Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management
Abstract
1. Introduction
2. Containment and Eradication Measures
2.1. Xylella fastidiosa and EU Legislation
2.2. Italy
2.3. Spain
2.4. Considerations for Existing Measures in the Framework of the “Farm to Fork Strategy”
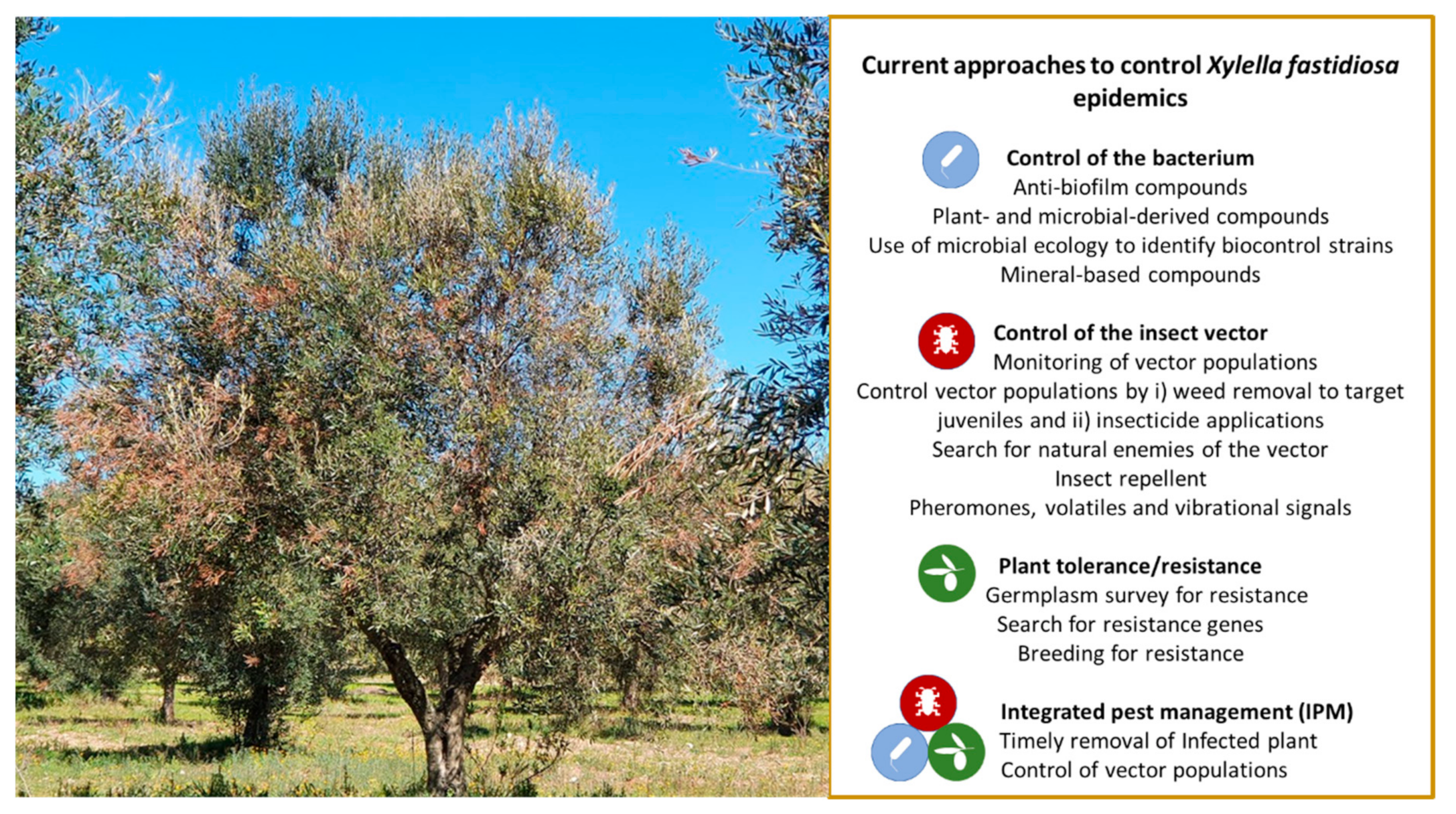
3. Current Attempts to Control X. fastidiosa in Olive—State of the Art
3.1. Minerals and Compounds Control: Moving beyond Conventional Agrochemicals
3.2. Plant- and Microbial-Derived Compounds
3.3. Microbial Control of X. fastidiosa Infections
4. Current Attempts to Control the Insect Vector(s) in Olives—State of the Art
4.1. Survey of the Insect Vectors
4.2. Weed Management
4.3. Insecticide Use to Control the Vector
4.4. Natural Enemies
4.5. Insect Repellent
4.6. Pheromones and Volatiles as Control Measures
5. Plant Breeding as a Sustainable Solution
Integrated Pest Management (IPM)
6. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wells, J.M.; Raju, B.C.; Hung, H.-Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Evol. Microbiol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- EPPO Global Database. Distribution of Xylella fastidiosa (XYLEFA). Available online: https://gd.eppo.int/taxon/XYLEFA/distribution (accessed on 12 April 2021).
