Abstract
Plant parasitic nematodes cause severe damage to crops. Endoparasitic nematophagous fungi (ENF) are a type of important biocontrol fungi, which can cause disease or kill nematodes by producing various spores. As a major ENF, Drechmeria coniospora displays certain potential for controlling plant-parasitic nematodes. In this study, the pathogenicity and secondary metabolites of the endoparasitic fungus D. coniospora YMF1.01759 were investigated. The strain D. coniospora YMF1.01759 had high infection efficiency against nematodes. The process of infecting nematodes by the strain was observed under an electron microscope. Here, 13 metabolites including one new compound 4(S)-butoxy-3-(butoxymethyl)-2-hydroxycyclopent-2-en-1-one (2) were isolated and identified from the fermentation products of D. coniospora YMF1.01759 cultured in a SDAY solid medium. Furthermore, a bioassay showed that 5-hydroxymethylfuran-2-carboxylic acid (1) is toxic to the root knot nematode Meloidogyne incognita and affects the hatching of its egg. Thereby, the nematicidal mortality attained 81.50% at 100 μg/mL for 48 h. Furthermore, egg hatching was inhibited at the tested concentrations, compared with water control eggs. This is the first report on the secondary metabolites of the ENF D. coniospora. The results indicated that D. coniospora could infect nematodes by spores and produce active metabolites to kill nematodes. The biological control potential of D. coniospora against nematodes was expounded further.
1. Introduction
Plant parasitic nematodes cause severe damage to crops. Nematode disease is a global phenomenon. The annual economic losses owing to nematode infection of crop production are estimated at $173 billion [1]. These are parasitic to a variety of crops such as cucumber, melon, pepper, tomato and fruit, and infect the entire plant including the roots, stems, leaves, flowers, fruit and seeds [2,3]. This scenario demands further research to identify effective albeit environment-friendly alternatives to replace legislatively withdrawn highly toxic nematicides [4,5,6].
Biological control agents such as nematophagous fungi may be a solution when applied in the context of integrated pest management systems. Endoparasitic fungi are a group of nematophagous fungi that produce a variety of special spores to infect free nematodes. Either the spores are swallowed by nematodes and then infect, or these infect by adhering to nematode epidermis [7,8,9]. Drechmeria coniospora is an obligate parasitic fungus belonging to the family of Clavicipitaceae. It forms spores that adhere to the cuticle of a range of different nematodes and infects a variety of nematode species [7,10,11,12]. The endoparasitic nematode fungus D. coniospora is highly aggressive to nematodes. In greenhouse experiments, it can reduce the number of root-knot nematodes forming galls on tomatoes and alfalfa [9].
In general, endoparasitic nematophagous fungi (ENF) infect their hosts using conidia. However, a lot of metabolites including nematicidal compounds were isolated from ENF. A polyketone (phomalactone) was identified from the endoparasitic Verticillium chlamydosporium and showed prominently nematicidal activity [13]. Several aurovertins were isolated from Pochonia chlamydosporia, and Aurovertins F and D displayed toxicity to the free-living nematode Panagrellus redivivus [14]. A series of indole diterpenoids were obtained from the endophytic fungus Drechmeria sp., and Drechmerins B and I displayed antimicrobial activity [15,16,17]. According to the genomic data, D. coniospora could produce abundant secondary metabolites [11,18]. The species has not been investigated with regard to metabolites. In the present study, the infecting process of D. coniospora YMF1.01759 against nematode was observed using scanning electron microscopy. Moreover, a chemical investigation was performed on the strain D. coniospora YMF1.01759. As a result, 13 metabolites including one new compound were isolated. In addition, a bioassay showed that 5-hydroxymethylfuran-2-carboxylic acid displayed prominent nematicidal activity and could affect the egg hatching of M. incognita. This implies that D. coniospora YMF1.01759 can infect nematodes by producing active metabolites.
2. Materials and Methods
2.1. Materials and Normal Culture
D. coniospora YMF1.01759 was deposited in the culture collection of the Key Laboratory for Conservation and Utilization of Bio-resource, and Key Laboratory for Microbial Resources of the Ministry of Education, Yunnan University.
Meloidogyne incognita: M. incognita was cultured on susceptible tomatoes (Solanum lycopersicum) for 40 days under greenhouse conditions (25 ± 3 °C). Infested tomatoes were uprooted, and roots showing galls and egg masses were washed to remove soil. Egg masses were selected and placed on a plastic plate. Then, adequate sterile water was added to cover the egg mass to permit the eggs to hatch. Finally, the second-stage juveniles were available for hatching at 28 °C for five days [2].
Caenorhabditis elegans: C. elegans were transferred onto a freshly prepared nematode growth medium (NGM) agar plate with Escherichia coli strain OP50 spread over the surface as a food source. C. elegans were obtained by gentle washing from the NGM plate with M9 buffer after culturing at 20 °C for three days.
