Tick-Borne Pathogens and Diseases in Greece
Abstract
1. Introduction
2. Prevalence of Pathogen-Transmitting Tick Species in Greece
3. Tick-Transmitted Viruses and Diseases, and Associated Risks
3.1. Tick-Borne Encephalitis Virus
3.1.1. TBE Virus and Its Reservoirs
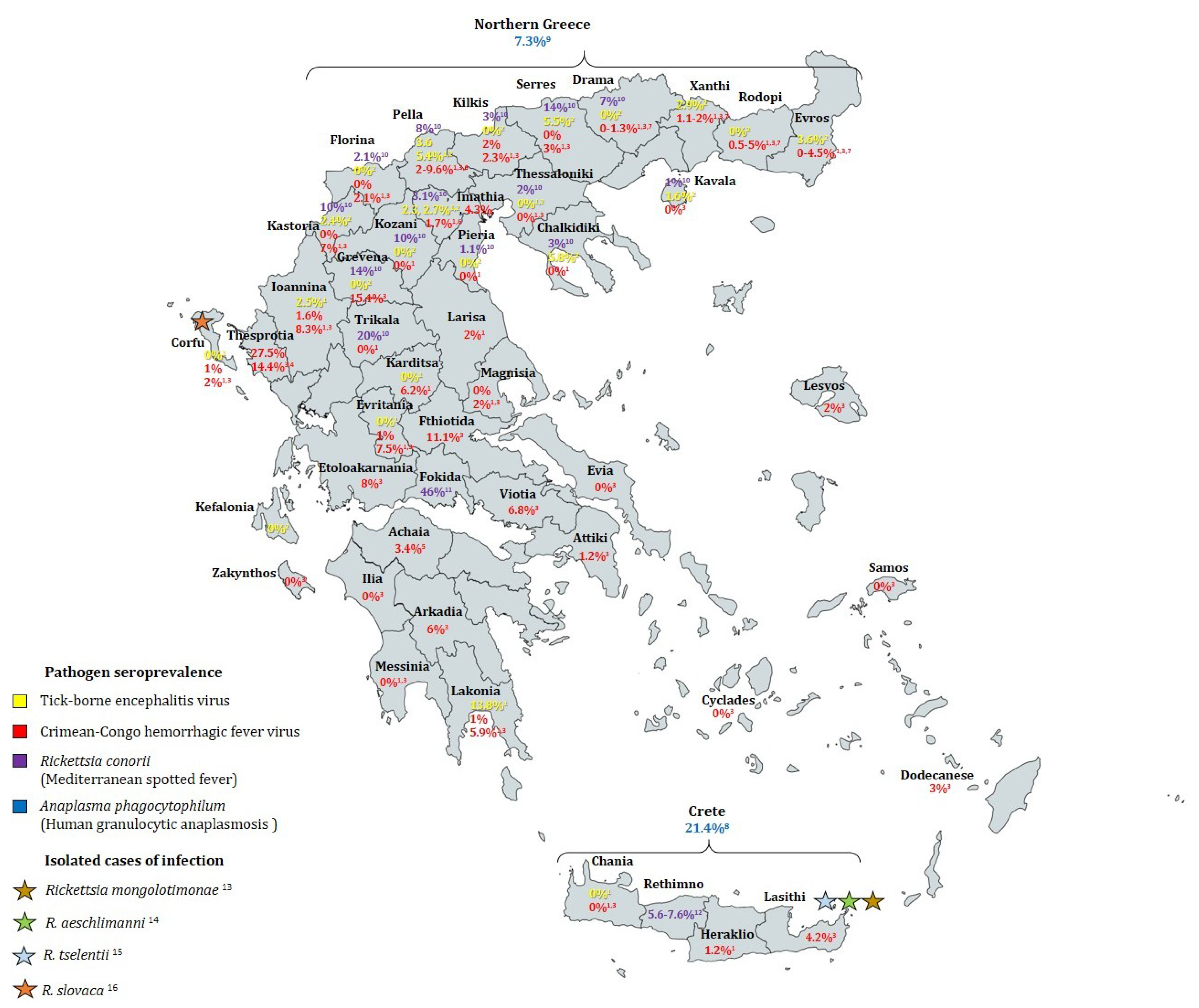
3.1.2. Prevalence of TBE Virus in Humans
3.2. Crimean-Congo Hemorrhagic Fever Virus
3.2.1. The CCHFV and the Greek Nucleoprotein AP 92
3.2.2. CCHFV Tick Vectors and Seroprevalence in Animals
3.2.3. Seroprevalence of CCHFV in Humans
3.3. Tick-Borne Phleboviruses and Diseases
Tick-Borne Phlebovirus Prevalence in Ticks
4. Tick-Transmitted Bacteria and Diseases, and Associated Tick Species
4.1. Spotted Fever Group Rickettsioses
4.1.1. Tick Vectors for SFGR
4.1.2. The Rickettsia Infectious Disease: Mediterranean Spotted Fever
4.1.3. The Rickettsia Infectious Disease: Tick-Borne Lymphadenopathy
4.1.4. Lymphangitis-Associated Rickettsiosis and Other SFG Rickettsia Infections in Humans
4.2. Anaplasma and Human Granulocytic Anaplasmosis
4.3. Borrelia and Associated Diseases
4.3.1. Lyme Disease (Lyme Borreliosis)
4.3.2. Tick-Borne Relapsing Fever Borrelia and the Diseases
4.3.3. Another Borrelia Clade: Borrelia Turcica
5. Tick-Transmitted Protozoa
5.1. Piroplasms and the Diseases Babesiosis and Theileriosis
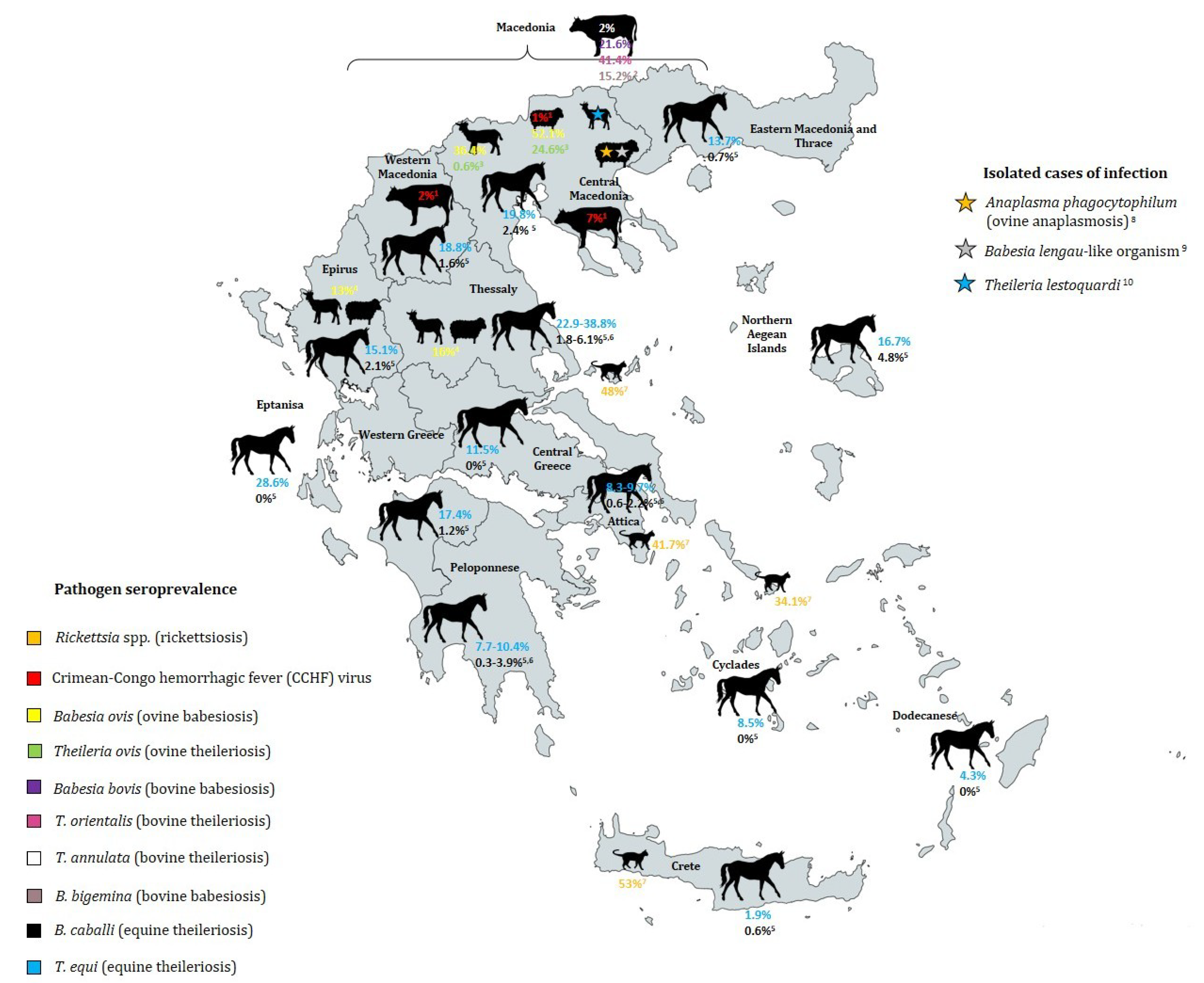
5.1.1. Prevalence of Piroplasms in Cattle
5.1.2. Prevalence of Piroplasms in Sheep and Goats
5.1.3. Equine Piroplasmosis-Associated Species and Their Prevalence
5.2. Tick-Borne Pathogens in Dogs and Cats
5.2.1. Tick-Borne Pathogens in Dogs
5.2.2. Tick-Borne Pathogens in Cats
5.3. Tick-Borne Pathogens in Wild Birds
6. Discussion
6.1. Perspectives in the Context of Social and Political Changes and Current Situation in Greece
6.2. Conclusions
Funding
Conflicts of Interest
References
- Heyman, P.; Cochez, C.; Hofhuis, A.; Van Der Giessen, J.; Sprong, H.; Porter, S.R.; Losson, B.; Saegerman, C.; Donoso-Mantke, O.; Niedrig, M.; et al. A clear and present danger: Tick-borne diseases in Europe. Expert Rev. Anti-Infect. Ther. 2010, 8, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G. The Hard Ticks of the World; Springer: Dordrecht, The Netherlands, 2014; Volume 10, pp. 978–994. [Google Scholar]
- Apanaskevich, D.A.; Oliver, J.H. Life cycles and natural history of ticks. Biol. Ticks 2013, 1, 59–73. [Google Scholar]
- Sonenshine, D.E.; Roe, R.M. (Eds.) Biology of Ticks Volume 2; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Süss, J.; Klaus, C.; Gerstengarbe, F.W.; Werner, P.C. What makes ticks tick? Climate change, ticks, and tick-borne diseases. J. Travel Med. 2008, 15, 39–45. [Google Scholar] [CrossRef]
- Lindgren, E.; Andersson, Y.; Suk, J.E.; Sudre, B.; Semenza, J.C. Monitoring EU emerging infectious disease risk due to climate change. Science 2012, 336, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. Evidence that climate change has caused ‘emergence’of tick-borne diseases in Europe? Int. J. Med. Microbiol. Suppl. 2004, 293, 5–15. [Google Scholar]
- Kioutsioukis, I.; Melas, D.; Zerefos, C. Statistical assessment of changes in climate extremes over Greece (1955–2002). Int. J. Climatol. 2010, 30, 1723–1737. [Google Scholar] [CrossRef]
- Lelieveld, J.; Hadjinicolaou, P.; Kostopoulou, E.; Chenoweth, J.; El Maayar, M.; Giannakopoulos, C.; Hannides, C.; Lange, M.A.; Tanarhte, M.; Tyrlis, E.; et al. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Change 2012, 114, 667–687. [Google Scholar] [CrossRef]
- Hellenic Republic, Ministry of Environment and Energy. 7th National Communication and 3rd Biennial Report under the United Nations Framework Convention on Climate Change; Hellenic Republic, Ministry of Environment and Energy: Athens, Greece, 2018. [Google Scholar]
- World Health Organization. A Global Brief on Vector-Borne Diseases. 2014. Available online: http://apps.who.int/iris/bitstream/10665/111008/1/WHO_DCO_WHD_2014.1_eng.pdf (accessed on 2 July 2019).