- Nunney, L.; Schuenzel, E.L.; Scally, M.; Bromley, R.E.; Stouthamer, R. Large-scale intersubspecific recombination in the plant-pathogenic bacterium Xylella fastidiosa is associated with the host shift to mulberry. Appl. Environ. Microbiol. 2014, 80, 3025–3033. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Delbianco, A.; Gibin, D.; Pasinato, L.; Morelli, M. Update of the Xylella spp. host plant database—Systematic literature search up to 31 December 2020. EFSA J. 2021, 19, 6674. [Google Scholar] [CrossRef]
- Marcelletti, S.; Scortichini, M. Genome-wide comparison and taxonomic relatedness of multiple Xylella fastidiosa strains reveal the occurrence of three subspecies and a new Xylella species. Arch. Microbiol. 2016, 198, 803–812. [Google Scholar] [CrossRef]
- Burbank, L.P.; Ortega, B.C. Novel amplification targets for rapid detection and differentiation of Xylella fastidiosa subspecies fastidiosa and multiplex in plant and insect tissues. J. Microbiol. Methods 2018, 155, 8–18. [Google Scholar] [CrossRef]
- Maiden, M.C.J.; Bygraves, J.A.; Feil, E.; Morelli, G.; Russell, J.E.; Urwin, R.; Zhang, Q.; Zhou, J.; Zurth, K.; Caugant, D.A. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 1998, 95, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; Datome, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci. Rep. 2017, 7, 17723. [Google Scholar] [CrossRef] [PubMed]
- Giampetruzzi, A.; Saponari, M.; Almeida, R.P.P.; Essakhi, S.; Boscia, D.; Loconsole, G.; Saldarelli, P. Complete genome sequence of the olive-infecting strain Xylella fastidiosa subsp. pauca De Donno. Genome Announc. 2017, 5, e00569-17. [Google Scholar] [CrossRef] [PubMed]
- D’Attoma, G.; Morelli, M.; De La Fuente, L.; Cobine, P.A.; Saponari, M.; de Souza, A.A.; De Stradis, A.; Saldarelli, P. Phenotypic characterization and transformation attempts reveal peculiar traits of Xylella fastidiosa subspecies pauca strain De Donno. Microorganisms 2020, 8, 1832. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; De Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.P.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef]
- Luvisi, A.; Aprile, A.; Sabella, E.; Vergine, M.; Nicoli, F.; Nutricati, E.; Miceli, A.; Negro, C.; De Bellis, L. Xylella fastidiosa subsp. pauca (CoDiRO strain) infection in four olive (Olea europaea L.) cultivars: Profile of phenolic compounds in leaves and progression of leaf scorch symptoms. Phytopathol. Mediterr. 2017, 56, 259–273. [Google Scholar]
- Schneider, K.; Van der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Lansink, A.O. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Decision (EU) 2015/789 of 18 May 2015 as regards measures to prevent the introduction into and the spread within the Union of Xylella fastidiosa (Wells et al.). Off. J. Eur. Union 2015, 125, 36–53. [Google Scholar]
- Sportelli, G. Xylella, morte annunciata per la Piana degli olivi monumentali? Olivo Olio 2020, 10, 12–18. [Google Scholar]
- Loconsole, G.; Saponari, M.; Boscia, D.; Datome, G.; Morelli, M.; Martelli, G.P.; Almeida, R.P.P. Intercepted isolates of Xylella fastidiosa in Europe reveal novel genetic diversity. Eur. J. Plant Pathol. 2016, 146, 85–94. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Saponari, M.; Loconsole, G.; Boscia, D.; Savino, V.N.; Almeida, R.P.P.; Zicca, S.; Landa, B.B.; Chacón-Diaz, C.; Saldarelli, P. Genome-wide analysis provides evidence on the genetic relatedness of the emergent Xylella fastidiosa genotype in Italy to isolates from Central America. Phytopathology 2017, 107, 816–827. [Google Scholar] [CrossRef]
- Vanhove, M.; Retchless, A.C.; Sicard, A.; Rieux, A.; Coletta-Filho, H.D.; De La Fuente, L.; Stenger, D.C.; Almeida, R.P.P. Genomic diversity and recombination among Xylella fastidiosa subspecies. Appl. Environ. Microbiol. 2019, 85, e02972-18. [Google Scholar] [CrossRef]
- Olmo, D.; Nieto, A.; Borràs, D.; Montesinos, M.; Adrover, F.; Pascual, A.; Gost, P.A.; Quetglas, B.; Urbano, A.; García, J.d.D. Landscape epidemiology of Xylella fastidiosa in the Balearic Islands. Agronomy 2021, 11, 473. [Google Scholar] [CrossRef]
- MAPA—Spanish Ministry of Agriculture Fisheries and Food. Xylella fastidiosa. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/organismos-nocivos/xylella-fastidiosa/ (accessed on 12 April 2021).
- Generalitat Valenciana. Situación Xylella fastidiosa Comunitat Valenciana, Marzo de 2021. Available online: https://agroambient.gva.es/es/web/agricultura/xylella-fastidiosa#:~:text=Situaci%C3%B3n%20Xylella%20fastidiosa%20Comunitat%20Valenciana%2C%20marzo%20de%202021 (accessed on 14 July 2021).
