The Ecophysiological Performance and Traits of Genera within the Stichococcus-like Clade (Trebouxiophyceae) under Matric and Osmotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Salt Tolerance
2.2. Desiccation Tolerance and Recovery Experiments
2.3. Qualitative and Quantitative Osmolyte Analysis
3. Results
3.1. Morphological Changes under Salt Stress
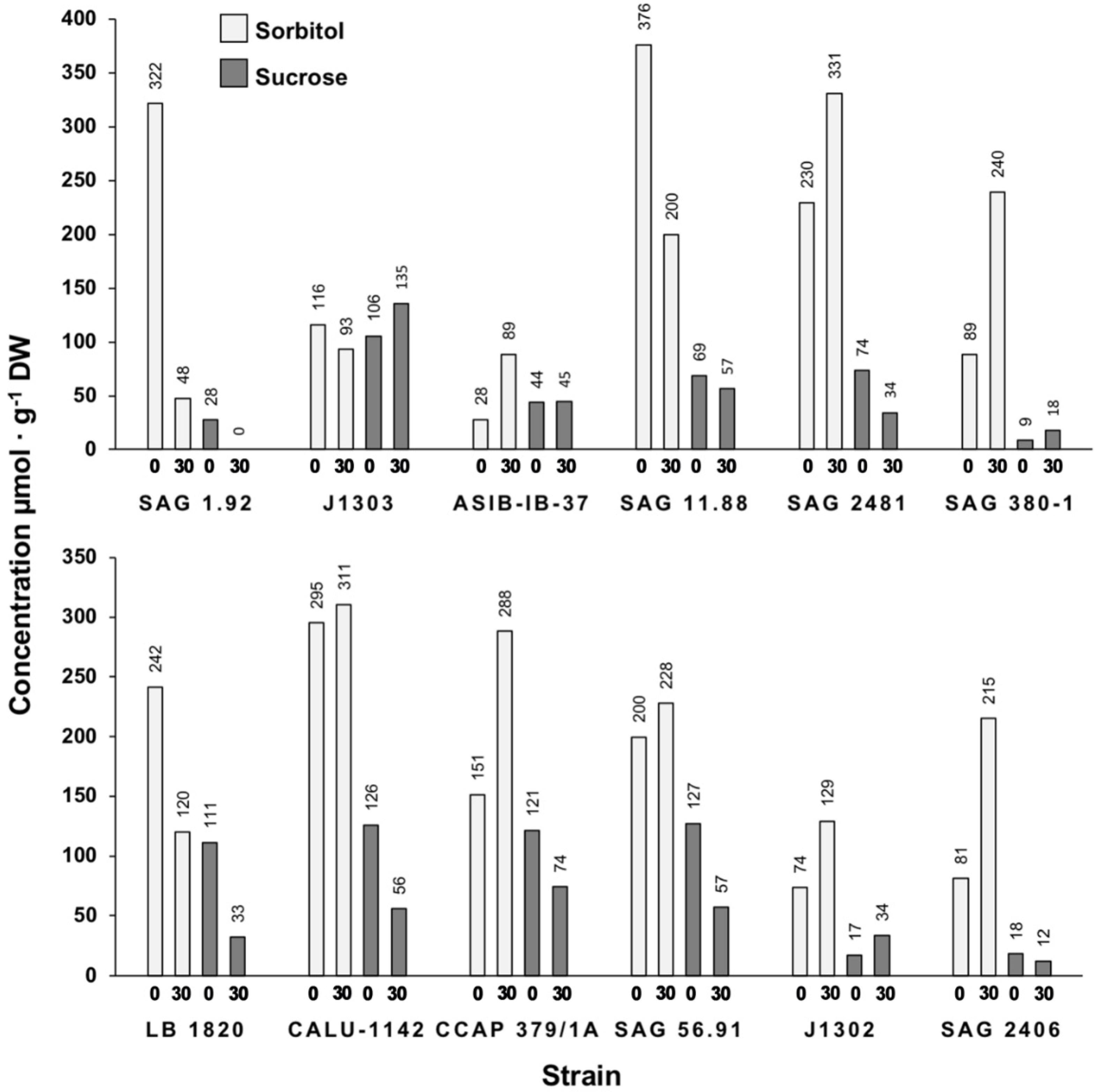
3.2. Low-Molecular-Weight Carbohydrate Analysis
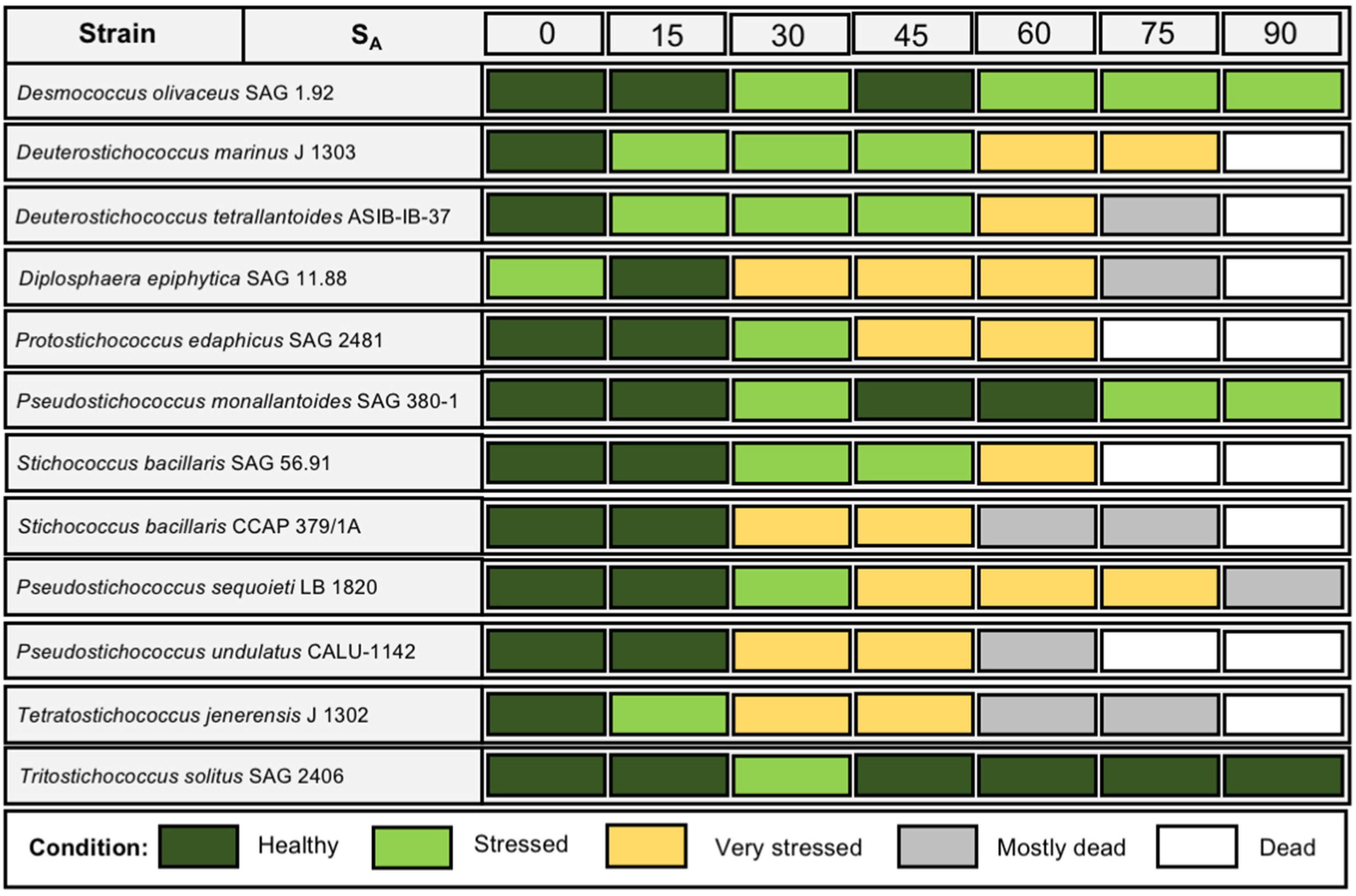
3.3. Dehydration and Rehydration
4. Discussion
4.1. Growth in Response to Saline Stress
4.2. Compatible Solute Production under Osmotic Stress
4.3. Dehydration and Rehydration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holzinger, A.; Karsten, U. Desiccation Stress and Tolerance in Green Algae: Consequences for Ultrastructure, Physiological and Molecular Mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef] [PubMed]
- Samolov, E.; Baumann, K.; Büdel, B.; Jung, P.; Leinweber, P.; Mikhailyuk, T.; Karsten, U.; Glaser, K. Biodiversity of Algae and Cyanobacteria in Biological Soil Crusts Collected along a Climatic Gradient in Chile Using an Integrative Approach. Microorganisms 2020, 8, 1047. [Google Scholar] [CrossRef]
- Padisák, J.; Naselli-Flores, L. Phytoplankton in Extreme Environments: Importance and Consequences of Habitat Permanency. Hydrobiologia 2021, 848, 157–176. [Google Scholar] [CrossRef]
- Häubner, N.; Schumann, R.; Karsten, U. Aeroterrestrial Microalgae Growing in Biofilms on Facades—Response to Temperature and Water Stress. Microb. Ecol. 2006, 51, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Colica, G.; Li, H.; Rossi, F.; Li, D.; Liu, Y.; De Philippis, R. Microbial Secreted Exopolysaccharides Affect the Hydrological Behavior of Induced Biological Soil Crusts in Desert Sandy Soils. Soil Biol. Biochem. 2014, 68, 62–70. [Google Scholar] [CrossRef]
- Rossi, F.; Mugnai, G.; De Philippis, R. Complex Role of the Polymeric Matrix in Biological Soil Crusts. Plant Soil 2018, 429, 19–34. [Google Scholar] [CrossRef]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial Role of Extracellular Polysaccharides in Desiccation and Freezing Tolerance in the Terrestrial Cyanobacterium Nostoc Commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef]