2.2. General Experimental Instruments
The optical rotations were measured with a Jasco DIP-370 digital polarimeter (JASCO Corporation, Tokyo, Japan). UV spectra were recorded on a Shimadzu UV-2401 PC spectrophotometer (Kyoto, Japan). NMR spectra were recorded on Avance III-600 spectrometers (Bruker Biospin, Rheinstetten, Germany) with tetramethylsilane (TMS) as an internal standard. The ESI-MS and HR-ESI-MS were recorded on a Thermo high resolution Q Exactive focus mass spectrometer (Thermo, Bremen, Germany). Column chromatography was performed on silica gel G (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China) and Sephadex LH-20 (Amersham Pharmacia). Precoated silica gel GF254 plates (Qingdao Marine Chemical Inc., Qingdao, China) were used for thin layer chromatography (TLC). HPLC was performed on an LC3000 (Beijing Chuangxintongheng Science and Technology Co., Ltd., Beijing, China). Abamectin was purchased as a positive control from Solarbio.
2.3. Assaying of Nematicidal Activity
2.3.1. Pathogenicity of D. coniospora YMF1.01759 against C. elegans
D. coniospora YMF1.01759 was cultured on a PDA plate at 25 °C for 14 days to observe the endoparasitic experiment. Subsequently, approximately 150–200 C. elegans were introduced to the plates. The strains and nematodes were co-cultured for seven days, and the plates were observed under a microscope every 12 h.
The endoparasitic phenomenon of the strain on nematodes was also recorded by scanning electron microscopy (SEM). Preparation of sample for SEM: samples of co-incubation for one-seven days were fixated with 4% glutaraldehyde for 30 min; and dehydrated through a graded series of ethanol (30%, 50%, 70%, 80% and 90%; 15 min in each step); and then immersed successively in 100% ethanol two times for 15 min each, in ethanol:isoamyl acetate (1:1, v/v) liquor for 10 min and in 100% isoamyl acetate for 10 min. The samples were then transferred to a critical point dryer using liquefied carbon dioxide as transitional fluid.
2.3.2. Nematicidal Activity of Extracts of D. coniospora YMF1.01759 on Different Media
Five types of solid media [LB (10.0 g tryptone, 5.0 g yeast extract, 10.0 g NaCl, 18.0 g agar, 1 L water), YMG (4.0 g yeast extract, 20.0 g glucose, 18.0 g agar, 1 L water), PDA (200.0 g potato, 20.0 g glucose, 18.0 g agar, 1 L water), SDAY (10.0 g bacterial peptone, 10.0 g yeast extract, 40.0 g glucose, 18.0 g agar, 1 L water) and RICE (rice 40.0 g, 18.0 g agar, 1 L water)] were used to culture D. coniospora YMF1.01759. The culturing was performed at 25 °C for 14 days and was then chopped and extracted with a mixed solvent (EtOAc/MeOH/AcOH, 80:15:5, v/v/v) at room temperature. Those crude extracts were dissolved in methanol for testing the nematicidal activity. The sample (30 μL) was added to a petri dish of diameter 3.5 cm containing 100–150 M. incognita and 970 μL distilled water. The final concentrations of the extracts were 5 mg mL−1. Distilled water containing 3% MeOH was used as a negative control. There were three replicates for each treatment. All the tested plates were incubated at 28 °C. M. incognita juveniles were observed under a light microscope after 24 h, 48 h and 72 h. The nematodes were considered to be dead when these did not move on physically stimulated with a fine needle. The mean percentage mortality was calculated.
2.3.3. Nematicidal Activity of Compounds
The isolated compounds were tested for the nematicidal activity on the second-stage juveniles of M. incognita using MeOH as the solvent. The tested compounds were diluted to different concentrations (400, 200, 100, 50 and 25 μg mL−1) to assay the nematicidal activity. Distilled water containing 3% MeOH was used as a negative control. Abamectin (10 μg mL−1) was served as a positive control. The compound solution and 100–150 nematodes were added to the 96-well plates. The plates were then placed in a 28 °C incubator. Subsequently, the dead nematodes and total nematodes were counted after 24 h, 48 h and 72 h. The mean percentage mortality was calculated. The nematodes were defined as dead when these showed no movement with physical stimulation and adopted a straight shape. The experiment using pure compound was conducted two times with five replicates. The test method is according to the literature [19].
2.3.4. Inhibition of Egg Hatching Activity of M. incognita
The compound was tested for inhibition of the egg hatching activity of M. incognita. Egg masses were obtained from the infested roots and washed with ddH2 O to remove soil. Fresh egg masses were assayed for inhibition of egg hatching activity using 96-well plates. An egg mass was placed in each well, and the compound dissolved in MeOH (the final concentration of MeOH in the test solution was at most 3.0%) was added to the tested wells at 200, 100 and 50 μg mL−1. Distilled water containing 3% MeOH was used as a control. The plates containing the various components were incubated at 28 °C. The number of hatched worms was counted under the microscope after one, two and three days. Each treatment was performed two times with three replicates [20].