- Tihomirova-Hristova, L.; Pérez-Díaz, M.; Antón-Iruela, O.; Bielsa-Lozoya, S.; García-Gutiérrez, S.; Monterde, A.; Navarro, I.; Montes Borrego, M.; Barbé, S.; Marco-Noales, E. Current situation after Xylella fastidiosa first outbreak in an olive grove in mainland Spain. In Proceedings of the 2nd European Conference on Xylella fastidiosa: How Research Can Support Solutions, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- Marchi, G.; Rizzo, D.; Ranaldi, F.; Ghelardini, L.; Ricciolini, M.; Scarpelli, I.; Drosera, L.; Emanuele, G.; Capretti, P.; Surico, G. First detection of Xylella fastidiosa subsp. multiplex DNA in Tuscany (Italy). Phytopathol. Mediterr. 2018, 57, 363–364. [Google Scholar]
- Giampetruzzi, A.; Datome, G.; Zicca, S.; Abou Kubaa, R.; Rizzo, D.; Boscia, D.; Saldarelli, P.; Saponari, M. Draft Genome sequence resources of three strains (TOS4, TOS5, and TOS14) of Xylella fastidiosa infecting different host plants in the newly discovered outbreak in Tuscany, Italy. Phytopathology 2019, 109, 1516–1518. [Google Scholar] [CrossRef]
- Soubeyrand, S.; de Jerphanion, P.; Martin, O.; Saussac, M.; Manceau, C.; Hendrikx, P.; Lannou, C. Inferring pathogen dynamics from temporal count data: The emergence of Xylella fastidiosa in France is probably not recent. New Phytol. 2018, 219, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; Milonas, P. Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) No 228/2013, (EU) No 652/2014 and (EU) No 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. Off. J. Eur. Union 2016, 317, 4–104. [Google Scholar]
- European Commission. Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. Off. J. Eur. Communities 2000, 169, 1–112. [Google Scholar]
- European Commission. Commission implementing regulation (EU) 2020/1201 of 14 August 2020, as regards measures to prevent the introduction into and the spread within the Union of Xylella fastidiosa (Wells et al.). Off. J. Eur. Union 2020, L 269, 2–39. [Google Scholar]
- Lázaro, E.; Parnell, S.; Civera, A.V.; Schans, J.; Schenk, M.; Abrahantes, J.C.; Zancanaro, G.; Vos, S.; European Food Safety Authority. General Guidelines for Statistically Sound and Risk-Based Surveys of Plant Pests; Wiley Online Library: Hoboken, NJ, USA, 2020; 65p. [Google Scholar]
- Emergenza Xylella. Available online: http://www.emergenzaxylella.it/ (accessed on 12 April 2021).
- MAPA—Spanish Ministry of Agriculture Fisheries and Food. Plan de Contingencia de Xylella fastidiosa (Well y Raju); Programa Nacional para la Aplicación de la Normativa Fitosanitaria. 2021. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/xylellafastidiosa_contingencia_marzo2021_tcm30-525545.pdf (accessed on 16 August 2021).
- European Commission. Farm to Fork Strategy. Available online: https://ec.europa.eu/food/farm2fork_en (accessed on 12 April 2021).
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Saponari, M.; Boscia, D. Recent advances on the control of Xylella fastidiosa and its vectors in olive groves: State of the art from the ongoing Europe’s Horizon 2020 research programs. In Proceedings of the BIOCONTROL, 4th International Symposium on Biological Control of Bacterial Plant Diseases, Viterbo, Italy, 9–11 July 2019. [Google Scholar] [CrossRef]
- Cruz, L.F.; Cobine, P.A.; De La Fuente, L. Calcium increases Xylella fastidiosa surface attachment, biofilm formation, and twitching motility. Appl. Environ. Microbiol. 2012, 78, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.A.; Cruz, L.F.; Navarrete, F.; Duncan, D.; Tygart, M.; De La Fuente, L. Xylella fastidiosa differentially accumulates mineral elements in biofilm and planktonic cells. PLoS ONE 2013, 8, e54936. [Google Scholar] [CrossRef]
- Navarrete, F.; De La Fuente, L. Zinc detoxification is required for full virulence and modification of the host leaf ionomer by Xylella fastidiosa. Mol. Plant-Microbe Interact. 2015, 28, 497–507. [Google Scholar] [CrossRef]
- Salt, D.E.; Baxter, I.; Lahner, B. Ionomics and the study of the plant ionomer. Annu. Rev. Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef]
- D’Attoma, G.; Saldarelli, P.; De La Fuente, L.; Cobine, P. Ionomes of plants infected with vascular pathogens: Xylella fastidiosa as a case study. In Proceedings of the Plant Health 2019, APS Annual Meeting, Cleveland, OH, USA, 3–7 August 2019. Paper 12629. [Google Scholar] [CrossRef]