- Gustavs, L.; Eggert, A.; Michalik, D.; Karsten, U. Physiological and Biochemical Responses of Green Microalgae from Different Habitats to Osmotic and Matric Stress. Protoplasma 2010, 243, 3–14. [Google Scholar] [CrossRef]
- Hellebust, J.A. Mechanisms of response to salinity in halotolerant microalgae. In Biosalinity in Action: Bioproduction with Saline Water; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1985; pp. 69–81. ISBN 978-94-010-8759-9. [Google Scholar]
- Kan, G.; Shi, C.; Wang, X.; Xie, Q.; Wang, M.; Wang, X.; Miao, J. Acclimatory Responses to High-Salt Stress in Chlamydomonas (Chlorophyta, Chlorophyceae) from Antarctica. Acta Oceanol. Sin. 2012, 31, 116–124. [Google Scholar] [CrossRef]
- Kirkham, M.B. Chapter 4—Soil–Water Terminology and Applications. In Principles of Soil and Plant Water Relations, 2nd ed.; Kirkham, M.B., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 41–52. ISBN 978-0-12-420022-7. [Google Scholar]
- Chang, W.; Li, X.; Halverson, L.J. Influence of Water Limitation on Endogenous Oxidative Stress and Cell Death within Unsaturated Pseudomonas Putida Biofilms. Environ. Microbiol. 2009, 11, 1482–1492. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive Effects of Na+ and Cl– Ions on Barley Growth under Salinity Stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef]
- Fujii, S.; Yamamoto, R.; Nakayama, S.; Yasui, S.; Broady, P.A. Effects of Salinity on Growth and Content of Intracellular Solutes in Heterococcus Sp.(Tribonennatales, Xanthophyceae) from Antarctica. Phycol. Res. 1999, 47, 65–69. [Google Scholar] [CrossRef]
- Ahmad, I.; Hellebust, J.A. Osmoregulation in the Extremely Euryhaline Marine Micro-Alga Chlorella Autotrophica. Plant Physiol. 1984, 74, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U. Seaweed Acclimation to Salinity and Desiccation Stress. In Seaweed Biology; Wiencke, C., Bischof, K., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2012; Volume 219, pp. 87–107. ISBN 978-3-642-28450-2. [Google Scholar]
- de Jaeger, L.; Carreres, B.M.; Springer, J.; Schaap, P.J.; Eggink, G.; Martins Dos Santos, V.A.; Wijffels, R.H.; Martens, D.E. Neochloris Oleoabundans Is Worth Its Salt: Transcriptomic Analysis under Salt and Nitrogen Stress. PLoS ONE 2018, 13, e0194834. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Nakahara, M.; Tsubota, H.; Deguchi, H.; Nakano, T. A New Aerial Alga, Stichococcus Ampulliformis Sp. Nov.(Trebouxiophyceae, Chlorophyta) from Japan. Phycol. Res. 2003, 51, 203–210. [Google Scholar] [CrossRef]