2.4. Extraction and Isolation of Metabolites from D. coniospora YMF1.01759
D. coniospora YMF1.01759 was grown on a SDAY solid medium (25 L) at 25 °C for 14 days. The solid fermentation products were cut into small pieces and extracted exhaustively with an EtOAc/MeOH/AcOH mixture solution (80:15:5, v/v/v) five times. The extracts were suspended in water and extracted three times by EtOAc, and n-butanol. The EtOAc extract (25.6 g) and n-butanol extract (80.4 g) were assayed for nematicidal activity against M. incognita. The results showed that the EtOAc extract had a strong nematicidal activity against M. incognita. Therefore, the EtOAc extract was used to study the metabolites further.
The EtOAc extract (25.6 g) was fractionated with a silica gel G (200–300 mesh) column eluted with a petroleum ether–EtOAc (50:1 to 0:100) gradient solvent system followed by EtOAc–MeOH (8:1 to 0:100), to yield 13 fractions (Fr.1–Fr.13). Fr.4 (240 mg) was chromatographed on a silica gel column eluted with a CHCl3–acetone (100:1 to 1:1) gradient solvent system to yield Fr.4.1–Fr.4.2. Fr.4.2 (14 mg) was separated by Sephadex LH-20 (MeOH) to obtain 11 (4.5 mg). Fr.5 (264 mg) was subjected to semipreparative gradient HPLC [detection wavelength of 254 nm and a mobile phase of MeOH–water (the water reduced from 90% to 50%) at a flow rate of 3 mL min−1]. It was then separated by preparational TLC plates (GF254) [the sample (7 mg) was dissolved in MeOH and repeatedly subjected to TLC separating plates, followed by CHCl3–acetone (10:1)] to yield 2 (4.0 mg). Fr.6 (240 mg) was loaded onto Sephadex LH-20 (MeOH) to obtain three fractions (Fr.6.1–Fr.6.3). Fr.6.1 (124 mg) was subjected to a silica gel column eluted with a petroleum ether–acetone (50:0 to 5:1) gradient solvent system and then purified by Sephadex LH-20 (MeOH) to give 4 (3.7 mg). Fr.6.3 (50 mg) was separated on a column of silica gel (200–300 mesh), eluted with petroleum ether–acetone (50:0 to 5:1) and purified by preparational TLC [the sample (6 mg) was dissolved in CHCl3–MeOH (1:1) and repeatedly subjected to TLC separating plates, followed by petroleum ether–EtOAc (4:1)] to yield 7 (4.5 mg). Fr.7 (239 mg) was separated on a Sephadex LH-20 column (MeOH) to give four fractions (Fr.7.1–Fr.7.4). Fr.7.2 (8 mg) was purified by preparational TLC [the sample was dissolved in CHCl3–MeOH (1:1) and repeatedly subjected to TLC separating plates, followed by petroleum ether–acetone (3:1)] to yield 5 (3.2 mg). Fr.7.3 (36 mg) was loaded repeatedly on Sephadex LH-20 (MeOH) to yield 6 (4.4 mg). Fr.9 (552 mg) was purified on Sephadex LH-20 (MeOH) to give four fractions (Fr.9.1–Fr.9.4). Fr.9.2 (111 mg) was subjected to semipreparative gradient HPLC [detection wavelength of 254 nm and a mobile phase of MeOH–water (the water reduced from 80% to 40%) at a flow rate of 3 mL min−1] and purified by Sephadex LH-20 (MeOH) to obtain 3 (6.3 mg) and 8 (5.2 mg). Fr.9.4 (95 mg) was subjected to a silica gel column eluted with a petroleum ether–ethyl acetate (50:1 to 5:1) gradient solvent system. It was then purified by Sephadex LH-20 (MeOH) to obtain 9 (4.3 mg). Fr.10 (1.0 g) was loaded onto a column of Sephadex LH-20 (MeOH) and then separated by semipreparative gradient HPLC [detection wavelength of 254 nm and a mobile phase of MeOH–water (the water reduced from 90% to 50%) at a flow rate of 3 mL min−1]. It was then purified by Sephadex LH-20 (MeOH) to obtain 10 (15.2 mg). Fr.11 (1.748 g) was first chromatographed on a Sephadex LH-20 column (MeOH). It was then subjected to semipreparative gradient HPLC [detection wavelength of 254 nm and a mobile phase of MeOH–water (the water reduced from 95% to 65%) at a flow rate of 3 mL min−1]. Subsequently, it was purified by Sephadex LH-20 (MeOH) to obtain 1 (33.5 mg). Fr.12 (1.784 g) was subjected to Sephadex LH-20 (MeOH) to give four fractions (Fr.12.1–Fr.12.4). Fr.12.3 (816 mg) was separated on a column of silica gel (200–300 mesh) and eluted with petroleum ether–ethyl acetate (from 20:1 to 3:1). It was then purified by preparational TLC [the sample (20 mg) was dissolved in MeOH and repeatedly subjected to TLC separating plates, followed by petroleum ether–acetone (5:1)] to yield 12 (3.6 mg). Fr.13 (1.06 g) was loaded on Sephadex LH-20 (CHCl3–MeOH, 1:1) to give three fractions (Fr.13.1–Fr.13.3). Fr.13.3 (816 mg) was subjected on a silica gel column (200–300 mesh), eluted with CHCl3–MeOH (30:1 to 5:1) and purified by semipreparative gradient HPLC [the mobile phase was MeOH–water (the water reduced from 90% to 50%) at a flow rate of 3 mL min−1] to obtain 13 (40.5 mg).
