Laboratory In-Situ Production of Autochthonous and Allochthonous Fluorescent Organic Matter by Freshwater Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Environmental Freshwater Sampling Location and Collection
2.2. Environmental Freshwater Microbial Cultures
2.2.1. Environmental Microbial Community Inoculum
2.2.2. Isolation of Environmental Bacterial Strains
2.3. Minimal Media Composition
2.4. Bacterial Culture Analysis
2.5. Fluorescence Measurements
2.5.1. Fluorescence Data Analysis
3. Results
3.1. Environmental Microbial Community Cultures
3.2. Environmental Bacterial Isolate Monocultures
4. Discussion
4.1. FOM Production Potential of a Freshwater Environmental Microbial Community
4.2. FOM Production by Environmental Freshwater Bacterial Monocultures
4.3. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aiken, G.R. Fluorescence and Dissolved Organic Matter. In Aquatic Organic Matter Fluorescence; Baker, A., Reynolds, D.M., Lead, J.R., Coble, P.G., Spencer, R.G.M., Eds.; Cambridge Environmental Chemistry Series; Cambridge University Press: Cambridge, UK, 2014; pp. 35–74. ISBN 9780521764612. [Google Scholar]
- Pan, Q.; Zhuo, X.; He, C.; Zhang, Y.; Shi, Q. Validation and Evaluation of High-Resolution Orbitrap Mass Spectrometry on Molecular Characterization of Dissolved Organic Matter. ACS Omega 2020, 5, 5372–5379. [Google Scholar] [CrossRef] [Green Version]
- Lavonen, E.E.; Kothawala, D.N.; Tranvik, L.J.; Gonsior, M.; Schmitt-Kopplin, P.; Köhler, S.J. Tracking changes in the optical properties and molecular composition of dissolved organic matter during drinking water production. Water Res. 2015, 85, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, A.; Lapierre, J.; Berggren, M.; Prairie, Y.T.; Dittmar, T.; del Giorgio, P.A. What’s in an EEM? Molecular Signatures Associated with Dissolved Organic Fluorescence in Boreal Canada. Environ. Sci. Technol. 2014, 48, 105598–110606. [Google Scholar] [CrossRef] [PubMed]
- Lechtenfeld, O.J.; Kattner, G.; Flerus, R.; McCallister, S.L.; Schmitt-Kopplin, P.; Koch, B.P. Molecular transformation and degradation of refractory dissolved organic matter in the Atlantic and Southern Ocean. Geochim. Cosmochim. Acta 2014, 126, 321–337. [Google Scholar] [CrossRef] [Green Version]
- Hudson, N.; Baker, A.; Reynolds, D.M. Fluorescence Analysis of Dissolved Organic Matter in Natural, Waste and Polluted Water—A Review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Murphy, K.R.; Hambly, A.; Stuetz, R.M.; Khan, S.J. Fluorescence as a potential monitoring tool for recycled water systems: A review. Water Res. 2009, 43, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G.; Lead, J.; Baker, A.; Reynolds, D.M.; Spencer, R.G.M. Aquatic Organic Matter Fluorescence; Cambridge University Press: Cambridge, UK, 