- Neustupa, J.; Škaloud, P. Diversity of Subaerial Algae and Cyanobacteria on Tree Bark in Tropical Mountain Habitats. Biologia 2008, 63, 806–812. [Google Scholar] [CrossRef]
- Gustavs, L.; Görs, M.; Karsten, U. Polyol Patterns in Biofilm-Forming Aeroterrestrial Green Algae (Trebouxiophyceae, Chlorophyta): Polyols in Aeroterrestrial Trebouxiophyceae. J. Phycol. 2011, 47, 533–537. [Google Scholar] [CrossRef]
- Hallmann, C.; Stannek, L.; Fritzlar, D.; Hause-Reitner, D.; Friedl, T.; Hoppert, M. Molecular Diversity of Phototrophic Biofilms on Building Stone. FEMS Microbiol. Ecol. 2013, 84, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Uher, B. Spatial Distribution of Cyanobacteria and Algae from the Tombstone in a Historic Cemetery in Bratislava, Slovakia. Fottea 2008, 9, 81–92. [Google Scholar] [CrossRef]
- Hodač, L.; Hallmann, C.; Spitzer, K.; Elster, J.; Faßhauer, F.; Brinkmann, N.; Lepka, D.; Diwan, V.; Friedl, T. Widespread Green Algae Chlorella and Stichococcus Exhibit Polar-Temperate and Tropical-Temperate Biogeography. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Pröschold, T.; Darienko, T. The Green Puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New Generic and Species Concept among This Widely Distributed Genus. Phytotaxa 2020, 441, 113–142. [Google Scholar] [CrossRef]
- George, E. A Note on Stichococcus bacillaris Naeg. and Some Species of Chlorella as Marine Algae. J. Mar. Biol. Assoc. UK 1957, 36, 111–114. [Google Scholar] [CrossRef]
- Hayward, J. Studies on the Growth of Stichococcus bacillaris Naeg in Culture. J. Mar. Biol. Assoc. UK 1974, 54, 261–268. [Google Scholar] [CrossRef]
- Sommer, V.; Karsten, U.; Glaser, K. Halophilic Algal Communities in Biological Soil Crusts Isolated from Potash Tailings Pile Areas. Front. Ecol. Evol. 2020, 8, 46. [Google Scholar] [CrossRef]
- Brown, L.M.; Hellebust, J.A. Sorbitol and Proline as Intracellular Osmotic Solutes in the Green Alga Stichococcus bacillaris. Can. J. Bot. 1978, 56, 676–679. [Google Scholar] [CrossRef]
- Brown, L.M.; Hellebust, J.A. Some New Taxonomic Characteristics Applied to Stichococcus bacillaris (Chlorophyceae). Can. J. Bot. 1980, 58, 1405–1411. [Google Scholar] [CrossRef]