- D’Attoma, G.; Morelli, M.; Saldarelli, P.; Saponari, M.; Giampetruzzi, A.; Boscia, D.; Savino, V.N.; De La Fuente, L.; Cobine, P.A. Ionomic differences between susceptible and resistant olive cultivars infected by Xylella fastidiosa in the outbreak area of salento, italy. Pathogens 2019, 8, 272. [Google Scholar] [CrossRef]
- Del Coco, L.D.; Migoni, D.; Girelli, C.R.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Soil and leaf ionomer heterogeneity in Xylella fastidiosa subsp. pauca-infected, non-infected and treated olive groves in Apulia, Italy. Plants 2020, 9, 760. [Google Scholar]
- Girelli, C.R.; Del Coco, L.; Scortichini, M.; Petriccione, M.; Zampella, L.; Mastrobuoni, F.; Cesari, G.; Bertaccini, A.; D’amico, G.; Contaldo, N. Xylella fastidiosa and olive quick decline syndrome (CoDiRO) in Salento (southern Italy): A chemometric 1 H NMR-based preliminary study on Ogliarola salentina and Cellina di Nardò cultivars. Chem. Biol. Technol. Agric. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Scortichini, M.; Jianchi, C.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; L’Aurora, A.; Petriccione, M.; Zampella, L.; Mastrobuoni, F. A zinc, copper and citric acid biocomplex shows promise for control of Xylella fastidiosa subsp. pauca in olive trees in Apulia region (southern Italy). Phytopathol. Mediterr. 2018, 57, 48–72. [Google Scholar]
- Tatulli, G.; Modesti, V.; Pucci, N.; Scala, V.; L’Aurora, A.; Lucchesi, S.; Salustri, M.; Scortichini, M.; Loreti, S. Further in vitro assessment and mid-term evaluation of control strategy of Xylella fastidiosa subsp. pauca in olive groves of Salento (Apulia, Italy). Pathogens 2021, 10, 85. [Google Scholar]
- Dongiovanni, C.; Fumarola, G.; Zicca, S.; Surano, A.; Di Carolo, M.; Datome, G. In vitro and in vivo effects of ammonium chloride on Xylella fastidiosa subsp. pauca infecting olives. In Proceedings of the 3rd European Conference on Xylella fastidiosa and XF-ACTORS Final Meeting, Online Event, 26–30 April 2021. [Google Scholar] [CrossRef]
- Baldassarre, F.; De Stradis, A.; Altamura, G.; Vergaro, V.; Citti, C.; Cannazza, G.; Capodilupo, A.L.; Dini, L.; Ciccarella, G. Application of calcium carbonate nanocarriers for controlled release of phytodrugs against Xylella fastidiosa pathogen. Pure Appl. Chem. 2020, 92, 429–444. [Google Scholar] [CrossRef]
- Hafez, M.M.; Aboulwafa, M.M.; Yassien, M.A.; Hassouna, N.A. Activity of some mucolytics against bacterial adherence to mammalian cells. Appl. Biochem. Biotechnol. 2009, 158, 97–112. [Google Scholar] [CrossRef]
- Muranaka, L.S.; Giorgiano, T.E.; Takita, M.A.; Forim, M.R.; Silva, L.F.; Coletta-Filho, H.D.; Machado, M.A.; de Souza, A.A. N-Acetylcysteine in agriculture, a novel use for an old molecule: Focus on controlling the plant–pathogen Xylella fastidiosa. PLoS ONE 2013, 8, e72937. [Google Scholar] [CrossRef] [PubMed]
- Alves de Souza, A.; Coletta-Filho, H.D.; Dongiovanni, C.; Saponari, M. N-acetyl-cysteine for controlling Xylella fastidiosa in citrus and olive: Understanding the differences to improve management. In Proceedings of the 2nd European Conference on Xylella fastidiosa: How Research Can Support Solutions, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- Cattò, C.; De Vincenti, L.; Cappitelli, F.; Datome, G.; Saponari, M.; Villa, F.; Forlani, F. Non-Lethal Effects of N-Acetylcysteine on Xylella fastidiosa strain De Donno biofilm formation and detachment. Microorganisms 2019, 7, 656. [Google Scholar] [CrossRef]
- Baldassarre, F.; Tatulli, G.; Vergaro, V.; Mariano, S.; Scala, V.; Nobile, C.; Pucci, N.; Dini, L.; Loreti, S.; Ciccarella, G. Sonication-assisted production of fosetyl-al nanocrystals: Investigation of Human toxicity and in vitro antibacterial efficacy against Xylella fastidiosa. Nanomaterials 2020, 10, 1174. [Google Scholar] [CrossRef]
- Baro, A.; Badosa, E.; Montesinos, L.; Feliu, L.; Planas, M.; Montesinos, E.; Bonaterra, A. Screening and identification of BP100 peptide conjugates active against Xylella fastidiosa using a viability-qPCR method. BMC Microbiol. 2020, 20, 229. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Gallo, A.; Altomare, C.; Vurro, M.; Maiorano, G.; Cardinali, A.; D’Antuono, I.; Marchi, G.; Mita, G. In vitro activity of antimicrobial compounds against Xylella fastidiosa, the causal agent of the olive quick decline syndrome in Apulia (Italy). FEMS Microbiol. Lett. 2018, 365, fnx281. [Google Scholar] [CrossRef] [PubMed]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Wallis, C.M.; Rogers, E.E.; Burbank, L.P. Grapevine phenolic compounds influence cell surface adhesion of Xylella fastidiosa and bind to lipopolysaccharide. PLoS ONE 2020, 15, e0240101. [Google Scholar] [CrossRef]