2014; ISBN 9780521764612. [Google Scholar]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence spectroscopy for wastewater monitoring: A review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Khamis, K.; Bradley, C.; Hannah, D.M. Understanding dissolved organic matter dynamics in urban catchments: Insights from in situ fluorescence sensor technology. Wiley Interdiscip. Rev. Water 2017, 5, e1259. [Google Scholar] [CrossRef] [Green Version]
- Goto, S.; Tada, Y.; Suzuki, K.; Yamashita, Y. Production and reutilization of fluorescent dissolved organic matter by a marine bacterial strain, Alteromonas macleodii. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.R.; Fish, T.A.E.; Playle, R.C.; Smith, D.S.; Curtis, P.J. Photodegradation of natural organic matter from diverse freshwater sources. Aquat. Toxicol. 2007, 84, 215–222. [Google Scholar] [CrossRef]
- Yamashita, Y.; Boyer, J.N.; Jaffé, R. Evaluating the distribution of terrestrial dissolved organic matter in a complex coastal ecosystem using fluorescence spectroscopy. Cont. Shelf Res. 2013, 66, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Osburn, C.; Del Vecchio, R.; Boyd, T. Physicochemical Effects on Dissolved Organic Matter Fluorescence in Natural Waters. In Aquatic Organic Matter Fluorescence; Coble, P.G., Lead, J., Baker, A., Reynolds, D.M., Spencer, R.G.M., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 233–277. ISBN 978-0-521-76461-2. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 0387312781. [Google Scholar]
- Stolpe, B.; Zhou, Z.; Guo, L.; Shiller, A.M. Colloidal size distribution of humic- and protein-like fluorescent organic matter in the northern Gulf of Mexico. Mar. Chem. 2014, 164, 25–37. [Google Scholar] [CrossRef]
- Carstea, E.M. Fluorescence Spectroscopy as a Potential Tool for In-situ Monitoring of Dissolved Organic Matter in Surface Water Systems. In Water Pollution; Balkis, N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 47–68. ISBN 978-953-307-962-2. [Google Scholar]
- Shimotori, K.; Omori, Y.; Hama, T. Bacterial production of marine humic-like fluorescent dissolved organic matter and its biogeochemical importance. Aquat. Microb. Ecol. 2009, 58, 55–66. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Zhou, T.H.; Du, Y.; Ye, B.; Wang, W.L.; Hu, H.Y. Characterizing the molecular weight distribution of dissolved organic matter by measuring the contents of electron-donating moieties, UV absorbance, and fluorescence intensity. Environ. Int. 2020, 137, 105570. [Google Scholar] [CrossRef]
- Mayorga, E.; Aufdenkampe, A.K. Processing of Bioactive Elements in The Amazon River Basin. In The Ecohydrology of South American Rivers and Wetlands; McClain, M.E., Ed.; IAHS Press: Wallingford, UK, 2002; p. 209. [Google Scholar]