- Negev, M.; Paz, S.; Clermont, A.; Pri-Or, N.G.; Shalom, U.; Yeger, T.; Green, M.S. Impacts of climate change on vector borne diseases in the Mediterranean Basin—implications for preparedness and adaptation policy. Int. J. Environ. Res. Public Health 2015, 12, 6745–6770. [Google Scholar] [CrossRef]
- Campbell-Lendrum, D.; Manga, L.; Bagayoko, M.; Sommerfeld, J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20130552. [Google Scholar] [CrossRef]
- Papadopoulos, B.; Morel, P.C.; Aeschlimann, A. Ticks of domestic animals in the Macedonia region of Greece. Vet. Parasitol. 1996, 63, 25–40. [Google Scholar] [CrossRef]
- Papazahariadou, M.; Papadopoulos, E.; Himonas, C. Seasonal activity of ixodid ticks on goats in northern Greece. Vet. Rec. 1995, 136, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Ragiadakou, D.; Kouris, G.; Papadopoulos, B.; Chaniotis, B.; Tselentis, Y. Ticks, Tick-Borne Rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann. N. Y. Acad. Sci. 2006, 1078, 389–399. [Google Scholar] [CrossRef]
- Pavlidou, V.; Gerou, S.; Kahrimanidou, M.; Papa, A. Ticks infesting domestic animals in northern Greece. Exp. Appl. Acarol. 2008, 45, 195–198. [Google Scholar] [CrossRef]
- Chaligiannis, I.; Papa, A.; Sotiraki, S. Ticks feeding on ruminants and humans in Greece. Parasit. Vect. 2014, 7, O1. [Google Scholar] [CrossRef] [PubMed]
- Chaligiannis, I.; Musella, V.; Rinaldi, L.; Cringoli, G.; de la Fuente, J.; Papa, A.; Sotiraki, S. Species diversity and spatial distribution of ixodid ticks on small ruminants in Greece. Parasitol. Res. 2016, 115, 4673–4680. [Google Scholar] [CrossRef]
- Papa, A.; Tsioka, K.; Kontana, A.; Papadopoulos, C.; Giadinis, N. Bacterial pathogens and endosymbionts in ticks. Ticks Tick Borne Dis. 2017, 8, 31–35. [Google Scholar] [CrossRef]
- de Mera, I.G.F.; Chaligiannis, I.; Hernández-Jarguín, A.; Villar, M.; Mateos-Hernández, L.; Papa, A.; Sotiraki, S.; Ruiz-Fons, F.; Cabezas-Cruz, A.; Gortázar, C.; et al. Combination of RT-PCR and proteomics for the identification of Crimean-Congo haemorrhagic fever virus in ticks. Heliyon 2017, 3, e00353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaligiannis, Ι.; de Mera, I.G.F.; Papa, A.; Sotiraki, S.; de la Fuente, J. Molecular identification of tick-borne pathogens in ticks collected from dogs and small ruminants from Greece. Exp. Appl. Acarol. 2018, 74, 443–453. [Google Scholar] [CrossRef]
- Latrofa, M.S.; Angelou, A.; Giannelli, A.; Annoscia, G.; Ravagnan, S.; Dantas-Torres, F.; Capelli, G.; Halos, L.; Beugnet, F.; Papadopoulos, E.; et al. Ticks and associated pathogens in dogs from Greece. Parasit. Vect. 2017, 10, 301. [Google Scholar] [CrossRef]
- Papa, A.; Kontana, A.; Tsioka, K.; Saratsis, A.; Sotiraki, S. Novel phlebovirus detected in Haemaphysalis parva ticks in a Greek island. Ticks Tick Borne Dis. 2017, 8, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Germanakis, A.; Gikas, A.; Scoulica, E.; Tselentis, Y. First isolation and genotypic identification of Rickettsia conorii Malish 7 from a patient in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 297–298. [Google Scholar] [CrossRef]
- Diakou, A.; Norte, A.C.; de Carvalho, I.L.; Núncio, S.; Nováková, M.; Kautman, M.; Alivizatos, H.; Kazantzidis, S.; Sychra, O.; Literák, I. Ticks and tick-borne pathogens in wild birds in Greece. Parasitol. Res. 2016, 115, 2011–2016. [Google Scholar] [CrossRef]
- Hepner, S.; Fingerle, V.; Heylen, D.; Marosevic, D.; Ghaffari, K.; Okeyo, M.; Sing, A.; Margos, G. First investigations on serum resistance and sensitivity of Borrelia turcica. Ticks Tick Borne Dis. 2019, 10, 1157–1161. [Google Scholar] [CrossRef]
- Hepner, S.; Fingerle, V.; Duscher, G.G.; Felsberger, G.; Marosevic, D.; Rollins, R.E.; Okeyo, M.; Sing, A.; Margos, G. Population structure of Borrelia turcica fom Greece and Turkey. Inf. Gen. Evol. 2020, 77, 104050. [Google Scholar] [CrossRef]
- Antoniadis, A. Investigation of Crimean-Congo Hemorrhagic Fever and Hemorrhagic Fever with Renal Syndrome in Greece; Aristotle University of Thessaloniki (Greece) School of Medicine: Thessaloniki, Greece, 1988. [Google Scholar]
- Psaroulaki, A.; Germanakis, A.; Gikas, A.; Scoulica, E.; Tselentis, Y. Simultaneous detection of “Rickettsia mongolotimonae” in a patient and in a tick in Greece. J. Clin. Microbiol. 2005, 43, 3558–3559. [Google Scholar] [CrossRef][Green Version]
- Germanakis, A.; Chochlakis, D.; Angelakis, E.; Tselentis, Y.; Psaroulaki, A. Skin lesions and inoculation eschars at the tick bite site in spotted fever group rickettsioses: Experience from a patient series in eastern crete, Greece. Dermatology 2014, 228, 332–337. [Google Scholar] [CrossRef]
- Papa, A.; Chaligiannis, I.; Kontana, N.; Sourba, T.; Tsioka, K.; Tsatsaris, A.; Sotiraki, S. A novel AP92-like Crimean-Congo haemorrhagic fever virus strain, Greece. Ticks Tick Borne Dis. 2014, 5, 590–593. [Google Scholar] [CrossRef]
- Haralabidis, S. Veterinary Parasitology; University Studio Press: Thessaloniki, Greece, 2001; pp. 172–188. (In Greek) [Google Scholar]
- Papa, A.; Pavlidou, V.; Antoniadis, A. Greek goat encephalitis virus strain isolated from Ixodes ricinus, Greece. Emerg. Infect. Dis. 2008, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Chaligiannis, I.; Xanthopoulou, K.; Papaioakim, M.; Papanastasiou, S.; Sotiraki, S. Ticks parasitizing humans in Greece. Vector Borne Zoonotic Dis. 2011, 11, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Nyström, F.; Olsen, B.; Salaneck, E.; Nilsson, K. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasit. Vect. 2014, 7, 1–20. [Google Scholar] [CrossRef]
- Papa, A.; Xanthopoulou, K.; Kotriotsiou, T.; Papaioakim, M.; Sotiraki, S.; Chaligiannis, I.; Maltezos, E. Rickettsia species in human-parasitizing ticks in Greece. Trans. R Soc. Trop. Med. Hyg. 2016, 110, 299–304. [Google Scholar] [CrossRef]
- Kachrimanidou, M.; Papa, A.; Chochlakis, D.; Pavlidou, V.; Psaroulaki, A. Molecular evidence for Anaplasma phagocytophilum in Ixodes ricinus ticks from Greece. Vector Borne Zoonotic Dis. 2011, 11, 1391–1393. [Google Scholar] [CrossRef]
- Kachrimanidou, M.; Souliou, E.; Pavlidou, V.; Antoniadis, A.; Papa, A. First detection of Rickettsia slovaca in Greece. Exp. Appl. Acarol. 2010, 50, 93–96. [Google Scholar] [CrossRef]
- Lefkaditis, M.A.; Sossidou, A.V.; Panorias, A.H.; Koukeri, S.E.; Paştiu, A.I.; Athanasiou, L.V. Urban stray cats infested by ectoparasites with zoonotic potential in Greece. Parasitol. Res. 2015, 114, 3931–3934. [Google Scholar] [CrossRef]
- Papa, A.; Kontana, A.; Tsioka, K.; Chaligiannis, I.; Sotiraki, S. Molecular detection of Crimean-Congo haemorrhagic fever virus in ticks, Greece, 2012–2014. Parasitol. Res. 2017, 116, 3057–3063. [Google Scholar] [CrossRef]
- Papazahariadou, M.; Saridomichelakis, M.; Koutinas, A.; Papadopoulos, E.; Leontides, L. Tick infestation of dogs in Thessaloniki, northern Greece. Med. Vet. Entomol. 2003, 17, 110–113. [Google Scholar] [CrossRef]
- Psaroulaki, A.; Spyridaki, I.; Ioannidis, A.; Babalis, T.; Gikas, A.; Tselentis, Y. First isolation and identification of Rickettsia conorii from ticks collected in the region of Fokida in Central Greece. J. Clin. Microbiol. 2003, 41, 3317–3319. [Google Scholar] [CrossRef]
- Pavlidou, V.; Gerou, S.; Diza, E.; Antoniadis, A.; Papa, A. Genetic study of the distribution of Greek goat encephalitis virus in Greece. Vector-Borne Zoonotic Dis. 2008, 8, 351–354. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; Chaligiannis, Ι.; Cabezas-Cruz, A.; Papa, A.; Sotiraki, S.; de la Fuente, J.; de Mera, I.G.F. Molecular identification of spotted fever group Rickettsia in ticks collected from dogs and small ruminants in Greece. Exp. Appl. Acarol. 2019, 78, 421–430. [Google Scholar] [CrossRef]
- Antoniadis, A.; Alexiou-Daniel, S.; Malissiovas, N.; Doutsos, J.; Polyzoni, T.; LeDue, J.W.; Peters, C.J.; Saviolakis, G. Seroepidemiological survey for antibodies to arboviruses in Greece. In Haemorrhagic Fever with Renal Syndrome, Tick-and Mosquito-Borne Viruses; Springer: Vienna, Austria, 1990; pp. 277–285. [Google Scholar]
- Pavlidou, V.; Geroy, S.; Diza, E.; Antoniadis, A.; Papa, A. Epidemiological study of tick-borne encephalitis virus in northern Greece. Vector Borne Zoonotic Dis. 2007, 7, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, O.; Paschaleri-Papadopoulou, E.; Deligaris, N.; Doukas, G. Isolation of tick-borne encephalitis virus from a flock of goats with abortions and fatal disease (preliminary report). Vet. News Greece 1971, 3, 112–114. [Google Scholar]