- Bruno, G.L.; Cariddi, C.; Botrugno, L. Exploring a sustainable solution to control Xylella fastidiosa subsp. pauca on olive in the Salento Peninsula, Southern Italy. Crop Prot. 2020, 139, 105288. [Google Scholar]
- Beaulieu, E.D.; Ionescu, M.; Chatterjee, S.; Yokota, K.; Trauner, D.; Lindow, S. Characterization of a diffusible signaling factor from Xylella fastidiosa. MBio 2013, 4, e00539-12. [Google Scholar] [CrossRef]
- Chatterjee, S.; Newman, K.L.; Lindow, S.E. Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant-Microbe Interact. 2008, 21, 1309–1315. [Google Scholar] [CrossRef]
- Lindow, S.; Newman, K.; Chatterjee, S.; Baccari, C.; Iavarone, A.T.; Ionescu, M. Production of Xylella fastidiosa diffusible signal factor in transgenic grape causes pathogen confusion and reduction in severity of Pierce’s disease. Mol. Plant-Microbe Interact. 2014, 27, 244–254. [Google Scholar] [CrossRef]
- Caserta, R.; Souza-Neto, R.R.; Takita, M.A.; Lindow, S.E.; De Souza, A.A. Ectopic expression of Xylella fastidiosa rpfF conferring production of diffusible signal factor in transgenic tobacco and citrus alters pathogen behavior and reduces disease severity. Mol. Plant-Microbe Interact. 2017, 30, 866–875. [Google Scholar] [CrossRef]
- Vona, D.; Datome, G.; Cicco, S.; Morelli, M.; Saldarelli, P.; Saponari, M.; Farinola, G. Monitoring of biofilm production in Xylella fastidiosa strain De Donno via biochemical signalling modulation. In Proceedings of the 2nd European Conference on Xylella fastidiosa: How Research Can Support Solutions, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- Mitter, B.; Brader, G.; Pfaffenbichler, N.; Sessitsch, A. Next generation microbiome applications for crop production—Limitations and the need of knowledge-based solutions. Curr. Opin. Microbiol. 2019, 49, 59–65. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world–bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Morelli, M.; Bahar, O.; Papadopoulou, K.K.; Hopkins, D.L.; Obradović, A. Editorial: Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 2020, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, P.; Roper, C.; Maloney, K. Greenhouse Evaluation of Grapevine Microbial Endophytes and Fungal Natural Products for control of Pierce’s Disease; Final Report for CDFA Agreement Number: 16-0512-SA. 2017. Available online: https://www.semanticscholar.org/paper/Final-Report-for-CDFA-Agreement-Number%3A-16-0512-SA-Rolshausen/f41a73b56fd4a19ca03397345a909eeefb5c6097 (accessed on 14 July 2021).
- Rolshausen, P.; Roper, C.; Kirkpatrick, B.; Cooksey, D.; Borneman, J.; Maloney, K. Control of Pierce’s Disease with fungal endophytes of grapevines antagonistic to Xylella fastidiosa. In Proceedings of the Pierce’s Disease Research Symposium, San Diego, CA, USA, 15–17 December 2010; pp. 224–228. [Google Scholar]
- Kirkpatrick, B.; Jones, D.-D.; Civerolo, E.; Purcell, A.H. Characterize and assess the biocontrol potential of bacterial endophytes of grapevines in California. In Proceedings of the Pierce’s Disease Research Symposium, Coronado, CA, USA, 8–11 December 2003. [Google Scholar]
- Vergine, M.; Meyer, J.B.; Cardinale, M.; Sabella, E.; Hartmann, M.; Cherubini, P.; De Bellis, L.; Luvisi, A. The Xylella fastidiosa-resistant olive cultivar “Leccino” has stable endophytic microbiota during the Olive Quick Decline Syndrome (OQDS). Pathogens 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Giampetruzzi, A.; Baptista, P.; Morelli, M.; Cameirao, C.; Neto, T.L.; Costa, D.; Datome, G.; Abou Kubaa, R.; Altamura, G.; Saponari, M.; et al. Differences in the endophytic microbiome of olive cultivars infected by Xylella fastidiosa across Seasons. Pathogens 2020, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Zicca, S.; De Bellis, P.; Masiello, M.; Saponari, M.; Saldarelli, P.; Boscia, D.; Sisto, A. Antagonistic activity of olive endophytic bacteria and of Bacillus spp. strains against Xylella fastidiosa. Microbiol. Res. 2020, 236, 126467. [Google Scholar] [CrossRef] [PubMed]
