Role of Rhizospheric Microbiota as a Bioremediation Tool for the Protection of Soil-Plant Systems from Microcystins Phytotoxicity and Mitigating Toxin-Related Health Risk
Abstract
1. Introduction
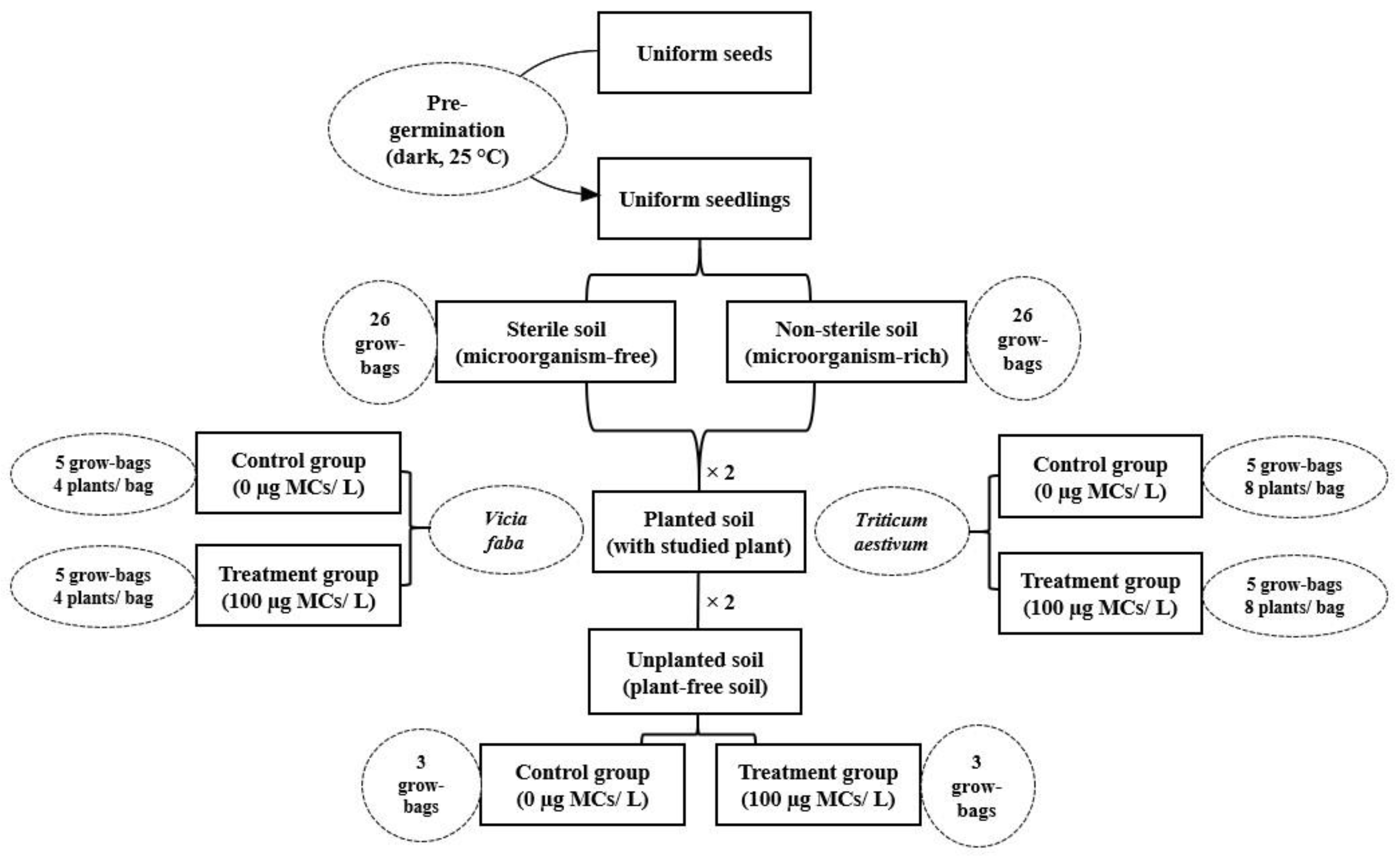
2. Materials and Methods
2.1. Bloom Sampling and Microcystins Quantification
2.2. Microcystins Extraction and Purification
2.3. Experimental Setup
2.3.1. Soil Collecting and Characterization
2.3.2. Plant Culture and Exposure Experiment
2.4. Plant Harvest and Growth Indicators’ Determination
2.5. Determination of Microcystins in Plant Tissues and Soil
2.6. Evaluation of Health Risk
2.6.1. Bioaccumulation Factor and Bioconcentration Level
2.6.2. Estimated Daily Intake and Health Risk Quotient
2.7. Statistical Analysis
3. Results
3.1. Effects of Microcystins on Plant Growth and Morphology
3.2. Accumulation of Microcystins in Soil-Plant System
3.3. Health Risk Assessment
4. Discussion
4.1. Impact of Microcystins on Plants’ Morphology
4.2. Impact of Microcystins on Plants’ Growth in Absence of Native Rhizospheric Microbiota
4.3. Impact of Microcystins on Plants’ Growth in Presence of Native Rhizospheric Microbiota
4.4. Bioaccumulation of Microcystins in Plants and Health Risk Assessment
4.5. Accumulation and Potential Removal of Microcystins in Agricultural Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, S.; He, Z.; Ma, C.; Zhang, H.; Xi, B.; Xia, X.; Xu, Y.; Wu, F. Stricter nutrient criteria are required to mitigate the impact of climate change on harmful cyanobacterial blooms. J. Hydrol. 2019, 569, 698–704. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Kurmayer, R.; Azevedo, S.M.F.O.; Wood, S.A.; Chorus, I.; Welker, M. Understanding the occurrence of cyanobacteria and cyanotoxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I., Welker, M., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2021; pp. 213–214. [Google Scholar]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Paerl, H.W.; Zhu, G.; Qin, B.; Hall, N.S.; Zhu, M. Long-term nutrient trends and harmful cyanobacterial bloom potential in hypertrophic Lake Taihu, China. Hydrobiologia 2017, 787, 229–242. [Google Scholar] [CrossRef]
- Paerl, H.W.; Havens, K.E.; Xu, H.; Zhu, G.; McCarthy, M.J.; Newell, S.E.; Scott, J.T.; Hall, N.S.; Otten, T.G.; Qin, B. Mitigating eutrophication and toxic cyanobacterial blooms in large lakes: The evolution of a dual nutrient (N and P) reduction paradigm. Hydrobiologia 2020, 847, 4359–4375. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef]
- Redouane, E.M.; El Amrani Zerrifi, S.; El Khalloufi, F.; Oufdou, K.; Oudra, B.; Lahrouni, M.; Campos, A.; Vasconcelos, V. Mode of action and fate of microcystins in the complex soil-plant ecosystems. Chemosphere 2019, 225, 270–281. [Google Scholar] [CrossRef]
- Pham, T.L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Oudra, B.; Dadi-El Andaloussi, M.; Vasconcelos, V.M. Identification and quantification of microcystins from a Nostoc muscorum bloom occurring in Oukaïmeden river (High-Atlas mountains of Marrakech, Morocco). Environ. Monit. Assess. 2009, 149, 437–444. [Google Scholar] [CrossRef]
- Massey, I.Y.; Wu, P.; Wei, J.; Luo, J.; Ding, P.; Wei, H.; Yang, F. A mini-review on detection methods of microcystins. Toxins 2020, 12, 641. [Google Scholar] [CrossRef]
- Fontanillo, M.; Köhn, M. Microcystins: Synthesis and structure–activity relationship studies toward PP1 and PP2A. Bioorg. Med. Chem. 2018, 26, 1118–1126. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, C.; Tuo, X.; Zhou, C.; Zhao, Q.; Shen, T. MCLR induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in Sertoli cells. Chemosphere 2021, 263, 127868. [Google Scholar] [CrossRef]