- Koptopoulos, G.; Papadopoulos, O. A Serological Survey for Antibodies to the Arboviruses of Tick-Borne Encephalitis and West Nile. Ph.D. Thesis, University of Thessaloniki, Thessaloniki, Greece, 1978. [Google Scholar]
- Maltezou, H.C.; Papa, A.; Tsiodras, S.; Dalla, V.; Maltezos, E.; Antoniadis, A. Crimean-Congo haemorrhagic fever in Greece: A public health perspective. Int. J. Infect. Dis. 2009, 13, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Sidira, P.; Maltezou, H.C.; Haidich, A.B.; Papa, A. Seroepidemiological study of Crimean-Congo haemorrhagic fever in Greece, 2009–2010. Clin. Microbiol. Infect. 2012, 18, E16–E19. [Google Scholar] [CrossRef]
- Papa, A.; Sidira, P.; Kallia, S.; Ntouska, M.; Zotos, N.; Doumbali, E.; Maltezou, H.C.; Demiris, N.; Tsatsaris, A. Factors associated with IgG positivity to Crimean-Congo haemorrhagic fever virus in the area with the highest seroprevalence in Greece. Ticks Tick Borne Dis. 2013, 4, 417–420. [Google Scholar] [CrossRef]
- Papa, A.; Tzala, E.; Maltezou, H.C. Crimean-Congo haemorrhagic fever virus, northeastern Greece. Emerg. Infect. Dis. 2011, 17, 141. [Google Scholar] [CrossRef]
- Sargianou, M.; Panos, G.; Tsatsaris, A.; Gogos, C.; Papa, A. Crimean-Congo haemorrhagic fever: Seroprevalence and risk factors among humans in Achaia, western Greece. Int. J. Infect. Dis. 2013, 17, e1160–e1165. [Google Scholar] [CrossRef]
- Papadopoulos, O.; Koptopoulos, G. Crimean-Congo haemorrhagic Fever (CCHF) in Greece: Isolation of the Virus from Rhipicephalus Bursa Ticks and a Preliminary Serological Survey. Arboviruses in the Mediterranean countries; Gustav Fisher Verlag: Stuttgart, Germany, 1980; pp. 117–121. [Google Scholar]
- Antoniadis, A.; Casals, J. Serological evidence of human infection with Congo-Crimean haemorrhagic fever virus in Greece. Am. J. Trop. Med. Hyg. 1982, 31, 1066–1067. [Google Scholar] [CrossRef]
- Papa, A.; Maltezou, H.C.; Tsiodras, S.; Dalla, V.G.; Papadimitriou, T.; Pierroutsakos, I.; Kartalis, G.N.; Antoniadis, A. A case of Crimean-Congo haemorrhagic fever in Greece, June 2008. Eurosurveillance 2008, 13, 18952. [Google Scholar] [CrossRef]
- Papa, A.; Sidira, P.; Larichev, V.; Gavrilova, L.; Kuzmina, K.; Mousavi-Jazi, M.; Mirazimi, A.; Ströher, U.; Nichol, S. Crimean-Congo haemorrhagic fever virus, Greece. Emerg. Infect. Dis. 2014, 20, 288. [Google Scholar] [CrossRef]
- Papa, A.; Papadopoulou, E.; Tsioka, K.; Kontana, A.; Pappa, S.; Melidou, A.; Giadinis, N.D. Isolation and whole-genome sequencing of a Crimean-Congo haemorrhagic fever virus strain, Greece. Ticks Tick Borne Dis. 2018, 9, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Markatou, F.; Maltezou, H.C.; Papadopoulou, E.; Terzi, E.; Ventouri, S.; Pervanidou, D.; Tsiodras, S.; Maltezos, E. Crimean-Congo haemorrhagic fever in a Greek worker returning from Bulgaria, June 2018. Eurosurveillance 2018, 23, 1800432. [Google Scholar] [CrossRef]
- Sidira, P.; Nikza, P.; Danis, K.; Panagiotopoulos, T.; Samara, D.; Maltezou, H.; Papa, A. Prevalence of Crimean-Congo haemorrhagic fever virus antibodies in Greek residents in the area where the AP92 strain was isolated. Hippokratia 2013, 17, 322. [Google Scholar] [PubMed]
- Babalis, T.; Tselentis, Y.; Roux, V.; Psaroulaki, A.; Raoult, D. Isolation and identification of a rickettsial strain related to Rickettsia massiliae in Greek ticks. Am. J. Trop. Med. Hyg. 1994, 50, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Dalla, V.; Petala, A.; Maltezou, H.C.; Maltezos, E. Fatal Mediterranean spotted fever in Greece. Clin. Microbiol. Infect. 2010, 16, 589–592. [Google Scholar] [CrossRef]
- Antoniou, M.; Tselentis, Y.; Babalis, T.; Gikas, A.; Stratigakis, N.; Vlachonikolis, I.; Kafatos, A.; Fioretos, M. The seroprevalence of ten zoonoses in two villages of Crete, Greece. Eur. J. Epidemiol. 1995, 11, 415–423. [Google Scholar] [CrossRef]
- Babalis, T.; Dupont, H.T.; Tselentis, Y.; Chatzichristodoulou, C.; Raoult, D. Rickettsia conorii in Greece: Comparison of a microimmunofluorescence assay and western blotting for seroepidemiology. Am. J. Trop. Med. Hyg. 1993, 48, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.; Economou, I.; Wang, X.; Psaroulaki, A.; Spyridaki, I.; Papadopoulos, B.; Christidou, A.; Tsafantakis, E.; Tselentis, Y. Fourteen-year seroepidemiological study of zoonoses in a Greek village. Am. J. Trop. Med. Hyg. 2002, 66, 80–85. [Google Scholar] [CrossRef][Green Version]
- Alexiou-Daniel, S.; Manika, K.; Arvanmdou, M.; Antoniadis, A. Prevalence of Rickettsia conorii and Rickettsia typhi infections in the population of northern Greece. Am. J. Trop. Med. Hyg. 2002, 66, 76–79. [Google Scholar] [CrossRef]
- Germanakis, A.; Psaroulaki, A.; Gikas, A.; Tselentis, Y. Mediterranean spotted fever in crete, Greece: Clinical and therapeutic data of 15 consecutive patients. Ann. N. Y. Acad. Sci. 2006, 1078, 263–269. [Google Scholar] [CrossRef]
- Tzavella, K.; Chatzizisis, Y.S.; Vakali, A.; Mandraveli, K.; Zioutas, D.; Alexiou-Daniel, S. Severe case of Mediterranean spotted fever in Greece with predominantly neurological features. J. Med. Microbiol. 2006, 55, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakos, D.; Papadopoulou, M.; Kanellopoulos, D.; Mamali, M.; Safarikas, M.; Katsianos, D.; Zisimopoulou, V. Chronic inflammatory demyelinating polyneuropathy associated with Rickettsia conorii: First case report. J. Neurol. Sci. 2016, 371, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Germanakis, A.; Chochlakis, D.; Angelakis, E.; Tselentis, Y.; Psaroulaki, A. Rickettsia aeschlimannii infection in a man, Greece. Emerg. Infect. Dis. 2013, 19, 1176. [Google Scholar] [CrossRef]
- Kostopoulou, V.; Chochlakis, D.; Kanta, C.; Katsanou, A.; Rossiou, K.; Rammos, A.; Papadopoulos, S.F.; Katsarou, T.; Tselentis, Y.; Psaroulaki, A.; et al. A case of human infection by Rickettsia slovaca in Greece. Jpn J. Infect. Dis. 2016, 69, 335–337. [Google Scholar] [CrossRef]
- Chochlakis, D.; Psaroulaki, A.; Kokkini, S.; Kostanatis, S.; Arkalati, E.; Karagrannaki, E.; Tsiatis, K.; Tselentis, Y.; Gikas, A. First evidence of Anaplasma infection in Crete, Greece. Report of six human cases. Clin. Microbiol. Infect. 2009, 15, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Tsiodras, S.; Spanakis, N.; Spanakos, G.; Pervanidou, D.; Georgakopoulou, T.; Campos, E.; Petra, T.; Kanellopoulos, P.; Georgiadis, G.; Antalis, E.; et al. Fatal human anaplasmosis associated with macrophage activation syndrome in Greece and the Public Health response. J. Infect. Public Health 2017, 10, 819–823. [Google Scholar] [CrossRef]
- Chochlakis, D.; Papaeustathiou, A.; Minadakis, G.; Psaroulaki, A.; Tselentis, Y. A sero-survey of Anaplasma phagocytophilum in blood donors in Crete, Greece. Eur. J. Clin. Microbiol. Infect Dis. 2008, 27, 473–475. [Google Scholar] [CrossRef]
- Alexiou-Daniel, S.; Manika, K.; Arvanitidou, M.; Diza, E.; Symeonidis, N.; Antoniadis, A. Serologic evidence of human granulocytic ehrlichiosis, Greece. Emerg. Infect. Dis. 2002, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Giadinis, N.D.; Chochlakis, D.; Ioannou, I.; Kritsepi-Konstantinou, M.; Papadopoulos, E.; Psaroulaki, A.; Karatzias, H. Haemorrhagic diathesis in a ram with Anaplasma phagocytophilum infection. J. Comp. Pathol. 2011, 144, 82–85. [Google Scholar] [CrossRef]
- Stamouli, M.; Totos, G.; Braun, H.B.; Michel, G.; Gizaris, V. Very low seroprevalence of Lyme Borreliosis in young Greek males. Eur. J. Epidemiol. 2000, 16, 495–496. [Google Scholar] [CrossRef]
- Papadopoulos, B.; Brossard, M.; Perié, N.M. Piroplasms of domestic animals in the Macedonia region of Greece 2. Piroplasms of cattle. Vet. Parasitol. 1996, 63, 57–66. [Google Scholar] [CrossRef]
- Stylianopoulos, M.; Ananiades, B. Contribution a l’etude des piroplasmoses en Grece. IV Les piroplasmoses bovines. Bull. Soc. Pathol. Exot. 1933, 26, 1153–1156. (In French) [Google Scholar]
- Theodoropoulos, G.; Gazouli, M.; Ikonomopoulos, J.A.; Kantzoura, V.; Kominakis, A. Determination of prevalence and risk factors of infection with Babesia in small ruminants from Greece by polymerase chain reaction amplification. Vet. Parasitol. 2006, 135, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, B.; Brossard, M.; Perié, N.M. Piroplasms of domestic animals in the Macedonia region of Greece 3. Piroplasms of small ruminants. Vet. Parasitol. 1996, 63, 67–74. [Google Scholar] [CrossRef]