- Susi, P.; Aktuganov, G.; Himanen, J.; Korpela, T. Biological control of wood decay against fungal infection. J. Environ. Manag. 2011, 92, 1681–1689. [Google Scholar] [CrossRef]
- Antelmi, I.; Sion, V.; Lucchese, P.; Nigro, F. Methylobacterium spp., endophytes of olive trees, as potential biocontrol agents of Xylella fastidiosa subsp. pauca. In Proceedings of the 2nd European Conference on Xylella fastidiosa: How Research Can Support Solutions, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- Sessitsch, A.; Coenye, T.; Sturz, A.V.; Vandamme, P.; Barka, E.A.; Salles, J.F.; Van Elsas, J.D.; Faure, D.; Reiter, B.; Glick, B.R. Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. Int. J. Syst. Evol. Microbiol. 2005, 55, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Ait Barka, E.; Clément, C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: From the rhizosphere to inflorescence tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Mitter, B.; Petric, A.; Shin, M.W.; Chain, P.S.G.; Hauberg-Lotte, L.; Reinhold-Hurek, B.; Nowak, J.; Sessitsch, A. Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front. Plant Sci. 2013, 4, 120. [Google Scholar] [CrossRef]
- Baccari, C.; Antonova, E.; Lindow, S. Biological control of Pierce’s disease of grape by an endophytic bacterium. Phytopathology 2019, 109, 248–256. [Google Scholar] [CrossRef]
- Morelli, M.; Dongiovanni, C.; Datome, G.; Giampetruzzi, A.; Loconsole, G.; Montilon, V.; Altamura, G.; Angione, D.; Saponari, M.; Saldarelli, P. Assessment of Paraburkholderia phytorfirmans PsJN biocontrol potential against Xylella fastidiosa ‘De Donno’ strain in olive. In Proceedings of the 2nd European Conference on Xylella fastidiosa: How Research Can Support Solutions, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauro, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean olive groves: Host-plant exploitation throughout the year. Insects 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance becomes a new threat to European agriculture. J. Pest Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Pegoraro, M.; Altamura, G.; Canuto, F.; Zicca, S.; Fumarola, G.; Almeida, R.; Saponari, M.; Dongiovanni, C.; et al. Temporal dynamics of Xylella fastidiosa subsp. pauca vector transmission to olive plants. Entomol. Gen. 2021, 117, 9250–9259. [Google Scholar]
- Dongiovanni, C.; Di Carolo, M.; Fumarola, G.; Tauro, D.; Altamura, G.; Cavalieri, V. Evaluation of insecticides for the control of juveniles of Philaenus spumarius L., 2015–2017. Arthropod Manag. Tests 2018, 43, tsy073. [Google Scholar] [CrossRef]
- Dáder, B.; Viñuela, E.; Moreno, A.; Plaza, M.; Garzo, E.; Del Estal, P.; Fereres, A. Sulfoxaflor and natural Pyrethrin with Piperonyl Butoxide are effective alternatives to Neonicotinoids against juveniles of Philaenus spumarius, the european vector of Xylella fastidiosa. Insects 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- POnTE Project Deliverable 9.1: Practical Solution for Management and Containment of Xf. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5cdd715c6&appId=PPGMS (accessed on 12 April 2021).
- Prabhaker, N.; Castle, S.J.; Toscano, N.C. Susceptibility of immature stages of Homalodisca coagulata (Hemiptera: Cicadellidae) to selected insecticides. J. Econ. Entomol. 2006, 99, 1805–1812. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Altamura, G.; Di Carolo, M.; Fumarola, G.; Saponari, M.; Cavalieri, V. Evaluation of efficacy of different insecticides against Philaenus spumarius L., vector of Xylella fastidiosa in olive orchards in Southern Italy, 2015–17. Arthropod Manag. Tests 2018, 43, tsy034. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Fumarola, G.; Di Carolo, M.; Tedone, B.; Ancona, S.; Palmisano, F.; Silletti, M.; Cavalieri, V. Ulteriori acquisizioni per il controllo di Philaenus spumarius vettore di Xylella fastidiosa. In Proceedings of the Giornate Fitopatologiche, Online Event, 27 October–12 November 2020; pp. 257–266. [Google Scholar]
- Izquierdo, J.; Sabaté, J.; Eficacia de deltametrín y flupiradifurona en el control de Philaenus spumarius. Phytoma. 2018, pp. 68–73. Available online: https://www.researchgate.net/publication/330540913_Eficacia_de_deltametrin_y_flupiradifurona_en_el_control_de_Philaenus_spumarius (accessed on 14 July 2021).