- Bischoff, H. Some Soil Algae from Enchanted Rock and Related Algal Species. Phycol. Stud. IV Univ. Tex. Publ. 1963, 6318, 1–95. [Google Scholar]
- Starr, R.C.; Zeikus, J.A. UTEX—The Culture Collection of Algae at the University of Texas at Austin 1993 List of Cultures 1. J. Phycol. 1993, 29, 1–106. [Google Scholar] [CrossRef]
- Karsten, U.; Herburger, K.; Holzinger, A. Living in Biological Soil Crust Communities of African Deserts—Physiological Traits of Green Algal Klebsormidium Species (Streptophyta) to Cope with Desiccation, Light and Temperature Gradients. J. Plant Physiol. 2016, 194, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Provasoli, L.; McLaughlin, J.; Droop, M. The Development of Artificial Media for Marine Algae. Arch. Für Mikrobiol. 1957, 25, 392–428. [Google Scholar] [CrossRef]
- Lüttge, U.; Büdel, B. Resurrection Kinetics of Photosynthesis in Desiccation-Tolerant Terrestrial Green Algae (Chlorophyta) on Tree Bark. Plant Biol. 2010, 12, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R.A. A Marine Stichococcus sp. Which Requires Vitamin BI2 (Cobalamin). J. Gen. Microbiol. 1954, 4, 93–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moazami-Goudarzi, M.; Colman, B. Inorganic Carbon Acquisition in Two Green Marine Stichococcus Species. Plant Cell Environ. 2011, 34, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Mikhailyuk, T.; Glaser, K.; Tsarenko, P.; Demchenko, E.; Karsten, U. Composition of Biological Soil Crusts from Sand Dunes of the Baltic Sea Coast in the Context of an Integrative Approach to the Taxonomy of Microalgae and Cyanobacteria. Eur. J. Phycol. 2019, 54, 263–290. [Google Scholar] [CrossRef]
- Sommer, V.; Mikhailyuk, T.; Glaser, K.; Karsten, U. Uncovering Unique Green Algae and Cyanobacteria Isolated from Biocrusts in Highly Saline Potash Tailing Pile Habitats, Using an Integrative Approach. Microorganisms 2020, 8, 1667. [Google Scholar] [CrossRef]
- Van, A.T.; Karsten, U.; Glaser, K. A Chemosystematic Investigation of Selected Stichococcus-like Organisms (Trebouxiophyta). Algae 2021, 36, 123–135. [Google Scholar] [CrossRef]
- Noiraud, N.; Maurousset, L.; Lemoine, R. Transport of Polyols in Higher Plants. Plant Physiol. Biochem. 2001, 39, 717–728. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of Salt Stress on Basic Processes of Photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P.J. Salinity, Osmolytes and Compatible Solutes. In Salinity: Environment-Plants-Molecules; Läuchli, A., Lüttge, U., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 181–204. ISBN 978-1-4020-0492-6. [Google Scholar]
