased plant biomass | IAA, GA, SA production, ACC activity by bacteria. | Microbacterium sp. 3J1 | Tomato (Solanum Lycopersicum L.) | [111] | |
| Pepper (Capsicum annum L.) | |||||
| Enhanced proline synthesis by plants. | Azospirillum brasilense | Maize (Zea mays L.) | [192] | ||
| Increased K+ and P+ uptake, as well as proline accumulation in plants. | Bacillus sp. | Maize (Zea mays L.) | [88] | ||
| Decreased level of GPOX, CAT and SOD in plants. | Bacillus sp. | Rice (Oryza sativa L.) | [200] | ||
| ACC deaminase production by bacteria. | Achromobacter piechaudii ARV8 | Tomato (Solanum Lycopersicum L.) | [202] | ||
| Pepper (Capsicum annum L.) | |||||
| Upregulation of stress protein genes Cadhn, VA, sHSP and CaPR-10 | B. licheniformis K11 → | Pepper (Capsicum annum L.) | [203] | ||
| Leaf water status improved | Phosphate solubilization, ACC deaminase, IAA, HCN and siderophores production by bacteria. Enhanced accumulation of antioxidant enzymes and proline in plants. | P. aeruginosa GGRJ21 | Mung bean (Vigna radiata L.) | [109] | |
| Auxin production by bacteria. | Azospirillum lipoferum AZ45 | Wheat (Triticum aestivum L.) | [63] | ||
| Enhanced proline synthesis by plant. | A. brasilense | Maize (Zea mays L.) | [192] | ||
| Enhanced proline synthesis by plant. Increased cytokinin level in shoots and root exudates in plants. | P. putida GAP-45 | Maize (Zea mays L.) | [195] | ||
| B. subtilis | Platycladus orientalis | [190] | |||
| IAA production, P solubilization, ACC deaminase activity by bacteria. | Azospirillum sp. | Wheat (Triticum aestivum L.) | [63] | ||
| Elevated ABA concentration in plants. | A. brasilense | Arabidopsis thaliana L. | [192,204] | ||
| Reduced water loss | 2R, 3R- butanediol released by bacteria. | P. chlororaphis O6 | Arabidopsis thaliana L. | [205] | |
| Choline accumulation, as a precursor of glycine betaine in plants. | Klebsiella variicola F2, P. fluorescens YX2 and Raoultella planticola YL2 | Maize (Zea mays L.) | [198] | ||
| Increased water content | Choline accumulation, as a precursor of glycine betaine in plants.N fixation, IAA and ACC deaminase production by bacteria. | B. subtilis GB03 | Arabidopsis thaliana L. | [199] | |
| Azospirillum sp. AZ1 | Garden pea (Pisum sativum L.) | [63] | |||
| EPS production by bacteria. | P. putida GAP-45 | Sunflower (Helianthus annuus L.) | [195] | ||
| Upregulation of stress specific genes APX1, SAMS1, and HSP17.8 | Bacillus amyloliquefaciens 5113 and Azospirillum brasilense NO40 | Wheat (Triticum aestivum L.) | [206] | ||
| IAA-production by bacteria. | Klebsiella sp. IG 3 | Wheat (Triticum aestivum L.) | [20] | ||
| Enhanced length and number of roots | ACC deaminase and IAA production by bacteria. | P. aeruginosa GGRJ21 | Mung bean (Vigna radiata L.) | [109] | |
| ACC deaminase production by bacteria. | P. fluorescens | Garden pea (Pisum sativum L.) | [207] | ||
| Longer roots | Nitric oxide production by bacteria. | A. brasilense | Tomato (Solanum Lycopersicum L.) | [193] | |
| Enhanced adventitious root development | Increase of IAA content in plants. | B. subtilis LDR2 | Wheat (Triticum aestivum L.) | [20] | |
| Higher photosynthetic efficiency | N fixation; auxin and ACC deaminase production; P solubilization by bacteria. | A. lipoferum AZ45 | Wheat (Triticum aestivum L.) | [63] | |
| Higher growth and yield | Decreased level of GPOX, CAT and SOD in plants. | Bacillus sp. | Rice (Oryza sativa L.) | [200] | |