Physicochemical properties of compounds 1 and 2:
Compound 1: white solid; + 3.9 (c 0.21, MeOH); UV (MeOH) λmax (log ε) 197 (3.09), 233 (2.68), 251 (2.80) nm; 1 H-NMR (CD3 OD, 600 MHz) δ 4.56 (1 H, s, H-6), 6.46 (1 H, d, J = 3.4 Hz, H-5), 7.13 (1 H, d, J = 3.4 Hz, H-4); 13 C-NMR (CD3 OD, 150 MHz) δ 162.0 (s, C-1), 145.9 (s, C-2), 119.8 (d, C-3), 110.3 (d, C-4), 160.0 (s, C-5), 57.5 (t, C-6); Negative ESI-MS m/z 141 [M − H]−; HR-ESI-MS m/z 141.01821 [M − H]− (calcd for C6 H5 O4, 141.01824).
Compound 2: Colorless amorphism; + 6.17 (c 0.09, MeOH); UV (MeOH) λmax (log ε) 195 (3.40), 212 (3.29), 251 (3.67) nm; 1 H-NMR (CD3 OD, 600 MHz) and 13 C-NMR (CD3 OD, 150 MHz) data, see Table 1; positive ESI-MS m/z 279 [M + Na]+; HR-ESI-MS m/z 279.15491 [M + Na]+ (calcd for C14 H24 O4 Na, 279.15668).

Table 1.
The NMR Data of Compound 2 in CD3 OD (600 MHz).
2.5. Statistical Analysis
Nematicidal activity and egg hatching inhibition assay investigations were performed in two independent experiments. Statistics were obtained by comparing the control performance for each concentration and time. The data were subjected to single-factor ANOVA using SPSS Statistics 17 software. a p < 0.001, b p < 0.01 and c p < 0.05 were considered to be statistically significant.
3. Results
3.1. Pathogenicity of D. coniospora YMF1.01759 against Nematodes
In China, the species D. coniospora was isolated from soil and first described by Prof. Zhang in our group [21]. The infection cycle of D. coniospora on nematodes and the formation of adhesive knobs were observed [11,22]. In our experiment, the pathogenicity of D. coniospora YMF1.01759 against the nematodes was first observed under microscope. After C. elegans were added to D. coniospora YMF1.01759 for 12 h, there was no evident difference in the nematode motility (Figure 1A). At 24 h, the nematode’s activity was weakened significantly, and it displayed a distorted S-shape (Figure 1B). Then, the nematode essentially remained motionless (Figure 1C). After approximately 48 h, the nematode was observed to have sprouted mycelium on the body surface (Figure 1D). Finally, the mycelia spread over the body of the nematodes and produced spores to begin a new infection process (Figure 1E,F). The strain can infect C. elegans over various periods.
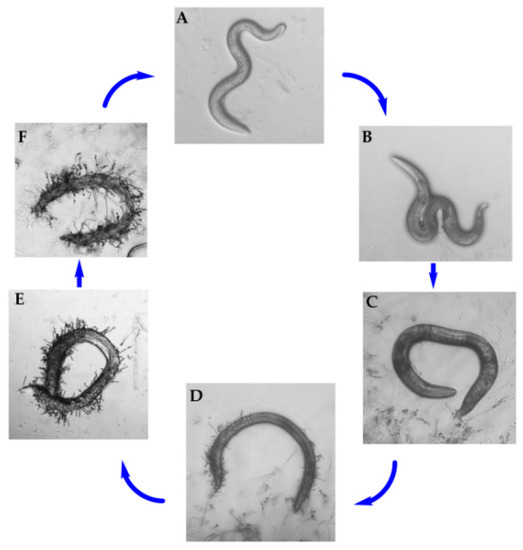
Figure 1.
Pathogenicity process of D. coniospora YMF1.01759 against C. elegans.
SEM showed that the mycelia of D. coniospora YMF1.01759 were septate (Figure 2A, yellow arrow) and that the spores were conical (Figure 2B). Adhesive knobs were produced at the tips of the spores when these matured (Figure 2B, yellow arrow). This mainly facilitates the identification of nematodes and adherence to these. When nematodes are added to plates containing D. coniospora YMF1.01759, spores produced by the stain simply attach to the nematodes’ body when coming in direct contact. The mature spores gradually adhere to the nematode body wall as the nematodes move (Figure 3A,B), which can adhere to various parts of the nematode body. After the adhesion is completed, the sticky spheres form budding tubes to infiltrate the nematode epidermis (Figure 3C). These then gradually infiltrate the nematode interior, and finally break through the nematode body wall to sprout (Figure 3D–F). Finally, the nematodes decompose (Figure 3G), and mycelia from nematodes produce spores to start a new cycle (Figure 3H). The formation of adhesive knobs is a spontaneous process. It plays a major role in the infestation process [22]. According to a report, chymotrypsin-like proteases are also involved in the infestation process [23].
