Feline Coronaviruses Identified in Feline Effusions in Suspected Cases of Feline Infectious Peritonitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feline Effusion Samples
2.2. Indirect Immunofluorescence Assay (IFA)
2.3. Viral RNA Extraction and RT-PCR
2.4. Sequencing and Phylogenetic Analysis
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
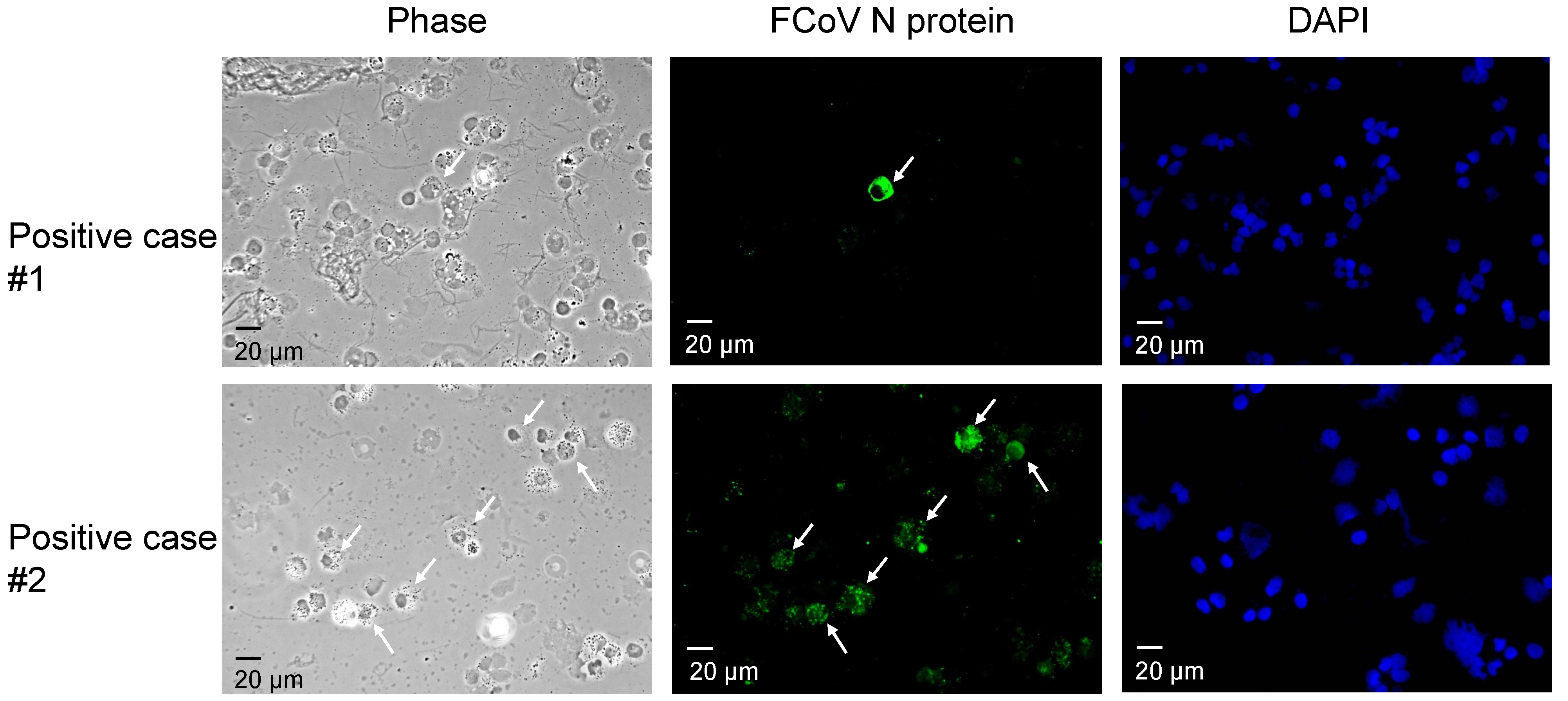
3.1. Detection of FCoV by IFA and RT-PCR
3.2. Association of Positive Cases with Clinical Characteristics and A/G Ratio
3.3. FCoV Genotyping and Sequencing
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, M.A. Feline infectious Peritonitis: Update on pathogenesis, diagnostics, and treatment. Vet. Clin. Small Anim. Pract. 2020, 50, 1001–1011. [Google Scholar] [CrossRef]
- Tasker, S. Diagnosis of feline infectious peritonitis: Update on evidence supporting available tests. J. Feline Med. Surg. 2018, 20, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Felten, S.; Hartmann, K. Diagnosis of feline infectious peritonitis: A review of the current literature. Viruses 2019, 11, 1068. [Google Scholar] [CrossRef] [Green Version]
- Haake, C.; Cook, S.; Pusterla, N.; Murphy, B. Coronavirus infections in companion animals: Virology, epidemiology, clinical and pathologic features. Viruses 2020, 12, 1023. [Google Scholar] [CrossRef]
- Jaimes, J.A.; Whittaker, G.R. Feline coronavirus: Insights into viral pathogenesis based on the spike protein structure and function. Virology 2018, 517, 108–121. [Google Scholar] [CrossRef]
- Brown, M.A. Genetic determinants of pathogenesis by feline infectious peritonitis virus. Vet. Immunol. Immunopathol. 2011, 143, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-W.; Egberink, H.F.; Halpin, R.; Spiro, D.J.; Rottier, P.J. Spike protein fusion peptide and feline coronavirus virulence. Emerg. Infect. Dis. 2012, 18, 1089. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. An update on feline infectious peritonitis: Diagnostics and therapeutics. Vet. J. 2014, 201, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, K.; Binder, C.; Hirschberger, J.; Cole, D.; Reinacher, M.; Schroo, S.; Frost, J.; Egberink, H.; Lutz, H.; Hermanns, W. Comparison of different tests to diagnose feline infectious peritonitis. J. Vet. Intern. Med. 2003, 17, 781–790. [Google Scholar] [CrossRef]
- Hirschberger, J.; Hartmann, K.; Wilhelm, N.; Frost, J.; Lutz, H.; Kraft, W. Clinical symptoms and diagnosis of feline infectious peritonitis. Tierarztl. Prax. 1995, 23, 92–99. [Google Scholar]