- Tsuji, K.; Watanuki, T.; Kondo, F.; Watanabe, M.F.; Suzuki, S.; Nakazawa, H.; Suzuki, M.; Uchida, H.; Harada, K.I. Stability of microcystins from cyanobacteria-II. Effect of UV light on decomposition and isomerization. Toxicon 1995, 33, 1619–1631. [Google Scholar] [CrossRef]
- Harada, K.; Tsuji, K.; Watanabe, M.F.; Kondo, F. Stability of microcystins from cyanobacteria—III.* Effect of pH and temperature. Phycologia 1996, 35, 83–88. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, P.; Chen, J. Effects of temperature on the stability of microcystins in muscle of fish and its consequences for food safety. Bull. Environ. Contam. Toxicol. 2010, 84, 202–207. [Google Scholar] [CrossRef]
- Ufelmann, H.; Krüger, T.; Luckas, B.; Schrenk, D. Human and rat hepatocyte toxicity and protein phosphatase 1 and 2A inhibitory activity of naturally occurring desmethyl-microcystins and nodularins. Toxicology 2012, 293, 59–67. [Google Scholar] [CrossRef]
- Janssen, E.M.L. Cyanobacterial peptides beyond microcystins—A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Li, J. Current research scenario for microcystins biodegradation—A review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 2017, 595, 615–632. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef]
- Ho, L.; Tang, T.; Hoefel, D.; Vigneswaran, B. Determination of rate constants and half-lives for the simultaneous biodegradation of several cyanobacterial metabolites in Australian source waters. Water Res. 2012, 46, 5735–5746. [Google Scholar] [CrossRef]
- Zastepa, A.; Pick, F.R.; Blais, J.M. Fate and Persistence of Particulate and Dissolved Microcystin-LA from Microcystis Blooms. Hum. Ecol. Risk Assess. 2014, 20, 1670–1686. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.; Kim, J.; Hong, S.; Shin, K.H. Distribution of microcystins in environmental multimedia and their bioaccumulation characteristics in marine benthic organisms in the Geum River Estuary, South Korea. Sci. Total Environ. 2021, 757, 143815. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef]
- Tian, D.; Zheng, W.; Wei, X.; Sun, X.; Liu, L.; Chen, X.; Zhang, H.; Zhou, Y.; Chen, H.; Zhang, H.; et al. Dissolved microcystins in surface and ground waters in regions with high cancer incidence in the Huai River Basin of China. Chemosphere 2013, 91, 1064–1071. [Google Scholar] [CrossRef]
- Zhang, Y.; Husk, B.R.; Duy, S.V.; Dinh, Q.T.; Sanchez, J.S.; Sauvé, S.; Whalen, J.K. Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec, Canada. Chemosphere 2021, 274, 129781. [Google Scholar] [CrossRef]
- WHO. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments. Cyanobacterial Toxins: Microcystins; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Chen, W.; Song, L.; Gan, N.; Li, L. Sorption, degradation and mobility of microcystins in Chinese agriculture soils: Risk assessment for groundwater protection. Environ. Pollut. 2006, 144, 752–758. [Google Scholar] [CrossRef]
- Bibo, L.; Yan, G.; Bangding, X.; Jiantong, L.; Yongding, L. A laboratory study on risk assessment of microcystin-RR in cropland. J. Environ. Manag. 2008, 86, 566–574. [Google Scholar] [CrossRef]
- Cao, Q.; Steinman, A.D.; Wan, X.; Xie, L. Bioaccumulation of microcystin congeners in soil-plant system and human health risk assessment: A field study from Lake Taihu region of China. Environ. Pollut. 2018, 240, 44–50. [Google Scholar] [CrossRef]