- Inaba, M.; Sakamoto, A.; Murata, N. Functional Expression in Escherichia Coli of Low-Affinity and High-Affinity Na+ (Li+)/H+ Antiporters of Synechocystis. J. Bacteriol. 2001, 183, 1376–1384. [Google Scholar] [CrossRef]
- Uji, T.; Hirata, R.; Mikami, K.; Mizuta, H.; Saga, N. Molecular Characterization and Expression Analysis of Sodium Pump Genes in the Marine Red Alga Porphyra Yezoensis. Mol. Biol. Rep. 2012, 39, 7973–7980. [Google Scholar] [CrossRef]
- Gimmler, H. Primary Sodium Plasma Membrane ATPases in Salt-tolerant Algae: Facts and Fictions. J. Exp. Bot. 2000, 51, 1171–1178. [Google Scholar] [CrossRef]
- Koster, K.L. Glass Formation and Desiccation Tolerance in Seeds. Plant Physiol. 1991, 96, 302–304. [Google Scholar] [CrossRef]
- Yancey, P.H. Water Stress, Osmolytes and Proteins. Am. Zool. 2001, 11. [Google Scholar] [CrossRef][Green Version]
- Kaplan, F.; Lewis, L.A.; Wastian, J.; Holzinger, A. Plasmolysis Effects and Osmotic Potential of Two Phylogenetically Distinct Alpine Strains of Klebsormidium (Streptophyta). Protoplasma 2012, 249, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Matsui, K.; Uemura, M. Klebsormidium Flaccidum, a Charophycean Green Alga, Exhibits Cold Acclimation That Is Closely Associated with Compatible Solute Accumulation and Ultrastructural Changes. Plant Cell Environ. 2008, 31, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Lütz, C.; Holzinger, A. Ecophysiological Performance of the Aeroterrestrial Green Alga Klebsormidium Crenulatum (Charophyceae, Streptophyta) Isolated from an Alpine Soil Crust with an Emphasis on Desiccation Stress. J. Phycol. 2010, 46, 1187–1197. [Google Scholar] [CrossRef]
- Kremer, B.P. Taxonomic Implications of Algal Photoassimilate Patterns. Br. Phycol. J. 1980, 15, 399–409. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.-G.; Wu, G.-H. Effects of Salinity Changes on the Growth of Dunaliella Salina and Its Isozyme Activities of Glycerol-3-Phosphate Dehydrogenase. J. Agric. Food Chem. 2009, 57, 6178–6182. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mandoli, A.; Jha, B. Physiological Characterization and Stress-Induced Metabolic Responses of Dunaliella Salina Isolated from Salt Pan. J. Ind. Microbiol. Biotechnol. 2008, 35, 1093. [Google Scholar] [CrossRef] [PubMed]