- Malik, A.A.; Roth, V.N.; Hébert, M.; Tremblay, L.; Dittmar, T.; Gleixner, G. Linking molecular size, composition and carbon turnover of extractable soil microbial compounds. Soil Biol. Biochem. 2016, 100, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Amon, R.M.W.; Benner, R. Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 1996, 41, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Schnitzer, M.; Riffaldi, R. The Determination of Quinone Groups in Humic Substances. Soil Sci. Soc. Am. J. 1972, 36, 772–777. [Google Scholar] [CrossRef]
- Asmala, E.; Massicotte, P.; Carstensen, J. Identification of dissolved organic matter size components in freshwater and marine environments. Limnol. Oceanogr. 2021, 66, 1381–1393. [Google Scholar] [CrossRef]
- Yue, L.; Wu, F.; Liu, C.; Li, W.; Wang, J.; Mei, Y. Molecular weight distribution of dissolved organic matter in Lake Hongfeng determined by high performance size exclusion chromatography (HPSEC) with on-line UV-vis absorbance and fluorescence detection. Chin. J. Geochem. 2004, 23, 275–283. [Google Scholar]
- Sorensen, J.P.R.; Baker, A.; Cumberland, S.A.; Lapworth, D.J.; MacDonald, A.M.; Pedley, S.; Taylor, R.G.; Ward, J.S.T. Real-time detection of faecally contaminated drinking water with tryptophan-like fluorescence: Defining threshold values. Sci. Total Environ. 2018, 622–623, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.P.R.; Lapworth, D.J.; Read, D.S.; Nkhuwa, D.C.W.; Bell, R.A.; Chibesa, M.; Chirwa, M.; Kabika, J.; Liemisa, M.; Pedley, S. Tracing enteric pathogen contamination in sub-Saharan African groundwater. Sci. Total Environ. 2015, 538, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Shin, H.S.; Hur, J. Estimating the concentration and biodegradability of organic matter in 22 wastewater treatment plants using fluorescence excitation emission matrices and parallel factor analysis. Sensors 2014, 14, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.P.R.; Diaw, M.T.; Pouye, A.; Roffo, R.; Diongue, D.M.L.; Faye, S.C.; Gaye, C.B.; Fox, B.G.; Goodall, T.; Lapworth, D.J.; et al. In-situ fluorescence spectroscopy indicates total bacterial abundance and dissolved organic carbon. Sci. Total Environ. 2020, 738, 139419. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.P.R.; Carr, A.F.; Nayebare, J.; Diongue, D.M.L.; Pouye, A.; Roffo, R.; Gwengweya, G.; Ward, J.S.T.; Kanoti, J.; Okotto, J.O.; et al. Tryptophan-like and humic-like fluorophores are extracellular in groundwater: Implications as real-time faecal indicators. Sci. Rep. 2020, 10, 15379. [Google Scholar] [CrossRef]
- Goto, S.; Tada, Y.; Suzuki, K.; Yamashita, Y. Evaluation of the Production of Dissolved Organic Matter by Three Marine Bacterial Strains. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Repeta, D.J. Chapter 2—Chemical Characterization and Cycling of Dissolved Organic Matter A2. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Academic Press: Boston, MA, USA, 2015; pp. 21–63. ISBN 978-0-12-405940-5. [Google Scholar]