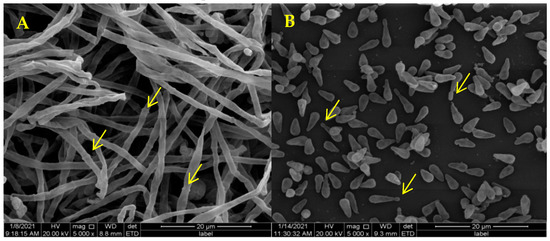
Figure 2.
Mycelia (A)and Spore (B) Morphology of D. coniospora YMF1.01759 using SEM.
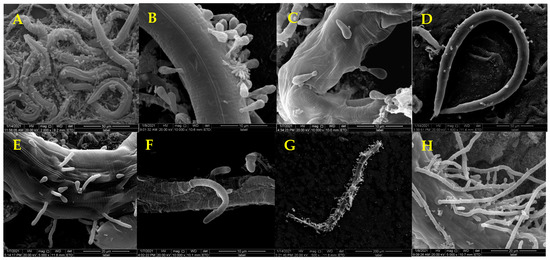
Figure 3.
Infection Process of C. elegans by Spores of D. coniospora YMF1.01759.
3.2. Nematicidal Activity of Medium Screening Extracts
Among the extracts from five media of D. coniospora YMF1.01759, the extract from the SDAY medium exhibited strong nematicidal activity against M. incognita: 99.1% mortality at a concentration of 5 mg mL−1 at 72 h. The other four extracts had no evident activity on M. incognita at 5 mg mL−1. To track active nematicidal compounds, the crude extract of SDAY was sequentially extracted with EtOAc and n-butanol. Two extracts were assayed for nematicidal activity against M. incognita. The results showed that the mortality of the EtOAc extracts attained 98.0% at 1 mg mL−1 at 72 h. However, the corrected mortality of the n-butanol extracts attained 13.3% under identical conditions. Therefore, the EtOAc extract from the SDAY medium was selected to study the active component.
3.3. Structure Identification
Compound 1 was obtained as a white solid. The HR-ESI-MS data revealed a molecular formula of C6 H5 O4 based on the [M − H]− ion signal at m/z 141.01821 [M − H]− (calculated for, 141.01824). 13 C-NMR and DEPT spectra indicated that compound 1 contained furan structure (δc 145.9, 119.8, 110.3 and 160.0), a methylene (δc 57.5) and a carboxyl group (δc 162.0). Based on the above data, the structure of compound 1 was identified as 5-hydroxymethylfuran-2-carboxylic acid (Figure 4) [24].
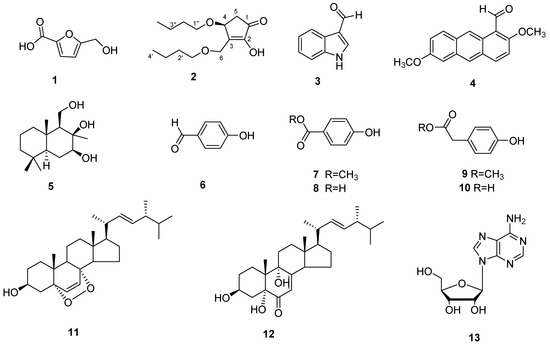
Figure 4.
The Metabolites isolated from D. coniospora YMF1.01759.
Compound 2 was obtained as a colorless amorphism. The HR-ESI-MS data revealed a molecular formula of C14 H24 O4 based on the [M + Na]+ ion signal at m/z 279.15491 (calcd for C14 H24 O4 Na, 279.15668). According to 1 H-, 13 C- and DEPT-NMR (Table 1), the compound contained two methyls, eight methylenes, one methane and one carbonyl. The 1 H−1 H COSY spectrum of compound 2 revealed three fragments (Figure 5) by the evident correlations of H-4/H-5, H-1′/H-2′/H-3′/H-4′ and H-1″/H-2″/H-3″/H-4″, respectively. The HMBC experiment (Figure 5) showed that the methine proton at δH 4.65 (H-4) is correlated with the carbons at δC 55.6 (C-6), 151.2 (C-3) and 202.7 (C-1); the methylene protons at δH 2.24 and 2.70 (H-4) are correlated with the carbons at δC 73.2 (C-4), 151.2 (C-3) and 202.7 (C-1); and the protons at δH 4.28 and 4.55 (H-6) are correlated with the carbons at δC 73.2 (C-4), 151.2 (C-3) and 153.6 (C-2). Those correlations established 2,4-dihydroxy-3-hydroxymethyl-cyclopent-2-en-1-one unit. Two straight-chain butyls are attached to 4-OH and 6-OH on the basis of the NOEs between H-6 to H-1″, and H-4 to H-1′ (Figure 5). The stereochemistry of 4-OH was determined by comparing the optical rotation values with (±)-4-hydroxy-3-methylcyclopent-2-enone (4 S-hydroxy-3-methyl-cyclopent-2-enone, , +3.36, c 0.375, CH2 Cl2; 4 R-hydroxy-3-methyl-cyclopent-2-enone, , − 4.7, c 0.35, CH2 Cl2) [25]. Therefore, compound 2 was determined as 4(S)-butoxy-3-(butoxymethyl)-2-hydroxycyclopent-2-en-1-one (Figure 4). This is a new compound.