- European Commission. Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off. J. Eur. Union L 2013, 139, 12–26. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. OJ L 2018, 61, 31–34. [Google Scholar]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef]
- Di Serio, F.; Bodino, N.; Cavalieri, V.; Demichelis, S.; Di Carolo, M.; Dongiovanni, C.; Fumarola, G.; Gilioli, G.; Guerrieri, E.; Picciotti, U. Collection of data and information on biology and control of vectors of Xylella fastidiosa. EFSA Support. Publ. 2019, 16. [Google Scholar] [CrossRef]
- Reis, C.; Villa, M.; Rodrigues, I.; Cameirão, C.; Baptista, P.; Pereira, J.A. Potential natural biocontrol agents of Aphrophoridae eggs. In Proceedings of the 2nd Joint Annual Meeting “European Research on Emerging Plant Diseases”, Valencia, Spain, 23–26 October 2018; p. 79. [Google Scholar]
- Mesmin, X.; Chartois, M.; Genson, G.; Rossi, J.-P.; Cruaud, A.; Rasplus, J.-Y. Ooctonus vulgatus (Hymenoptera, Mymaridae), a potential biocontrol agent to reduce populations of Philaenus spumarius (Hemiptera, Aphrophoridae) the main vector of Xylella fastidiosa in Europe. PeerJ 2020, 8, e8591. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, A.; Fierro, A.; Garganese, F.; Picciotti, U.; Porcelli, F. A biological control model to manage the vector and the infection of Xylella fastidiosa on olive trees. PLoS ONE 2020, 15, e0232363. [Google Scholar] [CrossRef] [PubMed]
- Molinatto, G.; Demichelis, S.; Bodino, N.; Giorgini, M.; Mori, N.; Bosco, D. Biology and prevalence in Northern Italy of Verrallia aucta (Diptera, Pipunculidae), a parasitoid of Philaenus spumarius (Hemiptera, Aphrophoridae), the main vector of Xylella fastidiosa in Europe. Insects 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Ruschioni, S.; Riolo, P.; Isidoro, N.; Romani, R. Fine structure of antennal sensilla of the spittlebug Philaenus spumarius L. (Insecta: Hemiptera: Aphrophoridae). I. Chemoreceptors and thermo-/hygroreceptors. Arthropod Struct. Dev. 2016, 45, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLoS ONE 2017, 12, e0190454. [Google Scholar] [CrossRef]
- Ganassi, S.; Cascone, P.; Di Domenico, C.; Pistillo, M.; Formisano, G.; Giorgini, M.; Grazioso, P.; Germinara, G.S.; De Cristofaro, A.; Guerrieri, E. Electrophysiological and behavioural response of Philaenus spumarius to essential oils and aromatic plants. Sci. Rep. 2020, 10, 3114. [Google Scholar] [CrossRef]
- Avosani, S.; Franceschi, P.; Ciolli, M.; Verrastro, V.; Mazzoni, V. Vibrational playbacks and microscopy to study the signalling behaviour and female physiology of Philaenus spumarius. J. Appl. Entomol. 2021, 145, 518–529. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Stefanizzi, F.; Perri, E. Evaluation of olives cultivated in southern Italy by simple sequence repeat markers. HortScience 2009, 44, 582–588. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Savino, V.N.; Martelli, G.P.; Saldarelli, P. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. pauca. BMC Genom. 2016, 17, 475. [Google Scholar] [CrossRef]
- Zaini, P.A.; Nascimento, R.; Gouran, H.; Cantu, D.; Chakraborty, S.; Phu, M.; Goulart, L.R.; Dandekar, A.M. Molecular profiling of Pierce’s disease outlines the response circuitry of Vitis vinifera to Xylella fastidiosa infection. Front. Plant Sci. 2018, 9, 771. [Google Scholar] [CrossRef]
- Choi, H.-K.; Iandolino, A.; da Silva, F.G.; Cook, D.R. Water deficit modulates the response of Vitis vinifera to the Pierce’s disease pathogen Xylella fastidiosa. Mol. Plant-Microbe Interact. 2013, 26, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; de Souza, A.A.; Takita, M.A.; Kishi, L.T.; Machado, M.A. RNA-Seq analysis of Citrus reticulata in the early stages of Xylella fastidiosa infection reveals auxin-related genes as a defense response. BMC Genom. 2013, 14, 676. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B.; Almeida-Souza, H.O.; Zaini, P.A.; Alves, M.N.; de Souza, A.G.; Pierry, P.M.; da Silva, A.M.; Goulart, L.R.; Dandekar, A.M.; Nascimento, R. Xylella fastidiosa subsp. pauca Strains Fb7 and 9a5c from Citrus display differential behavior, secretome, and plant virulence. Int. J. Mol. Sci. 2020, 21, 6769. [Google Scholar]
- Deyett, E.; Rolshausen, P.E. Temporal dynamics of the sap microbiome of grapevine under high Pierce’s disease pressure. Front. Plant Sci. 2019, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Huerta-Acosta, K.; Tenscher, A.C.; Walker, M.A. Genetic characterization of Vitis germplasm collected from the southwestern US and Mexico to expedite Pierce’s disease-resistance breeding. Theor. Appl. Genet. 2018, 131, 1589–1602. [Google Scholar] [CrossRef]
- Quinton, A. UC Davis Releases 5 New Wine Grape Varieties. Available online: https://www.ucdavis.edu/food/news/uc-davis-releases-five-new-wine-grape-varieties (accessed on 12 April 2021).