- Parodi, M.C.; Cammarata, G.; Paltrinieri, S.; Lavazza, A.; Ape, F. Using direct immunofluorescence to detect coronaviruses in peritoneal in peritoneal and pleural effusions. J. Small Anim. Pract. 1993, 34, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Paltrinieri, S.; Parodi, M.C.; Cammarata, G. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology, and direct immunofluorescence test on peritoneal and pleural effusions. J. Vet. Diagn. Investig. 1999, 11, 358–361. [Google Scholar] [CrossRef]
- Litster, A.; Pogranichniy, R.; Lin, T.-L. Diagnostic utility of a direct immunofluorescence test to detect feline coronavirus antigen in macrophages in effusive feline infectious peritonitis. Vet. J. 2013, 198, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-N.; Su, B.-L.; Wang, C.-H.; Hsieh, M.-W.; Chueh, T.-J.; Chueh, L.-L. Genetic diversity and correlation with feline infectious peritonitis of feline coronavirus type I and II: A 5-year study in Taiwan. Vet. Microbiol. 2009, 136, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-C.; Liu, I.-L.; Chen, Y.-T.; Chen, H.-W. Detection of Feline Coronavirus in Feline Effusions by Immunofluorescence Staining and Reverse Transcription Polymerase Chain Reaction. Pathogens 2020, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Herrewegh, A.; De Groot, R.; Cepica, A.; Egberink, H.F.; Horzinek, M.C.; Rottier, P. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR. J. Clin. Microbiol. 1995, 33, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addie, D.; Schaap, I.; Nicolson, L.; Jarrett, O. Persistence and transmission of natural type I feline coronavirus infection. J. Gen. Virol. 2003, 84, 2735–2744. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Worthing, K.A.; Wigney, D.I.; Dhand, N.K.; Fawcett, A.; McDonagh, P.; Malik, R.; Norris, J.M. Risk factors for feline infectious peritonitis in Australian cats. J. Feline Med. Surg. 2012, 14, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Soma, T.; Wada, M.; Taharaguchi, S.; Tajima, T. Detection of ascitic feline coronavirus RNA from clinically suspected cats of feline infectious peritonitis. J. Vet. Med. Sci. 2013. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Sasaki, T.; Matsuda, R.; Uematsu, Y.; Yamaguchi, T. Molecular epidemiological study of feline coronavirus strains in Japan using RT-PCR targeting nsp14 gene. BMC Vet. Res. 2015, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tekelioglu, B.; Berriatua, E.; Turan, N.; Helps, C.; Koçak, M.; Yilmaz, H. A retrospective clinical and epidemiological study on feline coronavirus (FCoV) in cats in Istanbul, Turkey. Prev. Vet. Med. 2015, 119, 41–47. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Kong, F.; Guo, D.; Zhai, J.; Su, M.; Sun, D. Circulation and genetic diversity of Feline coronavirus type I and II from clinically healthy and FIP-suspected cats in China. Transbound. Emerg. Dis. 2019, 66, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, U.; Deitz, K.; Hostetter, S. Positive predictive value of albumin: Globulin ratio for feline infectious peritonitis in a mid-western referral hospital population. J. Feline Med. Surg. 2012, 14, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Suri, A.S.; Rahman, O.A.; Mohd, H.B.; Faruku, B.; Saeed, S.; Azmi, T.I.T. Isolation and molecular characterization of type I and type II feline coronavirus in Malaysia. Virol. J. 2012, 9, 1–6. [Google Scholar]
- Duarte, A.; Veiga, I.; Tavares, L. Genetic diversity and phylogenetic analysis of Feline Coronavirus sequences from Portugal. Vet. Microbiol. 2009, 138, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Benetka, V.; Kübber-Heiss, A.; Kolodziejek, J.; Nowotny, N.; Hofmann-Parisot, M.; Möstl, K. Prevalence of feline coronavirus types I and II in cats with histopathologically verified feline infectious peritonitis. Vet. Microbiol. 2004, 99, 31–42. [Google Scholar] [CrossRef]
- Addie, D.D.; Jarrett, O. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Vet. Rec. 2001, 148, 649–653. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Diaz, A.; Damtie, D.; Xiu, L.; Toh, T.H.; Lee, J.S.; Saif, L.J.; Gray, G.C. Novel Canine Coronavirus Isolated from a Hospitalized Pneumonia Patient, East Malaysia. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]



| IFA and PCR | Sex | Breed | Age (m) # | |||||
|---|---|---|---|---|---|---|---|---|
| M | F | Purebred | Non-Purebred | 0–24 | 25–48 | 49–72 | >73 | |
| Positive (n = 45) | 25 | 20 | 21 | 24 | 33 | 4 | 2 | 5 |
| Negative (n = 50) | 27 | 23 | 23 | 27 | 16 | 4 | 2 | 27 |
| Total | 52 | 43 | 44 | 51 | 49 | 8 | 4 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, S.-J.; Chen, H.-W. Feline Coronaviruses Identified in Feline Effusions in Suspected Cases of Feline Infectious Peritonitis. Microorganisms 2021, 9, 1801. https://doi.org/10.3390/microorganisms9091801
Yen S-J, Chen H-W. Feline Coronaviruses Identified in Feline Effusions in Suspected Cases of Feline Infectious Peritonitis. Microorganisms. 2021; 9(9):1801. https://doi.org/10.3390/microorganisms9091801
Chicago/Turabian StyleYen, Shih-Jung, and Hui-Wen Chen. 2021. "Feline Coronaviruses Identified in Feline Effusions in Suspected Cases of Feline Infectious Peritonitis" Microorganisms 9, no. 9: 1801. https://doi.org/10.3390/microorganisms9091801
APA StyleYen, S.-J., & Chen, H.-W. (2021). Feline Coronaviruses Identified in Feline Effusions in Suspected Cases of Feline Infectious Peritonitis. Microorganisms, 9(9), 1801. https://doi.org/10.3390/microorganisms9091801