- Lee, S.; Jiang, X.; Manubolu, M.; Riedl, K.; Ludsin, S.A.; Martin, J.F.; Lee, J. Fresh produce and their soils accumulate cyanotoxins from irrigation water: Implications for public health and food security. Food Res. Int. 2017, 102, 234–245. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Y.W.; Liu, B.L.; Zhao, H.M.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H.; Li, Q.X. High ecological and human health risks from microcystins in vegetable fields in southern China. Environ. Int. 2019, 133, 105142. [Google Scholar] [CrossRef]
- Chen, G.; Li, Q.; Bai, M.; Chen, Y. Nitrogen Metabolism in Acorus calamus L. Leaves Induced Changes in Response to Microcystin–LR at Environmentally Relevant Concentrations. Bull. Environ. Contam. Toxicol. 2019, 103, 280–285. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, Z.; Bai, M.; Li, Q. Chronic effects of microcystin-LR at environmental relevant concentrations on photosynthesis of Typha angustifolia Linn. Ecotoxicology 2020, 29, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liu, H. Response of hormone in rice seedlings to irrigation contaminated with cyanobacterial extract containing microcystins. Chemosphere 2020, 256, 127157. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Jung, K.; Lundvall, L.; Neumann, S.; Peuthert, A. Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ. Toxicol. Chem. 2006, 25, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Arjona, T.; Ariza, R.R. Repair and tolerance of oxidative DNA damage in plants. Mutat. Res. Rev. Mutat. Res. 2009, 681, 169–179. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant. J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Gu, Y.; Liang, C. Responses of antioxidative enzymes and gene expression in Oryza sativa L and Cucumis sativus L seedlings to microcystins stress. Ecotoxicol. Environ. Saf. 2020, 193, 110351. [Google Scholar] [CrossRef]
- Cao, Q.; Steinman, A.D.; Yao, L.; Xie, L. Effects of light, microorganisms, farming chemicals and water content on the degradation of microcystin-LR in agricultural soils. Ecotoxicol. Environ. Saf. 2018, 156, 141–147. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Y.W.; Wang, Z.R.; Liu, B.L.; Zhao, H.M.; Li, H.; Cai, Q.Y.; Mo, C.H.; Li, Q.X. Bioaccumulation and Phytotoxicity and Human Health Risk from Microcystin-LR under Various Treatments: A Pot Study. Toxins 2020, 12, 523. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; Faghire, M.; Peix, A.; El Khalloufi, F.; Vasconcelos, V.; Oudra, B. Cyanobacterial extracts containing microcystins affect the growth, nodulation process and nitrogen uptake of faba bean (Vicia faba L., Fabaceae). Ecotoxicology 2012, 21, 681–687. [Google Scholar] [CrossRef]
- El Khalloufi, F.; Oufdou, K.; Lahrouni, M.; Faghire, M.; Peix, A.; Ramírez-Bahena, M.H.; Vasconcelos, V.; Oudra, B. Physiological and antioxidant responses of Medicago sativa-rhizobia symbiosis to cyanobacterial toxins (Microcystins) exposure. Toxicon 2013, 76, 167–177. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; El Khalloufi, F.; Benidire, L.; Albert, S.; Göttfert, M.; Caviedes, M.A.; Rodriguez-Llorente, I.D.; Oudra, B.; Pajuelo, E. Microcystin-tolerant Rhizobium protects plants and improves nitrogen assimilation in Vicia faba irrigated with microcystin-containing waters. Environ. Sci. Pollut. Res. 2016, 23, 10037–10049. [Google Scholar] [CrossRef]