- Sabella, E.; Luvisi, A.; Aprile, A.; Negro, C.; Vergine, M.; Nicolì, F.; Miceli, A.; De Bellis, L. Xylella fastidiosa induces differential expression of lignification related-genes and lignin accumulation in tolerant olive trees cv. Leccino. J. Plant Physiol. 2018, 220, 60–68. [Google Scholar] [CrossRef]
- Montilon, V.; Boscia, D.; Savino, V.; Saldarelli, P.; De Stradis, A. Evaluation of vascular occlusions in xylem vessels of olive cultivars infected with Xylella fastidiosa. In Proceedings of the 2nd European Conference on Xylella fastidiosa: How Research Can Support Solutions, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- Niza, B.; Coletta-Filho, H.; Merfa, M.; Takita, M.; De Souza, A. Differential colonization patterns of Xylella fastidiosa infecting citrus genotypes. Plant Pathol. 2015, 64, 1259–1269. [Google Scholar] [CrossRef]
- De Benedictis, M.; De Caroli, M.; Baccelli, I.; Marchi, G.; Bleve, G.; Gallo, A.; Ranaldi, F.; Falco, V.; Pasquali, V.; Piro, G. Vessel occlusion in three cultivars of Olea europaea naturally exposed to Xylella fastidiosa in open field. J. Phytopathol. 2017, 165, 589–594. [Google Scholar] [CrossRef]
- Cardinale, M.; Luvisi, A.; Meyer, J.B.; Sabella, E.; De Bellis, L.; Cruz, A.C.; Ampatzidis, Y.; Cherubini, P. Specific fluorescence in situ hybridization (FISH) test to highlight colonization of xylem vessels by Xylella fastidiosa in naturally infected olive trees (Olea europaea L.). Front. Plant Sci. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Boscia, D.; Altamura, G.; Ciniero, A.; Di Carolo, M.; Dongiovanni, C.; Fumarola, G.; Giampetruzzi, A.; Greco, P.; La Notte, P.; Loconsole, G. Resistenza a Xylella fastidiosa in diverse cultivar di olivo. L’inf. Agrar. 2017, 11, 59–63. [Google Scholar]
- Saponari, M.; Altamura, G.; Abou Kubaa, R.; Montilon, V.; Saldarelli, P.; Specchia, F.; Palmisano, F.; Silletti, M.R.; Pollastro, P.; Zicca, S.; et al. Further acquisition on the response of a large number of olive cultivars to infections caused by Xylella fastidiosa subsp. pauca, ST53. In Proceedings of the 2nd European Conference on Xylella fastidiosa: How Research Can Support Solutions, Ajaccio, France, 29–30 October 2019. [Google Scholar]
- XF-ACTORS Project: Screening of Olive Cultivars for Searching Sources of Resistance to Xylella fastidiosa. Available online: https://www.xfactorsproject.eu/screening-cultivars-resistance-xf/ (accessed on 12 April 2021).
- Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Tauro, D.; Ciniero, A.; Morelli, M.; Bosco, D.; Saponari, M. Evaluation of olive cultivar effect on the efficiency of the acquisition and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae). In Proceedings of the 3rd Hemipteran-Plant Interactions Symposium (HPIS 2017), Madrid, Spain, 4–8 June 2017; p. 39. [Google Scholar]
- POnTE Project (Pest Organisms Threatening Europe). Available online: https://www.ponteproject.eu/ (accessed on 12 April 2021).
- XF-ACTORS Project (Xylella Fastidiosa Active Containment through a Multidisciplinary-Oriented Research Strategy). Available online: https://www.xfactorsproject.eu/ (accessed on 12 April 2021).
- BIOVEXO Project (Biocontrol of Xylella and Its Vector in Olive Trees for Integrated Pest Management). Available online: https://biovexo.eu/ (accessed on 12 April 2021).
- Life Resilience Project. Available online: http://www.liferesilience.eu/home-eng/ (accessed on 12 April 2021).
- Cure XF Project (Capacity Building and Raising Awareness in Europe and in Third Countries to Cope with Xylella fastidiosa). Available online: http://www.cure-xf.eu/ (accessed on 12 April 2021).

| Geographic Area | Xylella fastidiosa Subspecies | Sequence Type |
|---|---|---|
| Apulia, Italy | pauca | ST53 |
| Provence-Alpes-Côte d’Azur, France | pauca | ST53 |
| Provence-Alpes-Côte d’Azur, France | multiplex | Unknown |
| Ibiza, Balearic Islands, Spain | pauca | ST80 |
| Mallorca, Balearic Islands, Spain | multiplex | ST81 |
| Madrid Community, Spain | multiplex | ST6 |
| Porto Metropolitan Area, Portugal | multiplex | ST7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S.; et al. Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management. Microorganisms 2021, 9, 1771. https://doi.org/10.3390/microorganisms9081771
Morelli M, García-Madero JM, Jos Á, Saldarelli P, Dongiovanni C, Kovacova M, Saponari M, Baños Arjona A, Hackl E, Webb S, et al. Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management. Microorganisms. 2021; 9(8):1771. https://doi.org/10.3390/microorganisms9081771
Chicago/Turabian StyleMorelli, Massimiliano, José Manuel García-Madero, Ángeles Jos, Pasquale Saldarelli, Crescenza Dongiovanni, Magdalena Kovacova, Maria Saponari, Alberto Baños Arjona, Evelyn Hackl, Stephen Webb, and et al. 2021. "Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management" Microorganisms 9, no. 8: 1771. https://doi.org/10.3390/microorganisms9081771
APA StyleMorelli, M., García-Madero, J. M., Jos, Á., Saldarelli, P., Dongiovanni, C., Kovacova, M., Saponari, M., Baños Arjona, A., Hackl, E., Webb, S., & Compant, S. (2021). Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management. Microorganisms, 9(8), 1771. https://doi.org/10.3390/microorganisms9081771