- Kouam, M.K.; Kantzoura, V.; Gajadhar, A.A.; Theis, J.H.; Papadopoulos, E.; Theodoropoulos, G. Seroprevalence of equine piroplasms and host-related factors associated with infection in Greece. Vet. Parasitol. 169, 273–278. [CrossRef] [PubMed]
- Mangana-Vougiouka, O.; Boutsini, S.; Ntousi, D.; Patakakis, M.; Orfanou, E.; Zafiropoulou, K.; Dilaveris, D.; Panagiotatos, D.; Nomikou, K. Epizootiological investigation of the most important infectious equine diseases in Greece. Rev. Sci. Tech. 2013, 32, 775–787. [Google Scholar] [CrossRef]
- Kouam, M.K.; Masuoka, P.M.; Kantzoura, V.; Theodoropoulos, G. Geographic distribution modeling and spatial cluster analysis for equine piroplasms in Greece. Infect. Genet. Evol. 2010, 10, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Cardassis, J.; Margaritis, J. Enzootia theileriaseos is aegas ofilomeni is Theileria ovis. Delt. Ellinikis Ktiniatrikis Eterias 1964, 15, 174–179. (In Greek) [Google Scholar]
- Fauquet, C.M.; Mayo, M.A.; Maniloff, J.; Desselberger, U.; Ball, L.A. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Monath, T.P.; Heinz, F.X. Flaviviruses. In Fields Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1996; pp. 961–1034. [Google Scholar]
- Haglund, M.; Forsgren, M.; Lindh, G.; Lindquist, L. A 10-year follow-up study of tick-borne encephalitis in the Stockholm area and a review of the literature: Need for a vaccination strategy. Scand. J. Infect. Dis. 1996, 28, 217–224. [Google Scholar] [CrossRef]
- Rubin, S.; Chumakov, M. New Data on the Antigenic Types of Tick-Borne Encephalitis Virus. Arboviruses in the Mediterranean Countries; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1980; pp. 231–236. [Google Scholar]
- Theiler, M.; Casals, J.; Moutousses, C. Etiology of t e 1927–28 epidemic of dengue in Greece. Proc. Soc. Exp. Biol. Med. 1960, 103, 244–246. [Google Scholar] [CrossRef]
- Ergönül, Ö. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef]
- Leblebicioglu, H. Crimean–Congo haemorrhagic fever in Eurasia. Int. J. Antimicrob. Agents 2010, 36, S43–S46. [Google Scholar] [CrossRef]
- Vorou, R.; Pierroutsakos, I.N.; Maltezou, H.C. Crimean-Congo haemorrhagic fever. Curr. Opin. Infect. Dis. 2007, 20, 495–500. [Google Scholar] [CrossRef]
- Rangunwala, A.; Samudzi, R.R.; Burt, F.J. Detection of IgG antibody against Crimean-Congo haemorrhagic fever virus using ELISA with recombinant nucleoprotein antigens from genetically diverse strains. Epidemiol. Infect. 2014, 142, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Vorou, R.M.; Papavassiliou, V.G.; Tsiodras, S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol. Infect. 2007, 135, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.; Chaintoutis, S.C.; Dovas, C.I.; Groschup, M.H.; Mertens, M. Detection of Crimean-Congo haemorrhagic fever virus-specific IgG antibodies in ruminants residing in Central and Western Macedonia, Greece. Ticks Tick Borne Dis. 2017, 8, 494–498. [Google Scholar] [CrossRef]
- Vorou, R.M. Crimean-Congo haemorrhagic fever in southeastern Europe. Int. J. Infect. Dis. 2009, 13, 659–662. [Google Scholar] [CrossRef][Green Version]
- Elliott, R.M.; Brennan, B. Emerging phleboviruses. Curr. Opin. Virol. 2014, 5, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef]
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albariño, C.G.; Zaki, S.R.; Rollin, P.E.; et al. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841. [Google Scholar] [CrossRef]
- Papa, A.; Kontana, A.; Tsioka, K.; Chaligiannis, I.; Sotiraki, S. Novel phleboviruses detected in ticks, Greece. Ticks Tick Borne Dis. 2016, 7, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Rovery, C.; Raoult, D. Mediterranean spotted fever. Infect. Dis. Clin. North Am. 2008, 22, 515–530. [Google Scholar] [CrossRef]
- Alioua, Z.; Bourazza, A.; Lamsyah, H.; Erragragui, Y.; Boudi, O.; Karouach, K.; Ghfir, M.; Mossedaq, R.; Sedrati, O. Manifestations neurologiques de la fièvreboutonneuseméditerranéenne: À propos de quatre observations. Rev. Med. Interne 2003, 24, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef]
- Caminopetros, J. La reaction scrotale du cobayeprovoquee par inoculation des tiques (R. sanguineus) infectees avec le virus de la fievreboutonneuse. Ext. CR Soc. Biol. 1932, 110, 344. (In French) [Google Scholar]
- Komitova, R.; Lakos, A.; Aleksandrov, A.; Christova, I.; Murdjeva, M. A case of tick-transmitted lymphadenopathy in Bulgaria associated with Rickettsia slovaca. Scand. J. Infect. Dis. 2003, 35, 213. [Google Scholar] [CrossRef]
- Selmi, M.; Bertolotti, L.; Tomassone, L.; Mannelli, A. Rickettsia slovaca in Dermacentor marginatus and tick-borne lymphadenopathy, Tuscany, Italy. Emerg. Infect. Dis. 2008, 14, 817. [Google Scholar] [CrossRef]
- Oteo, J.A.; Portillo, A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012, 3, 271–278. [Google Scholar] [CrossRef]
- Rieg, S.; Schmoldt, S.; Theilacker, C.; Wölfel, S.; Kern, W.V.; Dobler, G. Tick-borne lymphadenopathy (TIBOLA) acquired in Southwestern Germany. BMC Infect. Dis. 2011, 11, 167. [Google Scholar] [CrossRef]
- Lakos, A. Tick-borne lymphadenopathy–a new rickettsial disease? Lancet 1997, 350, 1006. [Google Scholar] [CrossRef]
- Oteo, J.; Ibarra, V.; Blanco, J.; Martínez de Artola, V.; Márquez, F.; Portillo, A.; Raoult, D.; Anda, P. Dermacentor-borne necrosis erythema and lymphadenopathy: Clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 2004, 10, 327–331. [Google Scholar] [CrossRef]
- Fernández Soto, P.; Encinas Grandes, A.; Pérez Sánchez, R. Rickettsia aeschlimannii in Spain: Molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerg. Infect. Dis. 2003, 9, 889–890. [Google Scholar] [PubMed]
- Sandalakis, V.; Chochlakis, D.; Ioannou, I.; Psaroulaki, A. Identification of a novel uncultured Rickettsia species strain (Rickettsia species strain Tselenti) in Cyprus. Am. J. Trop. Med. Hyg. 2013, 88, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Mansueto, S.; Rolain, J.M.; Raoult, D. Rickettsia massiliae human isolation. Emerg. Infect. Dis. 2006, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Socolovschi, C.; Jeanjean, L.; Bitam, I.; Fournier, P.E.; Sotto, A.; Labauge, P.; Raoult, D. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl. Trop. Dis. 2008, 2, e338. [Google Scholar] [CrossRef]
- García-García, J.C.; Portillo, A.; Núñez, M.J.; Santibáñez, S.; Castro, B.; Oteo, J.A. A patient from Argentina infected with Rickettsia massiliae. Am. J. Trop. Med. Hyg. 2010, 82, 691–692. [Google Scholar] [CrossRef]
- Cascio, A.; Torina, A.; Valenzise, M.; Blanda, V.; Camarda, N.; Bombaci, S.; Iaria, C.; De Luca, F.; Wasniewska, M. Scalp eschar and neck lymphadenopathy caused by Rickettsia massiliae. Emerg. Infect. Dis. 2013, 19, 836. [Google Scholar] [CrossRef]
- Chen, S.M.; Dumler, J.S.; Bakken, J.S.; Walker, D.H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994, 32, 589–595. [Google Scholar] [CrossRef]
- Christova, I.S.; Dumler, J.S. Human granulocytic ehrlichiosis in Bulgaria. Am. J. Trop. Med. Hyg. 1999, 60, 58–61. [Google Scholar] [CrossRef][Green Version]
- Fingerle, V.; Goodman, J.; Johnson, R.; Kurtti, T.; Munderloh, U.; Wilske, B. Epidemiological aspects of human granulocytic ehrlichiosis in southern Germany. Wien. Klin. Wochenschr. 1999, 111, 1000–1004. [Google Scholar] [PubMed]
- Cizman, M.; Avsic-Zupanc, T.; Petrovec, M.; Ruzic-Sabljic, E.; Pokorn, M. Seroprevalence of ehrlichiosis, Lyme borreliosis and tick-borne encephalitis infections in children and young adults in Slovenia. Wien. Klin. Wochenschr. 2000, 112, 842–845. [Google Scholar]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef]
- Dworkin, M.S.; Schwan, T.G.; Anderson, D.E.; Borchardt, S.M. Tick-borne relapsing fever. Infect. Dis. Clin. 2008, 22, 449–468. [Google Scholar] [CrossRef]
- Szekeres, S.; Lakos, A.; Földvári, G. Borrelia miyamotoi: A recently identified human pathogenic tick-borne relapsing fever spirochete. Orv. Hetil 2017, 158, 1124–1130. (In Hungarian) [Google Scholar] [CrossRef]
- Güner, E.S.; Watanabe, M.; Hashimoto, N.; Kadosaka, T.; Kawamura, Y.; Ezaki, T.; Kawabata, H.; Imai, Y.; Kaneda, K. Masuzawa, T. Borrelia turcica sp. nov., isolated from the hard tick Hyalomma aegyptium in Turkey. Int. J. Syst. Evol. Microbiol. 2004, 54, 1649–1652. [Google Scholar] [PubMed]
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R.; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef]
- Krause, P.J.; Spielman, A.; Telford, S.R.; Sikand, V.K.; McKay, K.; Christianson, D.; Pollack, R.J.; Brassard, P.; Magera, J.; Ryan, R.; et al. Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 1998, 339, 160–165. [Google Scholar] [CrossRef]