- Redouane, E.M.; Lahrouni, M.; Martins, J.C.; El Amrani Zerrifi, S.; Benidire, L.; Douma, M.; Aziz, F.; Oufdou, K.; Mandi, L.; Campos, A.; et al. Protective Role of Native Rhizospheric Soil Microbiota Against the Exposure to Microcystins Introduced into Soil-Plant System via Contaminated Irrigation Water and Health Risk Assessment. Toxins 2021, 13, 118. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Chézeau, A.; Turquet, J.; Quod, J.P.; Puiseux-Dao, S. Morphological and toxicological variability of Prorocentrum lima clones isolated from four locations in the south-west Indian Ocean. Toxicon 2001, 39, 1195–1202. [Google Scholar] [CrossRef]
- El Ghazali, I.; Saqrane, S.; Carvalho, A.P.; Ouahid, Y.; Del Campo, F.F.; Oudra, B.; Vasconcelos, V. Effect of different microcystin profiles on toxin bioaccumulation in common carp (Cyprinus carpio) larvae via Artemia nauplii. Ecotoxicol. Environ. Saf. 2010, 73, 762–770. [Google Scholar] [CrossRef]
- Bavithra, G.; Azevedo, J.; Oliveira, F.; Morais, J.; Pinto, E.; Ferreira, I.; Vasconcelos, V.; Campos, A.; Almeida, C.M.R. Assessment of ConstructedWetlands’ Potential for the Removal of Cyanobacteria and Microcystins (MC-LR). Water 2020, 12, 10. [Google Scholar] [CrossRef]
- Chen, W.; Li, L.; Gan, N.; Song, L. Optimization of an effective extraction procedure for the analysis of microcystins in soils and lake sediments. Environ. Pollut. 2006, 143, 241–246. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.A.; de Toledo Arruda-Neto, J.D.; Tornisielo, V.L.; Vilca, F.Z.; do Carmo Bittencourt-Oliveira, M. Microcystin-LR bioaccumulation and depuration kinetics in lettuce and arugula: Human health risk assessment. Sci. Total Environ. 2016, 566, 1379–1386. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Qu, Y.; Chen, W.; Song, L. Phytotoxicity, bioaccumulation and potential risks of plant irrigations using cyanobloom-loading freshwater. Sci. Total Environ. 2018, 624, 704–712. [Google Scholar] [CrossRef]
- FAO. New Food Balances; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- WHO. Cyanobacterial Toxins: Microcystin-LR. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- ANZECC. Water Quality Guidelines. In Livestock Drinking Water Guidelines; Australian and New Zealand Environment and Conservation Council: Canberra, Australia, 2000. [Google Scholar]
- Lahrouni, M.; Oufdou, K.; El Khalloufi, F.; Baz, M.; Lafuente, A.; Dary, M.; Pajuelo, E.; Oudra, B. Physiological and biochemical defense reactions of Vicia faba L.-Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ. Sci. Pollut. Res. 2013, 20, 5405–5415. [Google Scholar] [CrossRef]
- Peuthert, A.; Chakrabarti, S.; Pflugmacher, S. Uptake of Microcystins-LR and -LF (Cyanobacterial Toxins) in Seedlings of Several Important Agricultural Plant Species and the Correlation with Cellular Damage (Lipid Peroxidation). Environ. Toxicol. Int. J. 2007, 22, 436–442. [Google Scholar] [CrossRef]
- Llana-ruiz-cabello, M.; Jos, A.; Cameán, A.; Oliveira, F.; Barreiro, A.; Machado, J.; Azevedo, J.; Pinto, E.; Almeida, A.; Campos, A.; et al. Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia Oleracea and Lactuca Sativa. Toxins 2019, 11, 624. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, X.; Liu, H.; Liang, C. Effect of irrigation with microcystins-contaminated water on growth and fruit quality of Cucumis sativus L. and the health risk. Agric. Water Manag. 2018, 204, 91–99. [Google Scholar] [CrossRef]
- Saqrane, S.; Ghazali, I.E.; Oudra, B.; Bouarab, L.; Vasconcelos, V. Effects of cyanobacteria producing microcystins on seed germination and seedling growth of several agricultural plants. J. Environ. Sci. Heal. Part. B Pestic. Food Contam. Agric. Wastes 2008, 43, 443–451. [Google Scholar] [CrossRef]