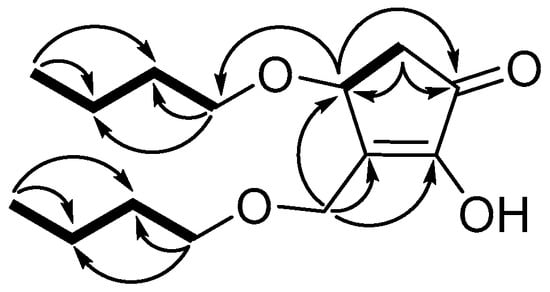
Figure 5.
Selected HMBC (arrows) and 1 H-1 H COSY (bold bond) Correlations of 2.
The other compounds were identified as indole-3-carboxaldehyde (3) [26], 2,6-dimethoxy-1-anthracenecarboxaldehyde (4) [27], 7β,8β,11-triol-drimane (5) [28], 4-hydroxy-benzaldehyde (6), 4-hydroxybenzoic acid methyl ester (7), 4-hydroxy-benzoic acid (8), 4-hydroxy-benzeneacetic acid methyl ester (9), 4-hydroxy-benzeneacetic acid (10), ergosterol peroxide (11) [29], 3β,5α,9α-trihydroxy-ergosta-7,22-dien-6-one (12) [30] and adenosine (13) through a comparison of their experimental and reported spectroscopic data.
3.4. Nematicidal Activity of Compounds and Their Inhibition of Egg Hatching
The nematicidal activity of the isolated compounds against M. incognita up to a concentration of 400 μg mL−1 was determined. With the exception of 5-hydroxymethylfuran-2-carboxylic acid (1), none of the compounds displayed toxic effect on M. incognita at 400 μg mL−1. After being exposed to compound 1 for 24 h, the nematodes began to die and their body shape straightened. It caused significant mortality of M. incognita at 400, 200 and 100 μg mL−1 (Table 2). The nematicidal mortality attained 81.50% at 100 μg mL−1 for 48 h. The nematicidal effects varied with the concentration and exposure time, and the nematicidal activity differed significantly among the exposure times of 24 h, 48 h and 72 h at an identical concentration (Table 3). The mortality of M. incognita by abamectin attained 100% at 10 μg mL−1 for 24 h.

Table 2.
Effect of Compound 1 on the Mortality (%) of M. incognita [mortality ± SD].

Table 3.
Variation in Percentage Mortality of M. incognita at each Concentration.
The inhibition of egg hatching by 5-hydroxymethylfuran-2-carboxylic acid (1) was also assayed in our experiment. The other compounds were not tested further because only compound 1 showed nematicidal activity against M. incognita. The inhibition activity was tested in 96-well plates. The egg hatching of M. incognita was inhibited remarkably by compound 1 at 200 μg mL−1. The average number of hatched juveniles from one egg mass after three days of treatment with 1 was 11.17 at 200 µg mL−1, whereas the average number of hatched juveniles from one egg mass of the control treatment was 63.33 (Table 4). Hence, compound 1 can inhibit egg hatching of M. incognita.

Table 4.
Inhibition of Egg hatching of M. incognita by compound 1.
4. Discussion
In the study, we isolated 13 metabolites from the EtOAc extract of D. coniospora YMF1.01759. Their structures displayed various compounds including polyketide, sesquiterpenoid, terpenes, nucleoside alkaloid and aromatic metabolites. Among these, 4(S)-butoxy-3-(butoxymethyl)-2-hydroxycyclopent-2-en-1-one (2) is a new natural product. The result shows that the strain can produce abundant metabolites.
An active assay demonstrated that 5-hydroxymethylfuran-2-carboxylic acid (1) had significant nematicidal activity and could affect the egg hatching of M. incognita. Compound 1 had been obtained from Aspergillus sp. earlier and showed effective nematicidal activities against the pine wood nematode Bursaphelenchus xylophilus and the free-living nematode C. elegans [31]. In our experiment, it showed activity against plant root-knot M. incognita. This indicated that the compound can kill different types of nematodes. Moreover, we observed it to display egg hatching inhibition activity, which had not been reported earlier. The compound 5-hydroxymethylfuran-2-carboxylic acid (1) was a furan, which belongs to polyketide. In previous studies, six furans were obtained from the fungus Coprinus comatus, which displayed nematicidal activity toward Meloidogyne arenaria and P. redivivus [32]. A nematicidal furan 5-(4-pentenyl)-2-furaldehyde was isolated from Irpex lacteus. It showed evident activity against Aphelenchoides besseyi [33]. This indicates that furans represent an important source for new natural nematicidal metabolites that may be developed as nematicidal agents.