- Uilenberg, G.; Irvin, A.D.; Cunningham, M.P.; Young, A.S. Advances in the Control of Theileriosis; Martinus Nijhoff: The Hague, The Netherlands, 1981; Volume 4, p. 37. [Google Scholar]
- Morel, P.; Uilenberg, G. Sur la nomenclature de quelques Theileria (Sporozoa, Babesioidea) des ruminants domestiques. Rev. Elev. Med. Vet. Pays 1981, 34, 139–143. (In French) [Google Scholar]
- Cardamatis, J. Des Piroplasmiases et leishmaniases. Zentralblatt fur Bakteriologie. Abt. I. Orig 1911, 60, 511–523. (In French) [Google Scholar]
- Cardamatis, J.P. Piroplasmoses des bovides en Grece. Le Bulletin de la Société de Pathologie Exotique 1912, 5, 87–88. (In French) [Google Scholar]
- Melanidis, C.; Stylianopoulos, M. Notes sur la peste bovine en Grece. Rev. Gen. Med. Vet. 1927, 36, 72–78. (In French) [Google Scholar]
- Pavlov, P. Das Vorkommen von Theileriose in Mazedonien. Dtsch. Tierärztl. Wschr. 1942, 40, 458–460. (In German) [Google Scholar]
- Cardassis, J. Essais de taitement de la theilériose bovine grecque par quelques antipaludiques de synthèse: La quinacrine et la paludrine. Bull. Acad. Vet. Fr. 1956, 39, 66–72. (In French) [Google Scholar]
- Cardassis, J. Epi tis Theileria annulata ofilomenis theileriaseos ton vooidon en Helladi. I. Epizootiologia ke kliniki meleti. Elliniki Ktiniatriki 1964, 7, 77–90. (In Greek) [Google Scholar]
- Joyner, L.; Davies, S.; Kendall, S. The experimental transmission of Babesia divergens by Ixodes ricinus. Exp. Parasitol. 1963, 14, 367–373. [Google Scholar] [CrossRef]
- Brocklesby, D.W.; Barnett, S.F. Large Babesia species transmitted to splenectomized calves by field collections of British ticks (Haemaphysalis punctata). Nature 1970, 228, 1215. [Google Scholar] [CrossRef]
- Ferrer, D.; Castellà, J.; Gutierrez, J. Seroprevalence of Babesia ovis in sheep in Catalonia, northeastern Spain. Vet. Parasitol. 1998, 79, 275–281. [Google Scholar] [CrossRef]
- Miaoulis, M.V. Co-existence du charbon bacteridien et de la babesiellose ovine. Rec. Med. Vet. 1931, 107, 463–464. (In French) [Google Scholar]
- Giadinis, N.; Chochlakis, D.; Kritsepi-Konstantinou, M.; Makridaki, E.; Tselentis, Y.; Kostopoulou, D.; Karatzias, H.; Psaroulaki, A. Haemolytic disease in sheep attributed to a Babesia lengau-like organism. Vet. Rec. 2012, 170, 155. [Google Scholar] [CrossRef]
- Bosman, A.-M.; Oosthuizen, M.C.; Peirce, M.A.; Venter, E.H.; Penzhorn, B.L. Babesia lengau sp. nov., a novel Babesia species in cheetah (Acinonyx jubatus, Schreber, 1775) populations in South Africa. J. Clin. Microbiol. 2010, 48, 2703–2708. [Google Scholar] [CrossRef]
- Leblond, A.; Pradier, S.; Pitel, P.; Fortier, G.; Boireau, P.; Chadoeuf, J.; Sabatier, P. An epidemiological survey of equine anaplasmosis Anaplasma phagocytophilum in southern France. Rev. Sci. Tech. 2005, 24, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Bashiruddin, J.; Camma, C.; Rebelo, E. Molecular detection of Babesia equi and Babesia caballi in horse blood by PCR amplification of part of the 16S rRNA gene. Vet. Parasitol. 1999, 84, 75–83. [Google Scholar] [CrossRef]
- Camacho, A.; Guitian, F.; Pallas, E.; Gestal, J.; Olmeda, A.; Habela, M.; Telford Iii, S.; Spielman, A. Theileria (Babesia) equi and Babesia caballi infections in horses in Galicia, Spain. Trop. Anim. Health Prod. 2005, 37, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Mangili, V.; Salvatori, R.; Maresca, C.; Scoccia, E.; Torina, A.; Moretta, I.; Gabrielli, S.; Tampieri, M.P.; Pietrobelli, M. Prevalence and diagnosis of Babesia and Theileria infections in horses in Italy: A preliminary study. Vet. J. 2010, 184, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Karatepe, B.; Karatepe, M.; Çakmak, A.; Karaer, Z.; Ergün, G. Investigation of seroprevalence of Theileria equi and Babesia caballi in horses in Nigde province, Turkey. Trop. Anim. Health Prod. 2009, 41, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kouam, M.K.; Kantzoura, V.; Masuoka, P.M.; Gajadhar, A.A.; Theodoropoulos, G. Genetic diversity of equine piroplasms in Greece with a note on speciation within Theileria genotypes (T. equi and T. equi-like). Inf. Gen. Evol. 2010, 10, 963–968. [Google Scholar] [CrossRef]
- Schein, E. Equine babesiosis. In Babesiosis of Domestic Animals and Man; Ristic, M., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1988; pp. 197–208. [Google Scholar]
- Criado-Fornelio, A.; Martinez-Marcos, A.; Buling-Sarana, A.; Barba-Carretero, J. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe: Part, I. Epizootiological aspects. Vet. Parasitol. 2003, 113, 189–201. [Google Scholar] [CrossRef]
- Hellenic Statistical Authority. 2016. Available online: http://www.statistics.gr/documents/20181/3500447/35.+Holdings+and+number+of+animals+by+kind%2C+region+and+regional+unit+%28+2016+%29/3d6ec63f-d867-45c6-b962-ed1132b15aba?version=1.0 (accessed on 2 July 2020).
- Siarkou, V.I.; Mylonakis, M.E.; Bourtzi-Hatzopoulou, E.; Koutinas, A.F. Sequence and phylogenetic analysis of the 16S rRNA gene of Ehrlichia canis strains in dogs with clinical monocytic ehrlichiosis. Vet. Microbiol. 2007, 125, 304–312. [Google Scholar] [CrossRef]
- Komnenou, A.A.; Mylonakis, M.E.; Kouti, V.; Tendoma, L.; Leontides, L.; Skountzou, E.; Dessiris, A.; Koutinas, A.F.; Ofri, R. Ocular manifestations of natural canine monocytic ehrlichiosis (Ehrlichia canis): A retrospective study of 90 cases. Vet. Ophthalmol. 2007, 10, 137–142. [Google Scholar] [CrossRef]
- Mylonakis, M.E.; Kritsepi-Konstantinou, M.; Dumler, J.S.; Diniz, P.P.V.P.; Day, M.J.; Siarkou, V.I.; Breitschwerdt, E.B.; Psychas, V.; Petanides, T.; Koutinas, A.F. Severe hepatitis associated with acute Ehrlichia canis infection in a dog. J. Vet. Intern. Med. 2010, 24, 633–638. [Google Scholar] [CrossRef]
- Angelou, A.; Gelasakis, A.I.; Verde, N.; Pantchev, N.; Schaper, R.; Chandrashekar, R.; Papadopoulos, E. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasit. Vect. 2019, 12, 283. [Google Scholar] [CrossRef]
- Athanasiou, L.V.; Kontos, V.I.; Kritsepi Konstantinou, M.; Polizopoulou, Z.S.; Rousou, X.A.; Christodoulopoulos, G. Cross-sectional serosurvey and factors associated with exposure of dogs to vector-borne pathogens in Greece. Vector Borne Zoonotic Dis. 2019, 19, 923–928. [Google Scholar] [CrossRef]
- Kostopoulou, D.; Gizzarelli, M.; Ligda, P.; Manzillo, V.F.; Saratsi, K.; Montagnaro, S.; Schunack, B.; Boegel, A.; Pollmeier, M.; Oliva, G.; et al. Mapping the canine vector-borne disease risk in a Mediterranean area. Parasit. Vect. 2020, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humansin Europe. Parasit. Vect. 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Brianti, E.; Latrofa, M.S.; Annoscia, G.; Weigl, S.; Lia, R.P.; Gaglio, G.; Napoli, E.; Giannetto, S.; Papadopoulos, E.; et al. On a Cercopithifilaria sp. transmitted by Rhipicephalus sanguineus: A neglected, but widespread filarioid of dogs. Parasit. Vect. 2012, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Diakou, A.; Di Cesare, A.; Accettura, P.M.; Barros, L.; Iorio, R.; Paoletti, B.; di Regalbono, A.F.; Halos, L.; Beugnet, F.; Traversa, D. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl. Trop. Dis. 2017, 11, e0005335. [Google Scholar] [CrossRef]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The potential rle of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [Google Scholar] [CrossRef]
- Semenza, J.C.; Rocklöv, J.; Penttinen, P.; Lindgren, E. Observed and projected drivers of emerging infectious diseases in Europe. Ann. N. Y. Acad. Sci. 2016, 1382, 73–83. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Papa, A. Crimean–Congo haemorrhagic fever: Risk for emergence of new endemic foci in Europe? Travel Med. Infect. Dis. 2010, 8, 139–143. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Jameson, L.; Medlock, J.; Vatansever, Z.; Tishkova, F. Unraveling the ecological complexities of tick-associated Crimean-Congo haemorrhagic fever virus transmission: A gap analysis for the western Palearctic. Vector-Borne Zoonotic Dis. 2012, 12, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Papa, A. Crimean-Congo haemorrhagic fever: Epidemiological trends and controversies in treatment. BMC Med. 2011, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Choi, K.S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Santino, I.; Dastoli, F.; Sessa, R.; Del Piano, M. Geographical incidence ofinfection with Borrelia burgdorferi in Europe. Panminerva Med. 1997, 39, 208–214. [Google Scholar]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals; Bailliere Tindall: London, UK, 1982. [Google Scholar]