- Carlson, C.A.; Hansell, D.A. Chapter 3—DOM Sources, Sinks, Reactivity, and Budgets. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Academic Press: Boston, MA, USA, 2015; pp. 65–126. ISBN 978-0-12-405940-5. [Google Scholar]
- Lønborg, C.; Álvarez-Salgado, X.A.; Davidson, K.; Miller, A.E.J. Production of bioavailable and refractory dissolved organic matter by coastal heterotrophic microbial populations. Estuar. Coast. Shelf Sci. 2009, 82, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Arai, K.; Wada, S.; Shimotori, K.; Omori, Y.; Hama, T. Production and degradation of fluorescent dissolved organic matter derived from bacteria. J. Oceanogr. 2018, 74, 39–52. [Google Scholar] [CrossRef]
- Koch, B.P.; Kattner, G.; Witt, M.; Passow, U. Molecular insights into the microbial formation of marine dissolved organic matter: Recalcitrant or labile? Biogeosciences 2014, 11, 4173–4190. [Google Scholar] [CrossRef] [Green Version]
- Elliott, S.; Lead, J.R.; Baker, A. Characterisation of the fluorescence from freshwater, planktonic bacteria. Water Res. 2006, 40, 2075–2083. [Google Scholar] [CrossRef]
- Fox, B.G.; Thorn, R.M.S.; Anesio, A.M.; Reynolds, D.M. The in situ bacterial production of fluorescent organic matter; an investigation at a species level. Water Res. 2017, 125, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.G.; Thorn, R.M.S.; Anesio, A.M.; Cox, T.; Attridge, J.W.; Reynolds, D.M. Microbial Processing and Production of Aquatic Fluorescent Organic Matter in a Model Freshwater System. Water 2019, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Marx, A.; Dusek, J.; Jankovec, J.; Sanda, M.; Vogel, T.; van Geldern, R.; Hartmann, J.; Barth, J.A.C. A review of CO2 and associated carbon dynamics in headwater streams: A global perspective. Rev. Geophys. 2017, 55, 560–585. [Google Scholar] [CrossRef]
- Brailsford, F.L.; Glanville, H.C.; Golyshin, P.N.; Johnes, P.J.; Yates, C.A.; Jones, D.L. Microbial uptake kinetics of dissolved organic carbon (DOC) compound groups from river water and sediments. Sci. Rep. 2019, 9, 11229. [Google Scholar] [CrossRef]
- Ridgwell, A.; Arndt, S. Why Dissolved Organics Matter: DOC in Ancient Oceans and Past Climate Change. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 1–20. [Google Scholar]
- Biddanda, B. Global Significance of the Changing Freshwater Carbon Cycle. Eos 2017. [Google Scholar] [CrossRef]
- Sigee, D.C. Freshwater Microbiology; Wiley: New York, NY, USA, 2004; ISBN 978-0-471-48529-2. [Google Scholar]