- Kirst, G. Salinity Tolerance of Eukaryotic Marine Algae. Annu. Rev. Plant Biol. 1990, 41, 21–53. [Google Scholar] [CrossRef]
- Gasulla, F.; de Nova, P.G.; Esteban-Carrasco, A.; Zapata, J.M.; Barreno, E.; Guéra, A. Dehydration Rate and Time of Desiccation Affect Recovery of the Lichenic Algae Trebouxia Erici: Alternative and Classical Protective Mechanisms. Planta 2009, 231, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.W.; Lewis, L.A.; Cardon, Z.G. Photosynthetic Recovery Following Desiccation of Desert Green Algae (Chlorophyta) and Their Aquatic Relatives. Plant Cell Environ. 2007, 30, 1240–1255. [Google Scholar] [CrossRef]
- Medwed, C.; Holzinger, A.; Hofer, S.; Hartmann, A.; Michalik, D.; Glaser, K.; Karsten, U. Ecophysiological, Morphological, and Biochemical Traits of Free-Living Diplosphaera Chodatii (Trebouxiophyceae) Reveal Adaptation to Harsh Environmental Conditions. Protoplasma 2021, 1–13. [Google Scholar] [CrossRef]
- Yamagishi, T.; Yamaguchi, H.; Suzuki, S.; Yoshikawa, M.; Jameson, I.; Lorenz, M.; Nobles, D.R.; Campbell, C.; Seki, M.; Kawachi, M. Comparative Genome Analysis of Test Algal Strain NIVA-CHL1 (Raphidocelis Subcapitata) Maintained in Microalgal Culture Collections Worldwide. PLoS ONE 2020, 15, e0241889. [Google Scholar]
- Müller, J.; Day, J.G.; Harding, K.; Hepperle, D.; Lorenz, M.; Friedl, T. Assessing Genetic Stability of a Range of Terrestrial Microalgae after Cryopreservation Using Amplified Fragment Length Polymorphism (AFLP). Am. J. Bot. 2007, 94, 799–808. [Google Scholar] [CrossRef]
- Müller, J.; Friedl, T.; Hepperle, D.; Lorenz, M.; Day, J.G. Distinction between Multiple Isolates of Chlorella Vulgaris (Chlorophyta, Trebouxiophyceae) and Testing for Conspecificity Using Amplified Fragment Length Polymorphism and Its Rdna Sequences. J. Phycol. 2005, 41, 1236–1247. [Google Scholar] [CrossRef]
- Holzinger, A.; Kaplan, F.; Blaas, K.; Zechmann, B.; Komsic-Buchmann, K.; Becker, B. Transcriptomics of Desiccation Tolerance in the Streptophyte Green Alga Klebsormidium Reveal a Land Plant-like Defense Reaction. PLoS ONE 2014, 9, e110630. [Google Scholar] [CrossRef]




| Strain ID | Species Assignment | Locality and Habitat | Collector/Isolator |