- Gray, J.; Dantas-Torres, F.; Estrada-Pena, A.; Levin, M. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013, 6, 872. [Google Scholar] [CrossRef]
- Boussalis, C.; Nelson, H.T.; Swaminathan, S. Towards comprehensive malaria planning: The effect of government capacity, health policy, and land use variables on malaria incidence in India. Soc. Sci. Med. 2012, 75, 1213–1221. [Google Scholar] [CrossRef]
- Bardosh, K.L.; Ryan, S.; Ebi, K.; Welburn, S.; Singer, B. Addressing vulnerability, building resilience: Community-based adaptation to vector-borne diseases in the context of global change. Infect. Dis. Poverty 2017, 6, 166. [Google Scholar] [CrossRef]
| Tick Species | Region | Host | Associated Pathogen(s) | References |
|---|---|---|---|---|
| Amblyomma variegatum | Macedonia | Goats | No pathogens reported | [15] |
| Dermacentor marginatus | Northern Greece (Macedonia), Cephalonia Island, Lesvos Island, Western and Central Greece | Cattle, sheep, goats, dogs, humans | CCHF virus, Rickettsia spp., Anaplasma spp., C. burnetii, Babesia sp., Theileria sp., T. annulata, T. bufeli/orientalis, T. ovis, T. lestoquardi, Coxiella-like endosymbionts | [16,17,18,19,20,21,22,23] |
| Haemaphysalis impeltatum | Central Greece | Sheep, goats, cattle | No pathogens reported | [19,20] |
| Haemaphysalis inermis | Northern Greece (Macedonia) | Sheep, goats, cattle, dogs | No pathogens reported | [15] |
| Haemaphysalis parva | Northern Greece (Macedonia, Thrace), Lesvos Island, Thessaly, Arta, Etoloakarnania, Evritania, Fokida, Ftiotida, Preveza | Cattle, sheep, goats, dogs | CCHF virus, Rickettsia spp., R. hoogstraalii, Anaplasma spp., Hepatozoon canis, H. felis, phleboviruses, C. burnetii, Babesia sp., B. ovis, B. crassa, Theileria sp., T. annulata, T. ovis, T. lestoquardi | [15,20,22,23,24,25] |
| Haemaphysalis punctata | Northern Greece (Macedonia), Cephalonia Island, Lesvos Island, Arta, Crete | Sheep, goats, cattle, dogs | No pathogens reported | [15,17,20,22,23,25,26] |
| Haemaphysalis sulcata | Northern Greece (Macedonia), Cephalonia Island, Arta, Etoloakarnania, Fokida, Preveza, Thessaloniki | Cattle, sheep, goats | Rickettsia spp., Anaplasma spp., C. burnetii, Babesia sp., B. divergens, Theileria sp., T. ovis | [15,17,20,22,23] |
| Hyalomma aegyptium | Serres region in Northern Greece (Lailias mountain region) | Wild birds, tortoises | Rickettsia spp., R. aeschlimannii, Borrelia turcica | [27,28,29] |
| Hyalomma anatolicum anatolicum | Northern Greece | Sheep, goats, cattle | No pathogens reported | [19,30] |
| Hyalomma anatolicum excavatum | Northern Greece (Macedonia), Cephalonia Island, Crete | Sheep, goats, cattle, horses, humans | Rickettsia aeschlimannii, R. sibirica mongolotimonae | [15,17,19,20,31,32], |
| Hyalomma concinna | Northern Greece (Thrace), Thessaly | Dogs | Hepatozoon canis | [24] |
| Hyalomma detritum scupense | Northern Greece (Macedonia, Thrace) | Dogs, cattle | No pathogens reported | [15,24] |
| Hyalomma dromedarii | Northern Greece | Sheep, goats, cattle | No pathogens reported | [19,20,33] |
| Hyalomma marginatum marginatum (syn. Hy. plumbeum plumbeum) | Northern Greece (Macedonia, Thrace), Cephalonia Island | Sheep, goats, horses, cattle, dogs, humanswild birds | CCHF virus, T. equi, Rickettsia spp. | [15,17,18,19,20,27,33,34,35,36] |
| Hyalomma marginatum rufipes | Northern Greece (Macedonia, Thrace), Antikythira Island | Sheep, goats, cattle, humans, wild birds | Rickettsia spp. | [15,19,20,36,37,38] |
| Hyalomma marginatum turanicum | Northern Greece (Macedonia) | Cattle | No pathogens reported | [15,19] |
| Ixodesacuminatus | Northern Greece | Wild birds | No pathogens reported | [27] |
| Ixodesfrontalis | Northern Greece | Wild birds | Rickettsia spp. | [27] |
| Ixodes gibbosus | Northern Greece (Macedonia), Komotini, Limnos Island, Cephalonia Island | Sheep, goats, cattle, horses, humans | Rickettsia spp., Anaplasma spp., C. burnetii, Theileria sp., T. lestoquardi | [15,16,17,18,20,22,23] |
| Ixodes hexagonus | Northern Greece (Macedonia) | Dogs | No pathogens reported | [15] |
| Ixodes ricinus | Chalkidiki, Rodopi, Macedonia, Thrace, Kastoria | Sheep, goats, dogs, cattle, humans | TBE virus, Rickettsia monacensis, R. massiliae, Anaplasma phagocytophilum, A. platys, B. divergens, Coxiella-like endosymbionts | [15,18,19,20,21,24,35,38,39] |
| Rhipicephalus (Boophilus) annulatus | Northern Greece (Macedonia, Thrace), Komotini, Chalkidiki | Cattle, sheep, goats, humans | No pathogens reported | [15,18,19,35,36,40] |
| Rhipicephalus bursa | Kastoria, Fokida, Macedonia, Thrace, Rodopi, Cephalonia Island | Sheep, goats, cattle, dogs, horses, humans | Rickettsia monacensis, R. massiliae, R. slovaca, Candidatus R. barbariae, R. rhipicephali, Anaplasma phagocytophilum, A. platys, CCHF virus, Theileria ovis, Coxiella-like endosymbionts | [15,16,17,18,19,20,21,22,23,24,33,38,40] |
| Rhipicephalus camicasi | Northern Greece | Sheep, goats, cattle | No pathogens reported | [19,33] |
| Rhipicephalus sanguineus | Country-wide | Sheep, goats, dogs, cats, cattle, humans | CCHF virus, Rickettsia spp. (R. conorii, R. aeschlimannii, R. africae, R. massiliae, R. rhipicephali), Ehrlichia canis, Hepatozoon canis, H. felis, Anaplasma spp., A. platys, Coxiella burnetii, Babesia sp., B. bigemina, B. ovis, B. caballi, Theileria sp., T. annulata, T. bufeli/orientalis, T. ovis, T. lestoquardi | [15,17,18,19,20,21,22,23,24,34,38,41,42] |
| Rhipicephalus turanicus | Northern Greece (Macedonia, Thrace), Cephalonia Island | Sheep, goats, cattle, dogs, horses, humans | Rickettsia aeschlimannii, R. massiliae, Hepatozoon canis | [15,17,18,19,24] |
| Study | Sampling Period | Geographic Region | Animals Sampled | Sampling Strategy | Total Ticks Sampled | Tick Species (Relative Species Distribution) |
|---|---|---|---|---|---|---|
| [15] | Mar–Jun and Nov–Dec 1983, Apr–May and Sep–Oct 1985, Apr–Jul and Oct–Nov 1986 | Northern Greece (Macedonia, 64 localities) | Cattle (602) | Tick collection from grazing herds of 5–20 animals | 5137 ticks (16 species) | Rhipicephalus bursa (29.38%); Boophilus annulatus (23.83%); Ixodes ricinus (10.47%); Rh. turanicus (8.92%); Hyalomma m. marginatum (7.96%); Haemaphysalis inermis (7.46%); l. gibbosus (7.40%); H. anatolicum excavatum (3.99%); H. detritum scupense (3.78%); H. punctata (1.77%); Dermacentor marginatus (0.93%); H. parva (0.56%); H. sulcata (0.12%); H. marginatum rufipes (0.04%); Rh. sannguineus (0.02%); H. marginatum turanicum (0.02%) |
| Sheep (721) | 2265 ticks (12 species) | Rhipicephalus bursa (47.95%); Rh. turanicus (18.59%); H. sulcata (14.79%); H. punctata (4.77%); Dermacentor marginatus (4.72%); Haemaphysalis inermis (4.19%); Ixodes ricinus (2.25%); l. gibbosus (1.99%); H. parva (0.53%); Rh. sannguineus (0.09%); Hy. anatolicum excavatum (0.09%); Hyalomma m. marginatum (0.04%) | ||||
| Goats (487) | 3231 ticks (12 species) | Rhipicephalus bursa (49.98%); l. gibbosus (23.03%); H. sulcata (9.97%); Rh. turanicus (6.47%); Ixodes ricinus (3.65%); Haemaphysalis inermis (2.88%); Dermacentor marginatus (2.38%); H. punctata (1.39%); H. parva (0.12%); Hyalomma m. marginatum (0.06%); Hy. anatolicum excavatum (0.03%); Amblyomma variegatum (0.03%) | ||||
| Dogs (70) | 987 ticks (6 species) | Rh. sannguineus (60.49%); Rh. turanicus (29.69%); Ixodes ricinus (8.41%); Rhipicephalus bursa (0.91%); I. hexagonus (0.30%); Haemaphysalis punctata (0.20%) | ||||
| [43] | 1996–1997 | Thessaloniki | Dogs (249) | Dogs admitted to local veterinary hospital and private practices | 2812 ticks (2 species) | Rh. sanguineus (89.30%); Rh. turanicus (5.55%); Rhipicephalus spp. nymphs and larvae (5.16%) |
| [44] | May–Aug 1998 | Northern Greece (3 villages in Fokida region) | Goats, sheep, and dogs (not specified) | Not specified | 439 ticks (3 species) | Rhipicephalus sanguineus (47.15%); Rh. bursa (31.44%); Rh. turanicus (21.41%) |
| [17] | Apr–Jul 1998, May–Jun and Oct 1999 | Corfu Island (32 localities) | Cattle (not specified) | Not specified | 145 ticks (5 species) | Hyalomma marginatum marginatum (31.03%); Hy. anatolicum excavatum (25.52%); Rhipicephalus bursa (20.69%); Rh. turanicus (20.00%); Ixodes gibbosus (2.76%) |
| Sheep (not specified) | 524 ticks (7 species) | Rh. turanicus (38.74%); Dermacentor marginatus (29.39%); Rhipicephalus bursa (11.45%), Hy. marginatum marginatum (11.07%); Hyalomma (Hy.) anatolicum excavatum (4.58%); Haemaphysalis (Ha.) punctata (4.39%); Ixodes gibbosus (0.38%) | ||||