- Black, J.G. Microbiology: Principles and Explorations, 6th ed.; Wiley: New York, NY, USA, 2005; ISBN 9780471420842. [Google Scholar]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; ISBN 0683035183. [Google Scholar]
- Davis, B.D.; Mingioli, E.S. Mutants of Escherichia coli requiring methionine or vitamin B12. J. Bacteriol. 1950, 60, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kragelund, L.; Nybroe, O. Culturability and Expression of Outer Membrane Proteins during Carbon, Nitrogen, or Phosphorus Starvation of Pseudomonas fluorescens DF57 and Pseudomonas putida DF14. Appl. Environ. Microbiol. 1994, 60, 2944–2948. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.; Elliott, S.; Lead, J.R. Effects of filtration and pH perturbation on freshwater organic matter fluorescence. Chemosphere 2007, 67, 2035–2043. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectroflourometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Kramer, G.D.; Herndl, G.J. Photo- and bioreactivity of chromophoric dissolved organic matter produced by marine bacterioplankton. Aquat. Microb. Ecol. 2004, 36, 239–246. [Google Scholar] [CrossRef]
- Shimotori, K.; Watanabe, K.; Hama, T. Fluorescence characteristics of humic-like fluorescent dissolved organic matter produced by various taxa of marine bacteria. Aquat. Microb. Ecol. 2012, 65, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Bro, R.; Kiers, H.A.L. A new efficient method for determining the number of components in PARAFAC models. J. Chemometr. 2003, 17, 274–286. [Google Scholar] [CrossRef]
- Asmala, E.; Kaartokallio, H.; Carstensen, J.; Thomas, D.N. Variation in Riverine Inputs Affect Dissolved Organic Matter Characteristics throughout the Estuarine Gradient. Front. Mar. Sci. 2016, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Zhao, W.; Lv, L.; Miao, H. Tracing the Variability of Dissolved Organic Matter Fluorescence in the East China Sea in the Red Tide Season with use of Excitation—Emission Matrix Spectroscopy and Parallel Factor Analysis. Mar. Sci. Res. Dev. 2013, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Koma, D.; Yamanaka, H.; Moriyoshi, K.; Ohmoto, T.; Sakai, K. Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway. Appl. Environ. Microbiol. 2012, 78, 6203–6216. [Google Scholar] [CrossRef] [Green Version]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Determann, S.; Lobbes, J.M.; Reuter, R.; Rullkötter, J. Ultraviolet fluorescence excitation and emission spectroscopy of marine algae and bacteria. Mar. Chem. 1998, 62, 137–156. [Google Scholar] [CrossRef]
- Jørgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Søndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Chen, M.; Yamashita, Y.; Jaffé, R. Fluorescence characteristics of size-fractionated dissolved organic matter: Implications for a molecular assembly based structure? Water Res. 2014, 55, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Romera-Castillo, C.; Sarmento, H.; Alvarez-Salgado, X.A.; Gasol, J.M.; Marrasé, C. Net Production and Consumption of Fluorescent Colored Dissolved Organic Matter by Natural Bacterial Assemblages Growing on Marine Phytoplankton Exudates. Appl. Environ. Microbiol. 2011, 77, 7490–7498. [Google Scholar] [CrossRef] [Green Version]
- Harun, S.; Baker, A.; Bradley, C.; Pinay, G. Spatial and seasonal variations in the composition of dissolved organic matter in a tropical catchment: The Lower Kinabatangan River, Sabah, Malaysia. Environ. Sci. Process. Impacts 2016, 18, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Markager, S. Resolving the variability of dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Roberts, T.A.; Moore, G.; Ward, J.M.; Muller, J.P. Fluorescence Characterization of Clinically-Important Bacteria. PLoS ONE 2013, 8, e75270. [Google Scholar] [CrossRef] [Green Version]
- Timko, S.A.; Maydanov, A.; Pittelli, S.L.; Conte, M.H.; Cooper, W.J.; Koch, B.P.; Schmitt-Kopplin, P.; Gonsior, M. Depth-dependent photodegradation of marine dissolved organic matter. Front. Mar. Sci. 2015, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, N.; Benner, R. Bacterial release of dissolved organic matter during cell growth and decline: Molecular origin and composition. Limnol. Oceanogr. 2006, 51, 2170–2180. [Google Scholar] [CrossRef] [Green Version]
- Suksomjit, M.; Nagao, S.; Ichimi, K.; Yamada, T.; Tada, K. Variation of dissolved organic matter and fluorescence characteristics before, during and after phytoplankton bloom. J. Oceanogr. 2009, 65, 835–846. [Google Scholar] [CrossRef]
- Zhi, E.; Yu, H.; Duan, L.; Han, L.; Liu, L.; Song, Y. Characterization of the composition of water DOM in a surface flow constructed wetland using fluorescence spectroscopy coupled with derivative and PARAFAC. Environ. Earth Sci. 2015, 73, 5153–5161. [Google Scholar] [CrossRef]
- Makarewicz, A.; Kowalczuk, P.; Sagan, S.; Granskog, M.A.; Pavlov, A.K.; Zdun, A.; Borzycka, K.; Zabłocka, M. Characteristics of Chromophoric and Fluorescent Dissolved Organic Matter in the Nordic Seas. Ocean Sci. Discuss. 2018, 14, 543–562. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, J.P.R.; Lapworth, D.J.; Marchant, B.P.; Nkhuwa, D.C.W.; Pedley, S.; Stuart, M.E.; Bell, R.A.; Chirwa, M.; Kabika, J.; Liemisa, M.; et al. In situ tryptophan-like fluorescence: A real-time indicator of faecal contamination in drinking water supplies. Water Res. 2015, 81, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridgeman, J.; Baker, A.; Brown, D.; Boxall, J.B. Portable LED fluorescence instrumentation for the rapid assessment of potable water quality. Sci. Total Environ. 