- Cao, Q.; Rediske, R.R.; Yao, L.; Xie, L. Effect of microcystins on root growth, oxidative response, and exudation of rice (Oryza sativa). Ecotoxicol. Environ. Saf. 2018, 149, 143–149. [Google Scholar] [CrossRef]
- Chen, J.; Han, F.X.; Wang, F.; Zhang, H.; Shi, Z. Accumulation and phytotoxicity of microcystin-LR in rice (Oryza sativa). Ecotoxicol. Environ. Saf. 2012, 76, 193–199. [Google Scholar] [CrossRef]
- Dai, H.; Yang, Z. Variation in Cd accumulation among radish cultivars and identification of low-Cd cultivars. Environ. Sci. Pollut. Res. 2017, 24, 15116–15124. [Google Scholar] [CrossRef]
- Xiang, L.; Chen, L.; Yu, L.Y.; Yu, P.F.; Zhao, H.M.; Mo, C.H.; Li, Y.W.; Li, H.; Cai, Q.Y.; Zhou, D.M.; et al. Genotypic variation and mechanism in uptake and translocation of perfluorooctanoic acid (PFOA) in lettuce (Lactuca sativa L.) cultivars grown in PFOA-polluted soils. Sci. Total Environ. 2018, 636, 999–1008. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; Khalloufi, F.E.; Pajuelo, E.; Oudra, B. Impact of cyanobacterial toxins (microcystins) on growth and root development of in vitro Vicia faba cultures. Int. J. Innov. Appl. Stud. 2015, 12, 2028–9324. [Google Scholar]
- Malaissi, L.; Vaccarini, C.A.; Hernández, M.P.; Ruscitti, M.; Arango, C.; Busquets, F.; Arambarri, A.M.; Giannuzzi, L.; Andrinolo, D.; Sedan, D. [D-Leu1]MC-LR and MC-LR: A Small–Large Difference: Significantly Different Effects on Phaseolus vulgaris L. (Fabaceae) Growth and Phototropic Response after Single Contact during Imbibition with Each of These Microcystin Variants. Toxins 2020, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Pappas, D.; Panou, M.; Adamakis, I.D.S.; Gkelis, S.; Panteris, E. Beyond microcystins: Cyanobacterial extracts induce cytoskeletal alterations in rice root cells. Int. J. Mol. Sci. 2020, 21, 9649. [Google Scholar] [CrossRef] [PubMed]
- Saqrane, S.; Ouahid, Y.; El Ghazali, I.; Oudra, B.; Bouarab, L.; del Campo, F.F. Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: A laboratory experimental approach. Toxicon 2009, 53, 786–796. [Google Scholar] [CrossRef]
- Petrou, M.; Karas, P.A.; Vasileiadis, S.; Zafiriadis, I.; Papadimitriou, T.; Levizou, E.; Kormas, K.; Karpouzas, D.G. Irrigation of radish (Raphanus sativus L.) with microcystin-enriched water holds low risk for plants and their associated rhizopheric and epiphytic microbiome. Environ. Pollut. 2020, 266, 115208. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.Q.; Hu, L.B.; Shi, Z.Q. Microcystin-LR-induced phytotoxicity in rice crown root is associated with the cross-talk between auxin and nitric oxide. Chemosphere 2013, 93, 283–293. [Google Scholar] [CrossRef]
- Liang, C.; Wang, W. Response and recovery of rice (Oryza sativa) seedlings to irrigation with microcystin-contaminated water. Environ. Earth Sci. 2015, 73, 4573–4580. [Google Scholar] [CrossRef]
- Liang, C.; Ma, X.; Liu, H. Effect of microcystins at different rice growth stages on its yield, quality, and safety. Environ. Sci. Pollut. Res. 2020, 28, 13942–13954. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Nélieu, S.; Delarue, G.; Bouaïcha, N. Evaluation of the transfer and the accumulation of microcystins in tomato (Solanum lycopersicum cultivar MicroTom) tissues using a cyanobacterial extract containing microcystins and the radiolabeled microcystin-LR (14C-MC-LR). Sci. Total Environ. 2016, 541, 1052–1058. [Google Scholar] [CrossRef]
- Levizou, E.; Papadimitriou, T.; Papavasileiou, E.; Papadimitriou, N.; Kormas, K.A. Root vegetables bioaccumulate microcystins-LR in a developmental stage-dependent manner under realistic exposure scenario: The case of carrot and radish. Agric. Water Manag. 2020, 240, 106274. [Google Scholar] [CrossRef]