| Goats (not specified) | 796 ticks (8 species) | Rh. turanicus (34.92%); Dermacentor marginatus (29.65%); Rhipicephalus bursa (29.15%); Ixodes gibbosus (3.02%); Haemaphysalis sulcata (1.38%); Ha. punctata (0.75%), Hyalomma marginatum marginatum (0.63%); Hy. anatolicum excavatum (0.50%) | ||||
| Horses (not specified) | 86 ticks (5 species) | Rh. turanicus (58.14%); Hy. marginatum marginatum (19.77%); Hy. anatolicum excavatum (17.44%); Rhipicephalus bursa (2.33%); Ixodes gibbosus (2.33%) | ||||
| Dogs (not specified) | 297 ticks (3 species) | Rh. sanguineus (96.97%); Hy. marginatum marginatum (1.68%); Rh. turanicus (1.35%) | ||||
| [45] | Apr–Jul and Sep–Dec of 2003–2006 | Northern Greece (11 prefectures of Macedonia and Thrace) | Sheep and goats (not specified) | Animals grazing permanently in the countryside | 3144 ticks (7 species) | Ixodes ricinus (46.06%); Rhipicephalus bursa (19.78%); Hyalomma marginatum (12.82%); Rh. sanguineus (6.93%); Rh. turanicus (5.66%); Boophilus annulatus (4.58%); I. gibbosus (4.17%) |
| [40] | Nov and Jun 2004–2006 | 4 localities of Halkidiki prefecture, Northern Greece | Goats, sheep, and dogs (not specified) | Not specified | 703 ticks (6 species) | Ixodes ricinus (50.50%); Hyalomma marginatum (18.92%); Rhipicephalus bursa (14.37%); Rh. sanguineus (10.81%); Rh. turanicus (3.56%); Boophilus annulatus (1.85%) |
| [36] | Jun–Sep 2008 | North-eastern Greece (Komotini, Alexandroupoli) | Humans (not specified, >300) | Individuals with tick bites visiting 2 hospitals in the region | 519 ticks (9 species) | Rhipicephalus sanguineus (81.50%); Rh. turanicus (5.20%); Hyalomma marginatum (5.20%); Rh. bursa (2.70%); Hy. rufipes (1.93%); Ixodes gibbosus (1.16%); Rh. annulatus (0.96%); I. rinicus (0.96%); Hy. anatolicum (0.39%) |
| [33] | Apr–Jun 2012 | Northern Greece (30 villages in Kastoria, Rodopi, Xanthi, Kavala and Chalkidiki) | Sheep and goats (not specified) | Not specified | 95 ticks (7 species) | Rhipicephalus sanguineus (55.79%); Rh. bursa (29.47%); Rh. turanicus (9.47%); Rh. camicasi (2.11%); Hyalomma marginatum (1.05%); Hy. dromedarii (1.05%); Dermacentor marginatus (1.05%) |
| [19] | Mar–Oct 2012–2013 | Country-wide (26 mainland prefectures and 5 Aegean islands) | Cattle (not specified) | Not specified | 142 ticks (11 species) | Hy. dromedarii (47.18%); Hy. marginatum (16.90%); Rh. sanguineus (10.56%); Hy. excavatum (5.63%); Rh. bursa (4.23%); Rh. turanicus (4.23%); Rh. camicasi (3.52%); Hy. rufipes (2.82%); Hy. anatolicum (2.11%); Hy. turanicum (2.11%); Hy. impeltatum (0.70%) |
| Humans (not specified) | Infected individuals who visited hospitals | 701 ticks (10 species) | Rh. sanguineus (80.17%); Rh. turanicus (4.85%); Hy. marginatum (4.28%); Rh. bursa (3.28%); Rhipicephalus nymphs (2.85%); Hy. rufipes (1.57%); Ixodes ricinus (0.85%); I. gibosus (0.85%); Rh. annulatus (0.71%); Hy. excavatum (0.28%); Dermacentor marginatus (0.28%) | |||
| [20] | Sheep (1201) | Random sampling of ~10 animals from each of 309 farms | 1339 ticks (13 species) | Rhipicephalus sanguineus (64.15%); Rh. bursa (26.59%); Dermacentor marginatus (2.39%); Hy. dromedarii (2.32%); Haemaphysalis parva (1.27%); Ha. sulcata (1.12%); I. gibbosus (0.82%); Hy. excavatum (0.52%); Hy. rufipes (0.30%); Hyalomma marginatum (0.22%); Ha. punctata (0.07%); Ixodes ricinus (0.07%); Hy. impeltatum (0.07%); Rhipicephalus nymph (0.07%) | ||
| Goats (681) | 769 ticks (6 species) | Rhipicephalus sanguineus (65.93%); Rh. bursa (24.71%); Dermacentor marginatus (7.15%); Haemaphysalis parva (1.04%); Ha. sulcata (0.91%); Hyalomma marginatum (0.26%) | ||||
| [24] | May–Aug 2015 | 6 provinces of mainland Greece (Larisa, Athens, Corinth, Thessaloniki, Xanthi, Alexandroupo-li) | Dogs (310) | Domestic dogs from rural areas, (shelters, temporary kennels, indoor environments, or hospitals) | 757 ticks (7 species) | Rhipicephalus sanguineus (s.l.) (70.15%); Haemaphysalis parva (14.66%); Rh. turanicus (11.36%); H. concinna (2.38%); Ixodes ricinus (0.26%); Hyalomma scupense (0.13%); Rh. bursa (0.13%) |
| [21] | Feb 2015–May 2016 | Northern Greece (5 prefectures) | Goats (28) | Not specified | 64 ticks (5 species) | Rh. bursa (57.81%); D. marginatus (17.19%); I. ricinus (15.63%); Rh. sanguineus (4.69%); Haemaphysalis parva (3.13%); Rhipicephalus spp. nymph (1.56%) |
| [23] | Mar–Oct 2014 | Central and Northern Greece (14 mainland prefectures) | Sheep (not specified) | Random sampling of 10 animals in each farm | 64 ticks (6 species) | Dermacentor marginatus (26.56%); Haemaphysalis parva (26.56%); H. sulcata (23.44%); Ixodes gibbosus (20.31%); H. punctata (1.56%), Rhipicephalus sanguineus (1.56%) |
| Goats (not specified) | 42 ticks (5 species) | Dermacentor marginatus (54.76%); Haemaphysalis parva (19.05%); H. sulcata (6.67%); Rhipicephalus sanguineus (7.14%); Rh. bursa (2.38%) | ||||
| Dogs (not specified) | Dogs presented to veterinary clinics | 73 ticks (1 species) | Rh. sanguineus s.l. (100%) | |||
| [46] | Mar–Oct 2014 | 13 prefectures of Central and Northern mainland Greece, and Limnos Island | Sheep (not specified) | Not specified | 63 ticks (5 species) | Dermacentor marginatus (26.98%); Haemaphysalis parva (26.98%); H. sulcata (23.81%); Ixodes gibosus (20.63%); Rhipicephalus sanguineus (1.59%) |
| Goats (not specified) | 43 ticks (6 species) | Dermacentor marginatus (53.49%); Haemaphysalis parva (18.60%); H. sulcata (16.28%); Rhipicephalus sanguineus (6.98%); Rh. bursa (2.33%); H. punctata (2.33%) | ||||
| Dogs (not specified) | 78 ticks (1 species) | Rhipicephalus sanguineus (93.59%); Rhipicephalus sp. (6.41%) |
| Pathogen | Disease | Tick Vectors (Transmitters) | Clinical Symptoms | Prevalence in Greece | Geographic Areas of High Prevalence | Season/Climate of High Prevalence | References |
|---|---|---|---|---|---|---|---|
| Tick-borne encephalitis (TBE) virus–Greek goat encephalitis virus (GGEV) | Tick-borne encephalitis | I. ricinus (European subtype) | Disorder of the nervous system with symptoms of meningitis and encephalitis | 1.7% general prevalence (1981–1988) [47] | October to November in Northern Greece, high relative humidity | [49,50] | |
| Crimean-Congo hemorrhagic fever (CCHF) virus | Crimean-Congo hemorrhagic fever | Hyalomma marginatum (main vector), Dermacentor marginatus, Rhipicephalus sanguineus, Rh. bursa (vector of Greek strain AP92), Haemaphysalis parva | Affects whole body by hemorrhage, kidney failure, pulmonary distress and shock, mortality of ~30%. First fatal case in Rodopi in June 2008 [51] | Altitude >400 m, special soil conditions, May to August, increased R. bursa activity with higher temperatures and lower humidity | [22,30,42,56,57,58,59,60,61,62] | ||
| Rickettsia massiliae | Rickettsiosis of spotted fever group (SFG) | Rhipicephalus sanguineus, Rh. turanicus, Rh. bursa, Ixodes ricinus | Fever, rash, headache, severe myalgia, weakness | No data | No data | Rhipicephalus sanguineus common in meso-Mediterranean bioclimatic zone, active during spring/early summer | [17,21,38,44,63] |
| Rickettsia conorii | Mediterranean Spotted Fever (MSF) | Rhipicephalus sanguineus | High fever, myalgia, lymphadeno-pathy, headache, maculopapular rash, neurological complications | Fatal case in Northeastern Greece in 2008 [64] | Adult Rh. sanguineus in May, larvae and nymphs active during summer | [26,69,70,71] | |
| Rickettsia sibirica mongolotimonae | Lymphangitis- associated Rickettsiosis (LAR) | Hyalomma anatolicum excavatum | Fever, maculopapular rash, enlarged regional lymph nodes, lymphangitis | First reported human case in Crete in 2002, [31] | No data | No data | [32] |
| Rickettsia aeschlimannii | Tick-borne lymphadeno-pathy (TIBOLA) | Rhipicephalus turanicus, Rh. sanguineus, H. anatolicum excavatum | Non-specific, inoculation eschar, fever, generalized maculopapular rash | First reported human case in Europe in Crete [72] | No data | No data | [17,32,38] |
| Rickettsia slovaca | Tick-borne lymphadeno-pathy (TIBOLA) | Rhipicephalus bursa | Enlarged regional lymph nodes, fever and malaise, maculopapular rash | First reported human case in Corfu in 2011 [73] | No data | May–August: Rh. bursa activity increased with high temperature and low humidity | [40] |