| N fixation, IAA and ACC deaminase production, P solubilization by bacteria. | Azospirillum sp. AZ45 | Wheat (Triticum aestivum L.) | [63] | ||
| ABA-dependent signaling genes activation | Gluconacetobacter diazotrophicus PAL5 | Sugarcane (Saccharum spp.) | [208] | ||
| ACC deaminase production by microbial consortium Downregulation of ACC-oxidase gene expression. | Ochrobactrum pseudogrignonense RJ12, Pseudomonas sp. RJ15 and B. subtilis RJ46 | Black gram (Vigna mungo L.), Garden pea (Pisum sativum L.) | [109] | ||
| Increased chlorophyll synthesis in leaf | Gibberellin production by bacteria. | P. putida H-2-3 | Soybean (Glycine max L.) | [201] | |
| Up-regulation of expression profile of rbcL gene and down-regulation of WRKY1 gene in plant. | Klebsiella sp. | Common oat (Avena sativa L.) | [151] | ||
| Salinity | Enhanced photosynthetic activity | IAA, siderophore production and phosphate solubilization by bacteria. | Microbacterium oleivorans KNUC7074, Brevibacterium iodinum KNUC7183, Rhizobium massiliae KNUC7586 | Pepper (Capsicum annum L.) | [160] |
| Enhanced chlorophyll content | IAA production, N–fixation, phosphate solubilization by bacteria. | Bacillus sp. UPMR7 and Citrobacter sp. UPMR17 | Rice (Oryza sativa L.) | [172] | |
| Upregulation of Toc GTPase genes | Pseudomonas putida UW4 | Tomato (Solanum lycopersicum) | [209] | ||
| IAA production, phosphate solubilization ACC deaminase activity by bacteria. | Streptomyces sp. PGPA39 | Tomato (Solanum Lycopersicum L.) | [173] | ||
| IAA and siderophore production, phosphate solubilization by bacteria. | M. oleivorans KNUC7074, B. iodinum KNUC7183, R. massiliae KNUC7586 | Pepper (Capsicum annum L.) | [160] | ||
| Increased leaf water content | Enhanced proline synthesis by plant with reduction in electrolyte leakage, increased K+ uptake and decreased Na+/K+ ratio. | Rhizobium sp., Pseudomonas sp. | Maize (Zea mays L.) | [210] | |
| Increased proline content and soluble sugars, lower MDA in plants. | Brachybacterium saurashtrense (JG-06), Brevibacterium casei (JG-08), Haererohalobacter (JG-11) | Peanut (Arachis hypogaea) | [90] | ||
| Increased shoot and root water content | EPS production by bacteria. | Enterobacter sp. MN17 and Bacillus sp. MN54 | Quinoa (Chenopodium quinoa L.) | [58] | |
| Improved production of IAA and decreased ABA synthesis in plants;increased Mg2+, K+,Ca2+ and decreased Na+ uptake by roots. | P. putida Rs-198 | Cotton (Gossypium hirsutum L.) | [158] | ||
| Promoted seedling growth | ACC deaminase production, higher antioxidant enzymatic activities and decreased ethylene production in plants. | Burkholderia sp. MTCC 12,259 | Rice (Oryza sativa L.) | [54] | |
| Lower level of ABA and SA in plants. | P. putida KT2440 or Novosphingobium sp. HR1a | Citrus plants | [175] | ||
| Salinity damage prevention | Higher activity of peroxidase, catalase and nitrate reductase in plants. | Pseudomonas sp. PF1, Pseudomonas sp. TDK1 | Rice (Oryza sativa L.) | [211] | |
| Upregulation of stress specific genes RBCS, RBCL, H+-PPase, HKT1, NHX1, NHX2 and NHX3, as well as downregulation of NCED gene expression. | Bacillus amyloliquefaciens SQR9 | Maize (Zea mays L.) | [212] | ||
| Upregulation of ABA-signaling cascade genes TaABARE and TaOPR1; Enhanced expression of stress-induced gene TaST, SOS1 and SOS4. | Dietzia natronolimnaea STR1 | Wheat (Triticum aestivum L.) | [213] | ||
| Higher accumulation of proteins, sugars, proline and glycine betaine in plants. | A. lipoferum FK1 | Chickpea (Cicer arietinum L.) | [168] | ||