As a typical endoparasitic fungus of nematodes, D. coniospora displayed strong pathogenic effect on nematodes by producing adhesive spores that attach to and penetrate the cuticle of nematodes. It exhibits certain potential for controlling plant-parasitic nematodes. This is the first report on the purification and identification of the main constituents of the species D. coniospora. The results indicate that metabolites have a synergistic effect with the pathogenic process of the ENF D. coniospora against nematodes. We demonstrated that D. coniospora YMF1.01759 could kill nematodes and inhibit egg hatching of M. incognita by producing spores or active metabolites. The biological control potential of the species D. coniospora against nematodes was expounded further.
Author Contributions
Conceptualization, J.W. and P.Z.; methodology, J.W. and G.L.; software, Z.D.; validation, G.L. and P.Z.; formal analysis, J.W.; investigation, G.L.; resources, K.Z.; data curation, Z.D.; writing—original draft preparation, J.W. and G.L.; writing—review and editing, G.L. and P.Z.; supervision, P.Z.; project administration, G.L. and P.Z.; funding acquisition, G.L. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31860015 and 31970060) and projects from the Department of Science and Technology of Yunnan Province (202001 BB050061 and 2018 FA006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eloh, K.; Demurtas, M.; Mura, M.G.; Deplano, A.; Onnis, V.; Sasanelli, N.; Maxia, A.; Caboni, P. Potent nematicidal activity of maleimide derivatives on Meloidogyne incognita. J. Agric. Food Chem. 2016, 64, 4876–4881. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.P.; Zhang, P.; Ruan, W.B.; Zhu, X. Nematicidal activity of chaetoglobosin A poduced by Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 41–46. [Google Scholar] [CrossRef]
- Waldo, B.; Soto-Adames, F.; Crow, W. Nematicide effects on arthropods in bermudagrass. Fla. Entomol. 2020, 103, 458–464. [Google Scholar]
- Pino-Otín, M.R.; Val, J.; Ballestero, D.; Navarro, E.; Sánchez, E.; Mainar, A.M. Impact of Artemisia absinthium hydrolate extracts with nematicidal activity on non-target soil organisms of different trophic levels. Ecotoxicol. Environ. Saf. 2019, 180, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Harder, W.; Wyss, U.; Veenhuis, M. Colonization and digestion of nematodes by the endoparasitic nematophagous fungus Drechmeria coniospora. Mycol. Res. 1991, 95, 873–878. [Google Scholar] [CrossRef]
- Tunlid, A.; Jansson, H.B.; Nordbringhertz-Hertz, B. Fungal attachment to nematodes. Mycol. Res. 1992, 96, 401–412. [Google Scholar] [CrossRef]
- Liu, X.Z.; Xiang, M.C.; Che, Y.S. The living strategy of nematophagous fungi. Mycoscience 2009, 50, 20–25. [Google Scholar] [CrossRef]
- Jansson, H.B.; Jeyaprakash, A.; Zuckerman, B.M. Differential adhesion and infection of nematodes by the Endoparasitic fungus Meria coniospora (Deuteromycetes). Appl. Environ. Microb. 1985, 49, 552–555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhou, Z.; Guo, Q.; Fokkens, L.; Miskei, M.; Pócsi, I.; Zhang, W.; Chen, M.; Wang, L.; Sun, Y.; et al. Insights into adaptations to a near-obligate nematode endoparasitic lifestyle from the finished genome of Drechmeria coniospora. Sci. Rep. 2016, 6, 23122. [Google Scholar] [CrossRef] [Green Version]
- Jansson, H.B. Adhesion of conidia of Drechmeria coniospora to Caenorhabditis elegans Wild type and mutants. J. Nematol. 1994, 26, 430–435. [Google Scholar]
- Khambay, B.P.S.; Bourne, J.M.; Cameron, S.; Kerry, B.R.; Zaki, M.J. Communication to the Editor—A nematicidal metabolite from Verticillium chlamydosporium. Pest Manag. Sci. 2000, 56, 1098–1099. [Google Scholar] [CrossRef]
- Niu, X.M.; Wang, Y.L.; Chu, Y.S.; Xue, H.X.; Li, N.; Wei, L.X.; Mo, M.H.; Zhang, K.Q. Nematodetoxic aurovertin-type metabolites from a root-knot nematode parasitic fungus Pochonia chlamydosporia. J. Agric. Food Chem. 2010, 58, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.C.; Wang, Y.L.; Zhang, T.Y.; Chen, Z.J.; Yang, T.M.; Wu, Y.Y.; Sun, C.P.; Ma, X.C.; Zhang, Y.X. Indole diterpenoids from the endophytic fungus Drechmeria sp. as natural antimicrobial agents. Phytochemistry 2018, 148, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.C.; Luan, Z.L.; Liang, J.H.; Cheng, Z.B.; Sun, C.P.; Wang, Y.L.; Zhang, M.Y.; Zhang, T.Y.; Wang, Y.; Yang, T.M.; et al. Drechmerin H, a novel 1(2), 2(18)-diseco indole diterpenoid from the fungus Drechmeria sp. as a natural agonist of human pregnane X receptor. Bioorg. Chem. 2018, 79, 250–256. [Google Scholar] [CrossRef]