| Anaplasma phagocytophilum | Human granulocytic anaplasmosis (ehrlichiosis), ovine anaplasmosis | Ixodes ricinus, Rhipicephalus bursa | Fever, chills, maculopapular rash, headache, malaise, splenomegaly, Cervical lymph-adenopathy, gastrointestinal disturbances | First reported case in Crete [74], first fatal case in Athens [75] | Peak tick activity in the summer | [39,78] | |
| Borrelia burgdorferi | Lyme disease | Ixodes ricinus complex | Multisystemic manifestation: Erythema migrans, neuroborreliosis with peripheral or central affection, joints (arthritis), skin (acro-dermatitis chronica atrophicans), Borrelia lymphocytoma, cardiovascular, renal (glomerulo-nephritis), chronification possible | 3.27% IgG positive by enzyme immunoessay and 0.27% IgG pos. Western Blot in 1100 Greek male Navy recruits | No data | No data | [79] |
| Borrelia turcica | Phylogenetic tree and goeBURST analysis showed differences to Lyme disease (LD) and relapsing fever (RF) species | Hyalomma aegyptium | Unknown pathogenicity for humans | 131 (38.6%) of 339 ticks Borrelia positive (conventional flaB PCR) (2000), and 58 (64.4%) (23S locus qPCR) of 90 screened ticks (of 358 collected in total) Borrelia positive | Location of tick collection in Serres region in Northern Greece (Lailias mountain region) (2000 and 2017) | Tick collection time during spring (May–June) (2000 and 2017) | [28,29] |
| Babesia bovis | Bovine babesiosis (most common babesiosis in Greece) | Boophilus annulatus (proposed) | Acute and very severe infection (high fever, anemia anorexia) | No data | 21.6% of 602 cattle in Macedonia (1984–1986) [80] | No data | [81] |
| Babesia bigemina | Bovine babesiosis (rare cases in Greece) | Boophilus annulatus (proposed) | Less severe than B. bovis infection | No data | 15.2% of 602 cattle in Macedonia (1984–1986) [80] | No data | [81] |
| Babesia ovis | Ovine babesiosis | Rhipicephalus bursa, Ixodes scapularis (proposed) | Apathy, fever, anemia, jaundice, haemoglobinuria, death | No data | No data | ||
| Babesia caballi | Equine piroplasmosis | Rhipicephalus sanguineus | Non-specific haemolytic conditions (fever, anaemia, jaundice) |
| No data | [34] | |
| Theileria ovis | Benign theileriosis of small ruminants | Rhipicephalus bursa | Non-pathogenic | No data | Macedonia: 24.6% of 721 sheep, 0.6% of 487 goats (1984–1985) [83] | No data | [87] |
| Theileria orientalis | Bovine theileriosis | Rh. bursa, H. punctata (proposed) | Non-pathogenic | No data | 41.4% of 602 cattle in Macedonia (1984–1986) [80] | No data | [83] |
| Theileria annulata | Mediterranean bovine theileriosis | Hyalomma d. detritum (proposed) | No clinical symptoms | No data | 2% of 602 cattle in Macedonia (1984–1986) [80] | Warm Mediterranean maquis | [83] |
| Theileria equi and T. equi-like genotype | Equine piroplasmosis | Hyalomma marginatum | Non-specific haemolytic conditions (fever, anaemia, jaundice), more severe than B. caballi | 38.8% of 49 equids in Thessaly [84] | Occurrence most predominantly in forest type land cover class | [34] |
| Citation | Sampling Period | Geographic Region | Humans Tested | Sampling Strategy | Detection Method | Positive Rate (Seroprevalence) | |
|---|---|---|---|---|---|---|---|
| TBE | [92] | 1958 | Athens | 56 | Not specified | Hemagglutination-inhibition (HI) and neutralization tests | 1.8% |
| [47] | 1981–1988 | Mainland Greece, islands of Corfu and Crete | 475 | Apparently healthy individuals (farmers, wood cutters & shepherds) | Indirect immunofluorescence (IFA) | Overall 1.7%. Regions of non-zero seroprevalence: Laconia (13.8%), Pella (3.57%), Ioannina (2.47%), Imathia (2.27%) | |
| [48] | 2003–2005 | Northern Greece (all prefectures) and Corfu Island | 921 | Apparently healthy permanent residents of urban and rural areas | Serum samples: IFA and ELISA (samples considered positive if positive by both). Cerebrospinal fluid samples: 2 RT-nPCR protocols | Overall 2.06%. Regions of non-zero seroprevalence: Chalkidiki (5.82%), Evros (3.57%), Imathia (2.7%), Kastoria (2.43%), Kavala (1.56%), Pella (5.35%), Serres (5.45%), Xanthi (2.94%) | |
| Jan 2003–May 2006 | 302 | Hospital patients with CNS infection | 0% | ||||
| CCHF | [57] | 1980–1981 | Imathia (Central Macedonia) | 65 | Not specified | IFA and HI | 6.2% |
| [30] | Apr 1987–Apr 1988 | Northern Greece (Evros, Rodopi, Halkidiki, Ioannina) | 597 | Not specified | IFA and ELISA | 1.2% | |
| [47] | 1981–1988 | Mainland Greece, islands of Corfu and Crete | 3388 | Apparently healthy individuals (farmers, wood cutters & shepherds) | IFA | Overall 1%. Regions of non-zero seroprevalence: Rodopi (0.5%), Xanthi (1.2%), Kilkis (2%), Pella (9.6%), Imathia (4.3%), Evritania (1%), Larisa (2%), Karditsa (6.2%), Ioannina (1.6%), Laconia (1%), Iraklio (1.2%). Corfu (1%) | |
| [52] | Jun 2009–Dec 2010 | Country-wide (28 prefectures) | 1611 | Random sampling of patients referred to primary health-care centers for blood testing with no signs of infectious disease | ELISA | Overall 4.2%. Regions of non-zero seroprevalence: Thesprotia (27.5%), Grevena (15.4%), Fthiotida (11.1%), Etoloakarnania (8.0%), Ioannina (8.3%), Evritania (7.5%), Kastoria (7.0%), Viotia (6.8%), Arkadia (6.0%), Lakonia (5.9%), Rodopi (4.95%), Evros (4.49%), Lasithi (4.2%), Dodecanese (3.0%), Serres (3.0%), Lesbos (2%), Magnesia (2.0%), Kilkis (2.3%), Corfu (2.0%), Florina (2.1%), Pella (2.0%), Xanthi (1.99%), Drama (1.34%), Attiki (1.2%) | |
| [53] | 2010–2012 | Thesprotia prefecture | 166 | Permanent residents randomly selected among persons who visited local health centers | ELISA | 14.4% | |
| [55] | Mar–Jul 2012 | Achaia prefecture | 207 | Random sampling of patients referred to primary health-care centers for blood testing with no signs of infectious disease | ELISA, confirmation by IFA | 3.4% | |
| [62] | 2010–2011 | Imathia and Pella prefectures | 277 | Random selection of residents who visited local health centers for testing (not for infectious diseases) or blood donation | ELISA, confirmation by IFA | 2.2% | |
| 51 | People belonging in groups of risk for CCHFV (19 slaughterhouse workers, 32 hunters) | 3.1% in hunters, 5.3% in slaughterhouse workers | |||||
| [33] | Sep–Oct 2012 | Kastoria regional unit | 100 | People who visited local health center with no signs of infectious disease | ELISA, confirmation by IFA | 6% | |
| [54] | Nov 2008–Apr 2009 | Northern Greece (Drama, Kavala, Xanthi, Rodopi, and Evros prefectures) | 1178 | Random sampling of patients referred to primary health-care centers for blood testing, regardless of reason for testing and CCHFV risk factors | ELISA | Overall 3.14%. Regions of non-zero seroprevalence: Rodopi 4.95%, Evros 4.49%, Drama 1.34%, Xanthi 1.09% | |
| MSF | [65] | 1985 and 1987 | 2 villages in Crete (Iraklio and Rethimno prefectures) | 419 | Random selection of 97 households | IFA | Tymbaki village: 5.6%, 2.2% after 2 years Anogia village 2: 0.5%, 0% after 2 years |
| [67] | Oct 1998 | Anogia village, Rethimno prefecture, Crete | 238 | Random selection of 84 households | IFA | 7.6% | |
| [66] | 1991 | 3 rural villages in Fokida province | 254 | Not specified | IFA and Western blot | 45.3% | |
| [68] | Apr–Oct 2000 | Northern Greece (14 prefectures) | 1584 | Random selection of samples collected by prefecture hospitals | IFA | Overall 7.9%. Regions of non-zero seroprevalence: Drama (7%), Florina (2.1%), Grevena (14%), Imathia (3.1%), Kastoria (10%), Kavala (1%), Khalkidiki (7.3%), Kilkis (3%), Kozani (10%), Pella (8%), Pieria (1.1%), Serres (14%), Thessaloniki (2%), Trikala (20%) | |
| HGA | [77] | Apr–Oct 2000 | Northern Greece | 300 | Healthy individuals hospitalized for routine blood tests | IFA | 7.3% |
| [76] | Aug 2005–Aug 2006 | Crete | 496 | Healthy blood donors | IFA | 21.4% | |
| LB | [79] | Sep 1997–Nov 1998 | Country-wide | 1100 | Randomly selected healthy Greek Navy recruits (18–25 years old) | ELISA | 3.27% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efstratiou, A.; Karanis, G.; Karanis, P. Tick-Borne Pathogens and Diseases in Greece. Microorganisms 2021, 9, 1732. https://doi.org/10.3390/microorganisms9081732
Efstratiou A, Karanis G, Karanis P. Tick-Borne Pathogens and Diseases in Greece. Microorganisms. 2021; 9(8):1732. https://doi.org/10.3390/microorganisms9081732
Chicago/Turabian StyleEfstratiou, Artemis, Gabriele Karanis, and Panagiotis Karanis. 2021. "Tick-Borne Pathogens and Diseases in Greece" Microorganisms 9, no. 8: 1732. https://doi.org/10.3390/microorganisms9081732
APA StyleEfstratiou, A., Karanis, G., & Karanis, P. (2021). Tick-Borne Pathogens and Diseases in Greece. Microorganisms, 9(8), 1732. https://doi.org/10.3390/microorganisms9081732