| IAA production, phosphate solubilization, siderophore production, ACC activity in bacteria. | Bacillus fortis | Pepper (Capsicum annum L.) | [174] | ||
| Improved root system | IAA production, N–fixation, phosphate solubilization by bacteria. | Bacillus sp. UPMR7 and Citrobacter sp. UPMR17 | Rice (Oryza sativa L.) | [172] | |
| P-solubilization, bacteriocin and siderophore production by bacteria. | Bacillus sp., A. pascens | Maize (Zea mays L.) | [214] | ||
| Increased proline content and totalsoluble sugar, decreased lipid peroxidation and electrolyte leakage in plants. | B. amyloliquefaciens SN13 | Rice (Oryza sativa L.) | [80] | ||
| Increased plant biomass | Increased IAA, GA, zeatin production; high proline and MDA content in plants. | Azotobacter vinellandii SRIAz3 | Rice (Oryza sativa L.) | [169] | |
| ACC deaminase production by bacteria. | Hartmannibacter diazotrophicus E19T | Summer barley (Hordeum vulgare L.) | [204] | ||
| IAA production, phosphate solubilization ACC deaminase activity by bacteria. | Streptomyces sp. PGPA39 | Tomato (Solanum Lycopersicum L.) | [173] | ||
| IAA and siderophores production, higher K+/Na+ ratio in shoot in plant. | P. fluorescens CECT 378T | Sunflower (Helianthus annuus L.) | [157] | ||
| EPS production by bacteria. Growth hormones production by plant. | Brevibacterium iodinum RS16, Micrococcus yunnanensis RS222, B. aryabhattai RS341 and B. licheniformis RS656 | Canola (Brassica napus L.) | [161] | ||
| IAA production, N2 fixation, ACC deaminase activity, HCN and EPS production by bacteria. | Curtobacterium albidum SRV4 | Rice (Oryza sativa L.) | [178] | ||
| Increased yield | Biofilm producing on roots, enhanced amount of EPS, IAA production, ACC-deaminase activity, phosphate solubilization by bacteria. | B. pumilus FAB10 | Wheat (Triticum aestivum L.) | [141] | |
| IAA production, N2 fixation, ACC deaminase activity, phosphate solubilization by bacteria. | Azospirillum sp. | Wheat (Triticum | [63] | ||
| EPS production by bacteria. | P. aeruginosa P23 | Sunflower (Helianthus annuus L.) | [161] | ||
| IAA production, phosphate solubilization, siderophore production and ACC activity by bacteria. | B. fortis | Pepper (Capsicum annum L.) | [174] | ||
| Increased shoot length | IAA production and phosphate solubilization by bacteria. | M. oleivorans KNUC7074 and R. massiliae KNUC7586 | Pepper (Capsicum annum L.) | [160] | |
| Decreased Na+ concentrations in plants. | B. pumilus | Rice (Oryza sativa L.) | [156] | ||
| Increased N, Fe, P and Mn uptake | IAA production, phosphate solubilization, siderophore production by bacteria. | Streptomyces sp. | Wheat (Triticum aestivum L.) | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiodor, A.; Singh, S.; Pranaw, K. The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture. Microorganisms 2021, 9, 1841. https://doi.org/10.3390/microorganisms9091841
Fiodor A, Singh S, Pranaw K. The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture. Microorganisms. 2021; 9(9):1841. https://doi.org/10.3390/microorganisms9091841
Chicago/Turabian StyleFiodor, Angelika, Surender Singh, and Kumar Pranaw. 2021. "The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture" Microorganisms 9, no. 9: 1841. https://doi.org/10.3390/microorganisms9091841
APA StyleFiodor, A., Singh, S., & Pranaw, K. (2021). The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture. Microorganisms, 9(9), 1841. https://doi.org/10.3390/microorganisms9091841