- Liang, J.H.; Huo, X.K.; Cheng, Z.B.; Sun, C.P.; Zhao, J.C.; Kang, X.H.; Zhang, T.Y.; Chen, Z.J.; Yang, T.M.; Wu, Y.Y.; et al. An indole diterpenoid isolated from the fungus Drechmeria sp. and its antimicrobial activity. Nat. Prod. Res. 2019, 33, 2770–2776. [Google Scholar] [CrossRef]
- Lebrigand, K.; He, L.D.; Thakur, N.; Arguel, M.J.; Polanowska, J.; Henrissat, B.; Record, E.; Magdelenat, G.; Barbe, V.; Raffaele, S.; et al. Comparative genomic analysis of Drechmeria coniospora reveals core and specific genetic requirements for fungal endoparasitism of nematodes. PLoS Genet. 2016, 12, e1006017. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Le, D.Q.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Cha, B.; Luu, N.H.; Kim, J.C. Nematicidal activities of 4-quinolone alkaloids isolated from the aerial part of Triumfetta grandidens against Meloidogyne incognita. J. Agric. Food Chem. 2014, 63, 68–74. [Google Scholar] [CrossRef]
- Huang, D.; Yu, C.; Shao, Z.; Cai, M.; Li, G.; Zheng, L.; Yu, Z.; Zhang, J. Identification and characterization of nematicidal volatile organic compounds from deep sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 2020, 25, 744. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.Q. Genus and species of endoparasitic fungi of Meloidogyne incognita newly recorded in China. Acta Mycol. Sin. 1994, 13, 75–76. [Google Scholar]
- Boogert, P.; Dijksterhuis, J.; Velvis, H.; Veenhuis, M. Adhesive knob formation by conidia of the nematophagous fungus Drechmeria coniospora. Antonie Leeuwenhoek 1992, 61, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jansson, H.B.; Friman, E. Infection-related surface proteins on conidia of the nematophagous fungus Drechmeria coniospora. Mycol. Res. 1999, 103, 249–256. [Google Scholar] [CrossRef]
- Mitsukura, K.; Sato, Y.; Yoshida, T.; Nagasawa, T. Oxidation of heterocyclic and aromatic aldehydes to the corresponding carboxylic acids by Acetobacter and Serratia strains. Biotechnol. Lett. 2004, 26, 1643–1648. [Google Scholar] [CrossRef]
- Soorukram, D.; Knochel, P. Formal enantioselective synthesis of (+)-estrone. Org. Lett. 2007, 9, 1021–1023. [Google Scholar] [CrossRef]
- Nakajima, E.; Nakano, H.; Yamada, K.; Shigemori, H.; Hasegawa, K. Isolation and identification of lateral bud growth inhibitor, indole-3-aldehyde, involved in apical dominance of pea seedlings. Phytochemistry 2002, 61, 863–865. [Google Scholar] [CrossRef]
- Bilger, C.; Demerseman, P.; Royer, R. Synthesis of new mutagenic compounds, nitro derivatives of anthrafurans. J. Heterocycl. Chem. 1985, 22, 735–739. [Google Scholar] [CrossRef]
- Panasenko, A.A.; Gorincioi, E.C.; Aricu, A.N.; Barcari, E.A.; Deleanu, K.; Vlad, P.F. H−1 and C−13 NMR spectra of some drimanic sesquiterpenoids. Russ. Chem. Bull. 2004, 53, 2700–2705. [Google Scholar] [CrossRef]
- Yue, J.M.; Chen, S.N.; Lin, Z.W.; Sun, H.D. Sterols from the fungus Lactarium volemus. Phytochemistry 2001, 56, 801–806. [Google Scholar] [CrossRef]
- Cai, H.H.; Liu, X.M.; Chen, Z.Y.; Liao, S.T.; Zou, Y.X. Isolation, purification and identification of nine chemical compounds from Flammulina velutipes fruiting bodies. Food Chem. 2013, 141, 2873–2879. [Google Scholar] [CrossRef]
- Kimura, Y.; Tania, S.; Hayashia, A.; Ohtania, K.; Fujiokab, S.; Kawanoa, T.; Shimada, A. Nematicidal activity of 5-hydroxymethyl-2-furoic acid against plant-parasitic nematodes. Z. Nat. C 2007, 62, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, Y.J.; Fang, L.; Li, X.; Tan, N.H.; Zhang, K.Q. Coprinus comatus damages nematode cuticles mechanically with spiny balls and produces potent toxins to immobilize nematodes. Appl. Environ. Microb. 2007, 73, 3916–3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, M.; Wada, K.; Munakata, K. New nematicidal metabolites from a fungus, Irpex lacteus. Agric. Biol. Chem. 1981, 45, 1527–1529. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).