- do Carmo Bittencourt-Oliveira, M.; Cordeiro-Araújo, M.K.; Chia, M.A.; de Toledo Arruda-Neto, J.D.; de Oliveira, Ê.T.; dos Santos, F. Lettuce irrigated with contaminated water: Photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol. Environ. Saf. 2016, 128, 83–90. [Google Scholar] [CrossRef]
- Chia, M.A.; Auta, Z.Z.; Esson, A.E.; Yisa, A.G.; Abolude, D.S. Assessment of microcystin contamination of Amaranthus hybridus, Brassica oleracea, and Lactuca sativa sold in markets: A case study of Zaria, Nigeria. Environ. Monit. Assess. 2019, 191, 1–9. [Google Scholar] [CrossRef]
- Neffling, M.R.; Lance, E.; Meriluoto, J. Detection of free and covalently bound microcystins in animal tissues by liquid chromatography-tandem mass spectrometry. Environ. Pollut. 2010, 158, 948–952. [Google Scholar] [CrossRef]
- Crush, J.R.; Briggs, L.R.; Sprosen, J.M.; Nichols, S.N. Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ. Toxicol. 2008, 23, 246–252. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J.; Hunter, R.A.; Berger, K.; De Paoli, D.A.; Orr, C.L.A. Ingestion of toxic Microcystis aeruginosa by dairy cattle and the implications for microcystin contamination of milk. Toxicon 2001, 39, 1847–1854. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J.; Hunter, R.A.; Berger, K. Exposure of beef cattle to sub-clinical doses of Microcystis aeruginosa: Toxin bioaccumulation, physiological effects and human health risk assessment. Toxicon 2003, 41, 613–620. [Google Scholar] [CrossRef]
- Miller, M.J.; Critchley, M.M.; Hutson, J.; Fallowfield, H.J. The adsorption of cyanobacterial hepatotoxins from water onto soil during batch experiments. Water Res. 2001, 35, 1461–1468. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Hu, Y.L.; Lee, J.F. Effects of humic acid and suspended soils on adsorption and photo-degradation of microcystin-LR onto samples from Taiwan reservoirs and rivers. J. Hazard. Mater. 2012, 217, 323–329. [Google Scholar] [CrossRef]
- Miller, M.J.; Hutson, J.; Fallowfield, H.J. The adsorption of cyanobacterial hepatoxins as a function of soil properties. J. Water Health 2005, 3, 339–347. [Google Scholar] [CrossRef][Green Version]
- Esterhuizen, M.; Schmitner, N.; Pflugmacher, S. Bioavailability of microcystin-LR in two different soil types to the legume Alfalfa Medicago sativa L. Int. J. Environ. Sci. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, Y.; Chen, X.; Hu, Y.O.O.; Xiang, H.; Tao, J.; Ling, Y. Biodegradation mechanism of microcystin-LR by a novel isolate of Rhizobium sp. TH and the evolutionary origin of the mlrA gene. Int. Biodeterior. Biodegrad. 2016, 115, 17–25. [Google Scholar] [CrossRef]
- Ramani, A.; Rein, K.; Shetty, K.G.; Jayachandran, K. Microbial degradation of microcystin in Florida’s freshwaters. Biodegradation 2012, 23, 35–45. [Google Scholar] [CrossRef]
- El Khalloufi, F.; Oufdou, K.; Bertrand, M.; Lahrouni, M.; Oudra, B.; Ortet, P.; Barakat, M.; Heulin, T.; Achouak, W. Microbiote shift in the Medicago sativa rhizosphere in response to cyanotoxins extract exposure. Sci. Total Environ. 2016, 539, 135–142. [Google Scholar] [CrossRef]