|---|---|---|---|
| SAG 1.92 * | Desmocococcus olivaceus | Vienna, Austria; subaerial | W. Vischer, before 1960 |
| J 1303 *, † SAG 2139 * | Deuterostichococcus marinus | Dauphin Island, Alabama, USA; soil | T.R. Deason, 1969 |
| ASIB-IB-37 * | Deuterostichococcus tetrallantoideus | Weißkugel Peak, Ötztal Valley, Austria; soil | H. Reisigl, 1964 |
| SAG 11.88 * | Diplosphaera epiphytica | Waweira Scenic Reserve, New Zealand; lichen phycobiont | E. Tschermak-Woess, 1984 |
| SAG 2481 * | Protostichococcus edaphicus | Swabian Alb, Germany; forest soil | L. Hodač, 2008 |
| SAG 380-1 * | Pseudostichoccocus monallantoides | Germany; marine | L. Moewus, 1951 |
| LB 1820 * | Pseudostichococcus sequoieti | USA; redwood forest soil | G. Arce, 1971 |
| CALU-1142 * | Pseudostichococcus undulatus | Dolomite Mountains, Italy | G. Vinatzer, 1975 |
| CCAP 379/1A * | Stichococcus bacillaris | Likely Switzerland | W. Vischer, before 1936 |
| J 1302 *, † SAG 2138 * | Tetratostichococcus jenerensis | Kampong Kuala Jenera, Kelantan, Malaysia; soil of rainforest tree | J. Neustupa, 2000 |
| SAG 2406 * | Tritostichococcus solitus | Northeim, Germany; karstwater stream rock surface | K. Mohr, 2003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van, A.T.; Sommer, V.; Glaser, K. The Ecophysiological Performance and Traits of Genera within the Stichococcus-like Clade (Trebouxiophyceae) under Matric and Osmotic Stress. Microorganisms 2021, 9, 1816. https://doi.org/10.3390/microorganisms9091816
Van AT, Sommer V, Glaser K. The Ecophysiological Performance and Traits of Genera within the Stichococcus-like Clade (Trebouxiophyceae) under Matric and Osmotic Stress. Microorganisms. 2021; 9(9):1816. https://doi.org/10.3390/microorganisms9091816
Chicago/Turabian StyleVan, Anh Tu, Veronika Sommer, and Karin Glaser. 2021. "The Ecophysiological Performance and Traits of Genera within the Stichococcus-like Clade (Trebouxiophyceae) under Matric and Osmotic Stress" Microorganisms 9, no. 9: 1816. https://doi.org/10.3390/microorganisms9091816
APA StyleVan, A. T., Sommer, V., & Glaser, K. (2021). The Ecophysiological Performance and Traits of Genera within the Stichococcus-like Clade (Trebouxiophyceae) under Matric and Osmotic Stress. Microorganisms, 9(9), 1816. https://doi.org/10.3390/microorganisms9091816