- Rapala, J.; Berg, K.A.; Lyra, C.; Niemi, R.M.; Manz, W.; Suomalainen, S.; Paulin, L.; Lahti, K. Paucibacter toxinivorans gen. nov., sp. nov., a bacterium that degrades cyclic cyanobacterial hepatotoxins microcystins and nodularin. Int. J. Syst. Evol. Microbiol. 2005, 55, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Lemes, G.A.F.; Kersanach, R.; da Pinto, L.S.; Dellagostin, O.A.; Yunes, J.S.; Matthiensen, A. Biodegradation of microcystins by aquatic Burkholderia sp. from a South Brazilian coastal lagoon. Ecotoxicol. Environ. Saf. 2008, 69, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Yan, H.; Pan, G. Microbial degradation of microcystin-LR by Ralstonia solanacearum. Environ. Technol. 2011, 32, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Lu, X.; Jacob, J.; Sun, S.; Heath, R. Metagenomic Identification of Bacterioplankton Taxa and Pathways Involved in Microcystin Degradation in Lake Erie. PLoS ONE 2013, 8, e61809. [Google Scholar] [CrossRef]
- Krishnan, A.; Zhang, Y.Q.; Mou, X. Isolation and Characterization of Microcystin-Degrading Bacteria from Lake Erie. Bull. Environ. Contam. Toxicol. 2018, 101, 617–623. [Google Scholar] [CrossRef]
- Chen, W.; Jia, Y.; Li, E.; Zhao, S.; Zhou, Q.; Liu, L.; Song, L. Soil-based treatments of mechanically collected cyanobacterial blooms from Lake Taihu: Efficiencies and potential risks. Environ. Sci. Technol. 2012, 46, 13370–13376. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Tian, C.; Xiao, B.; Song, L. Evaluation of the potential of anoxic biodegradation of intracellular and dissolved microcystins in lake sediments. J. Hazard. Mater. 2015, 286, 395–401. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, F.; Liu, C.; Wang, M. Biodegradation of microcystins by Bacillus sp. Strain EMB. Energy Procedia 2012, 16, 2054–2059. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.S.; Vo, T.; Park, C.; Choi, J.; Kwon, J.; Roh, S.W.; Choi, Y. Establishment of a new strategy against Microcystis bloom using newly isolated lytic and toxin-degrading bacteria. J. Appl. Phycol. 2018, 30, 1795–1806. [Google Scholar] [CrossRef]
- Wu, Y.; He, J.; Yang, L. Evaluating Adsorption and Biodegradation Mechanisms during the Removal of Microcystin-RR by Periphyton. Environ. Sci. Technol. 2010, 44, 6319–6324. [Google Scholar] [CrossRef]
| Measured Parameters | Corresponding Values |
|---|---|
| Physical Parameters | |
| Particle-size distribution (%) | |
| Sand (50–2000 µm) | 7.03 |
| Silt (2–50 µm) | 64.05 |
| Clay (<2 µm) | 28.92 |
| Texture: Silty clay loam | |
| Clay minerals (%) | |
| Hartite | 50 |
| Wollastonite | 20 |
| Laumonite | 11 |
| Sillimanite | 8 |
| Clinoptilotite | 7 |
| Periclase | 1 |
| Moisture content (%) | 12.65 ± 0.00 |
| Water-holding capacity (%) | 34 ± 1.00 |
| Chemical Parameters | |
| pH (1:5 H2O) | 8.17 ± 0.07 |
| Electrical conductivity (dS m−1) | 0.42 ± 0.06 |
| Organic matter (g kg−1) | 3.8 ± 1.00 |
| Humic acids (mg g−1) | 0.48 ± 0.02 |
| Macronutrients (g kg−1) | |
| Nitrogen (N) | 0.74 ± 0.15 |
| Phosphorus (P) | 0.42 ± 0.18 |
| Potassium (K) | 24.57 ± 0.42 |
| Biological Parameters | |
| Total bacterial count | 3.28 × 107 |
| Growth Indicators | MCs µg L−1 | Plants/Treatments | ||||
|---|---|---|---|---|---|---|
| Faba Bean | Common Wheat | |||||
| Sterile Soil | Non-Sterile Soil | Sterile Soil | Non-Sterile Soil | |||
| Shoot | TLN (plant−1) | 0 | 11 ± 1.00 | 12 ± 1.00 | 6 ± 0.00 | 6 ± 0.00 |
| 100 | 11 ± 1.00 | 12 ± 1.00 | 6 ± 0.00 | 6 ± 0.00 | ||
| % Change | 0.00 | 0.00 | 0.00 | 0.00 | ||
| SL (cm plant−1) | 0 | 38.66 ± 1.07 | 32.28 ± 1.31 | 23.02 ± 0.48 | 24.47 ± 0.26 | |
| 100 | 32.12 ± 1.76 * | 32.03 ± 1.53 | 20.03 ± 0.69 * | 24.4 ± 0.21 | ||
| % Change | 16.90 | 0.80 | 13.00 | 0.30 | ||
| SDW (g plant−1) | 0 | 0.8 ± 0.04 | 0.62 ± 0.04 | 0.24 ± 0.02 | 0.21 ± 0.00 | |
| 100 | 0.62 ± 0.03 * | 0.67 ± 0.04 | 0.16 ± 0.01 * | 0.214 ± 0.01 | ||
| % Change | 22.39 | −8.36 | 33.05 | −1.90 | ||
| Root | LRN and FRN (plant−1) | 0 | 36.3 ± 2.50 | 34.7 ± 1.20 | 7 ± 0.00 | 7 ± 0.00 |
| 100 | 23.7 ± 3.00 * | 34.7 ± 3.20 | 6 ± 0.00 | 8 ± 0.00 | ||
| % Change | 34.71 | 0.58 | 14.29 | −14.29 | ||
| RL (cm plant−1) | 0 | 21.68 ± 1.81 | 18.15 ± 0.82 | 23.72 ± 0.89 | 23.8 ± 1.73 | |
| 100 | 16.38 ± 0.53 * | 18.2 ± 1.40 | 20.03 ± 0.62 * | 22.85 ± 1.42 | ||
| % Change | 24.45 | −0.28 | 15.60 | 4.00 | ||
| RDW (g plant−1) | 0 | 0.28 ± 0.02 | 0.24 ± 0.05 | 0.025 ± 0.00 | 0.031 ± 0.00 | |
| 100 | 0.19 ± 0.04 * | 0.23 ± 0.04 | 0.018 ± 0.00 | 0.029 ± 0.00 | ||
| % Change | 33.10 | 3.36 | 26.98 | 6.54 | ||
| MC Content (µg kg−1 DW) | Planted Soil | Unplanted Soil (Bulk Soil) | ||||
|---|---|---|---|---|---|---|
| Faba Bean | Common Wheat | |||||
| Sterile Soil | Non-Sterile Soil | Sterile Soil | Non-Sterile Soil | Sterile Soil | Non-Sterile Soil | |
| Shoot | 7.65 ± 0.39 | 6.99 ± 0.02 | 88.12 ± 5.61 | 60 ± 7.73 | - | - |
| BAF (shoot) | 0.08 ± 0.00 | 0.07 ± 0.00 | 0.88 ± 0.06 | 0.6 ± 0.08 | - | - |
| BL | Low | Low | Low | Low | - | - |
| Root | 49.13 ± 3.88 | 20.38 ± 0.88 | 12.77 ± 8.48 | 5.47 ± 0.49 | - | - |
| BAF (root) | 0.49 ± 0.04 | 0.2 ± 0.01 | 0.13 ± 0.08 | 0.05 ± 0.00 | - | - |
| BL | Low | Low | Low | Low | - | - |
| Rhizospheric/bulk soil | 0.187 ± 0.04 | 0.11 ± 0.00 | 0.117 ± 0.07 | 0.08 ± 0.00 | 0.161 ± 0.09 | 0.114 ± 0.02 |
| Health Risk Parameters | Faba Bean | Common Wheat | ||||||
|---|---|---|---|---|---|---|---|---|
| Human | Cattle | Human | Cattle | |||||
| Sterile Soil | Non-Sterile Soil | Sterile Soil | Non-Sterile Soil | Sterile Soil | Non-Sterile Soil | Sterile Soil | Non-Sterile Soil | |
| EDI | 4.3 × 10−3 ± 0.00 | 4 × 10−3 ± 0.00 | 0.31 ± 0.02 | 0.28 ± 0.00 | 0.73 ± 0.05 | 0.5 ± 0.06 | 3.52 ± 0.22 | 2.4 ± 0.31 |
| RQ | 0.11 ± 0.00 | 0.1 ± 0.00 | 0.68 ± 0.03 | 0.62 ± 0.00 | 18.36 ± 1.10 | 12.5 ± 1.60 | 7.83 ± 0.50 | 5.33 ± 0.70 |
| Risk level | Moderate | Moderate | Moderate | Moderate | High | High | High | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redouane, E.M.; Mugani, R.; Lahrouni, M.; Martins, J.C.; Zerrifi, S.E.A.; Oufdou, K.; Campos, A.; Vasconcelos, V.; Oudra, B. Role of Rhizospheric Microbiota as a Bioremediation Tool for the Protection of Soil-Plant Systems from Microcystins Phytotoxicity and Mitigating Toxin-Related Health Risk. Microorganisms 2021, 9, 1747. https://doi.org/10.3390/microorganisms9081747
Redouane EM, Mugani R, Lahrouni M, Martins JC, Zerrifi SEA, Oufdou K, Campos A, Vasconcelos V, Oudra B. Role of Rhizospheric Microbiota as a Bioremediation Tool for the Protection of Soil-Plant Systems from Microcystins Phytotoxicity and Mitigating Toxin-Related Health Risk. Microorganisms. 2021; 9(8):1747. https://doi.org/10.3390/microorganisms9081747
Chicago/Turabian StyleRedouane, El Mahdi, Richard Mugani, Majida Lahrouni, José Carlos Martins, Soukaina El Amrani Zerrifi, Khalid Oufdou, Alexandre Campos, Vitor Vasconcelos, and Brahim Oudra. 2021. "Role of Rhizospheric Microbiota as a Bioremediation Tool for the Protection of Soil-Plant Systems from Microcystins Phytotoxicity and Mitigating Toxin-Related Health Risk" Microorganisms 9, no. 8: 1747. https://doi.org/10.3390/microorganisms9081747
APA StyleRedouane, E. M., Mugani, R., Lahrouni, M., Martins, J. C., Zerrifi, S. E. A., Oufdou, K., Campos, A., Vasconcelos, V., & Oudra, B. (2021). Role of Rhizospheric Microbiota as a Bioremediation Tool for the Protection of Soil-Plant Systems from Microcystins Phytotoxicity and Mitigating Toxin-Related Health Risk. Microorganisms, 9(8), 1747. https://doi.org/10.3390/microorganisms9081747