2015, 524–525, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Jeppesen, E.; Zhang, Y.; Shi, K.; Liu, X.; Zhu, G. Dissolved organic matter fluorescence at wavelength 275/342 nm as a key indicator for detection of point-source contamination in a large Chinese drinking water lake. Chemosphere 2016, 144, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Protein Fluorescence. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Yamashita, Y.; Fichot, C.G.; Shen, Y.; Jaffé, R.; Benner, R. Linkages among fluorescent dissolved organic matter, dissolved amino acids and lignin-derived phenols in a river-influenced ocean margin. Front. Mar. Sci. 2015, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: A focus on förster resonance energy transfer techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef]
- Möller, M.; Denicola, A. Protein tryptophan accessibility studied by fluorescence quenching. Biochem. Mol. Biol. Educ. 2002, 30, 175–178. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Maliwal, B.P. Oxygen quenching and fluorescence depolarization of tyrosine residues in proteins. J. Biol. Chem. 1983, 258, 4794–4801. [Google Scholar] [CrossRef]
- Coble, P.G.; Del Castillo, C.E.; Avril, B. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Ziervogel, K.; Osburn, C.; Brym, A.; Battles, J.; Joye, S.; D’souza, N.; Montoya, J.; Passow, U.; Arnosti, C. Linking Heterotrophic Microbial Activities with Particle Characteristics in Waters of the Mississippi River Delta in the Aftermath of Hurricane Isaac. Front. Mar. Sci. 2016, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.C.; Evans, R.D.; Dillon, P.J. Separation and Characterization of NOM by High-Performance Liquid Chromatography and On-Line Three-Dimensional Excitation Emission Matrix Fluorescence Detection. Environ. Sci. Technol. 2003, 37, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, Z.; Li, A.; Wu, W.; Zhou, Q.; Wang, J. HPLC/HPSEC-FLD with multi-excitation/emission scan for EEM interpretation and dissolved organic matter analysis. Water Res. 2012, 47, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
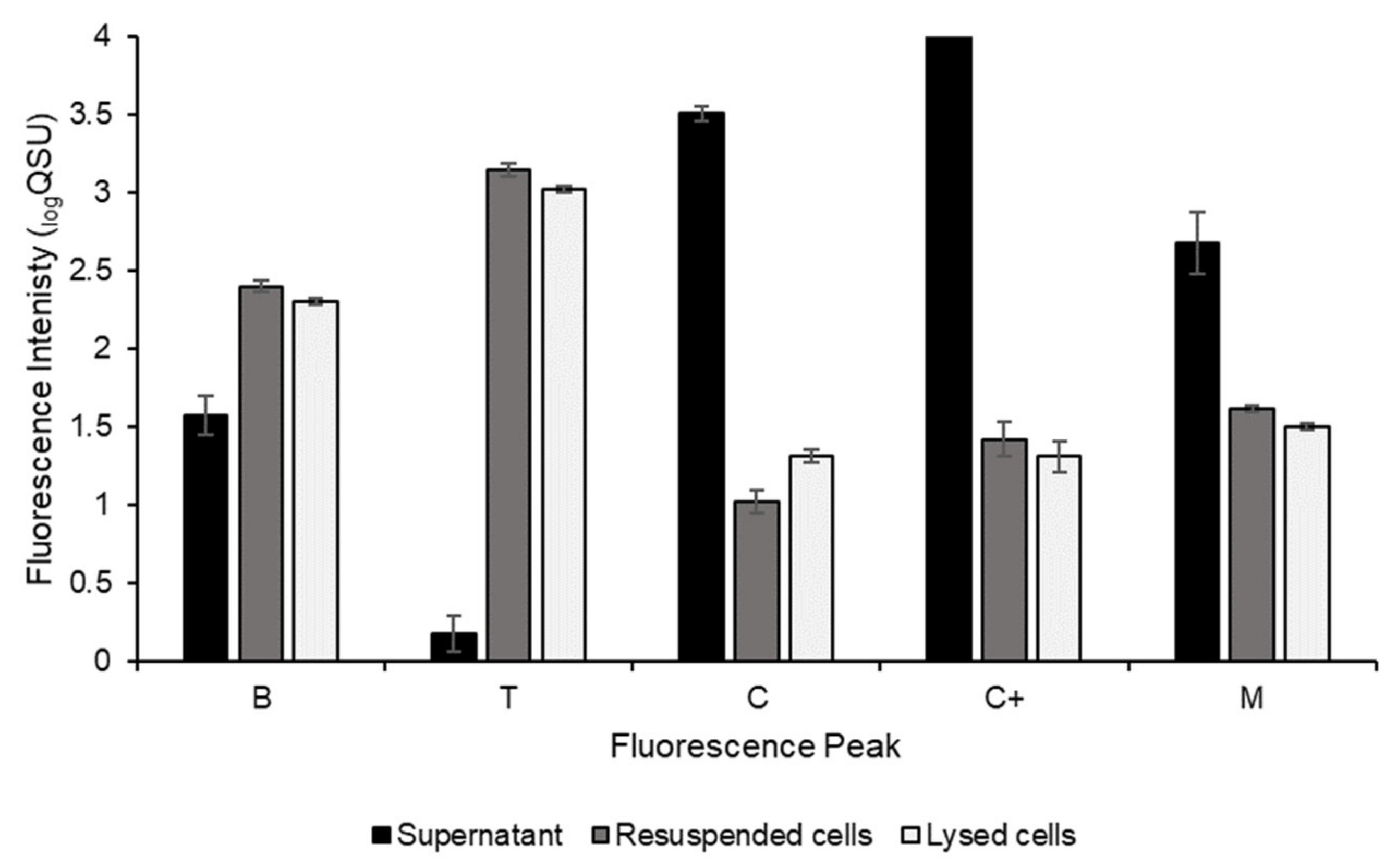
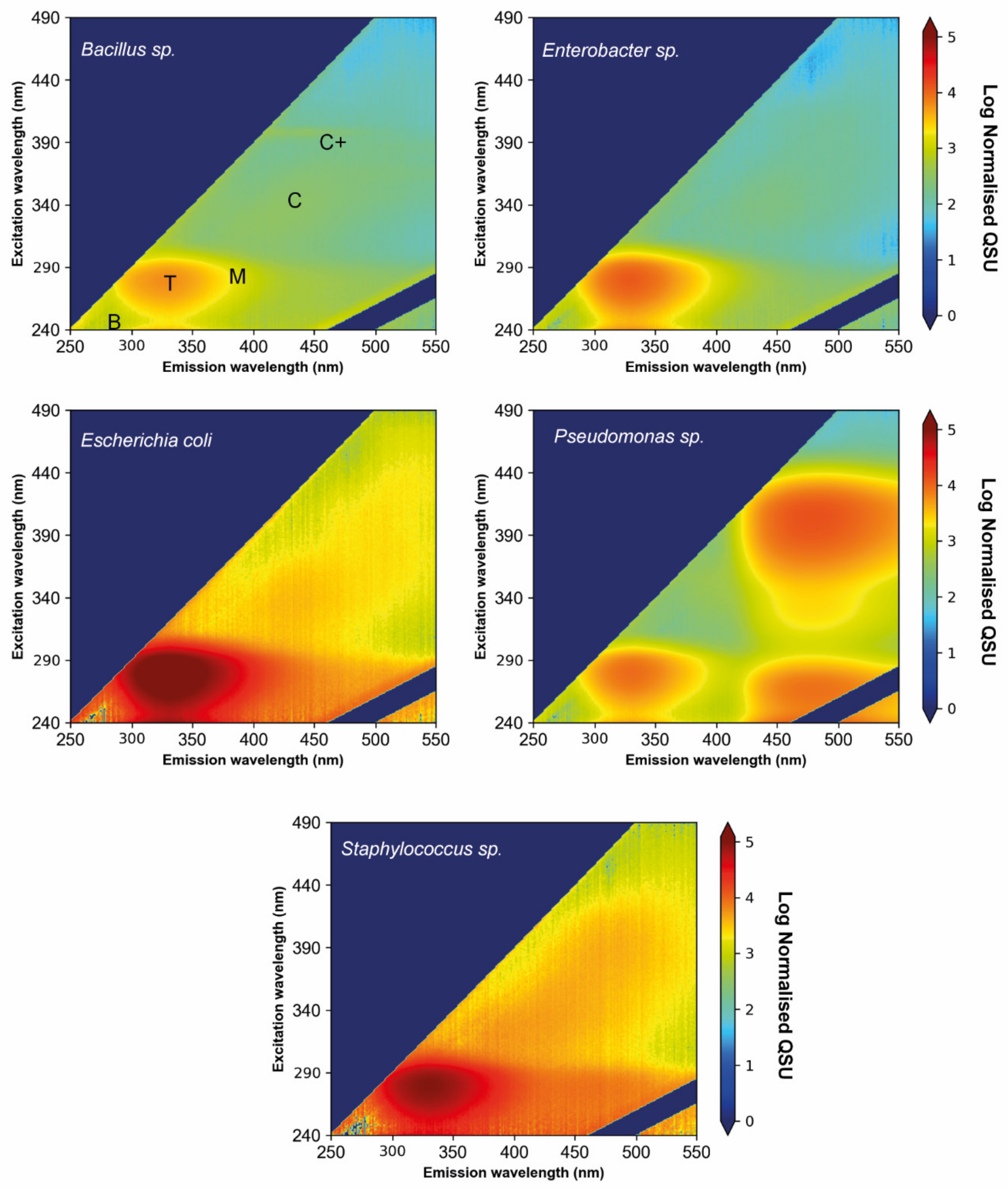
| λex/λem (nm) | Named Fluorescence Peak | Conventional Descriptions |
|---|---|---|
| 250/290–320 | B | Protein-like material, autochthonous, resembles tyrosine fluorescence, associated with amino acids |
| 280/320–360 | T | Protein-like material, autochthonous, resembles tryptophan fluorescence, associated with microbial processes |
| 300/380–420 | M | Humic-like material, autochthonous, associated with microbial degradation |
| 345/420–460 | C | Humic-like material, allochthonous, terrestrial in origin, resembles humic acids |
| 400/440–490 | C+ | Humic-like material, allochthonous, terrestrial in origin, resembles humic acids |
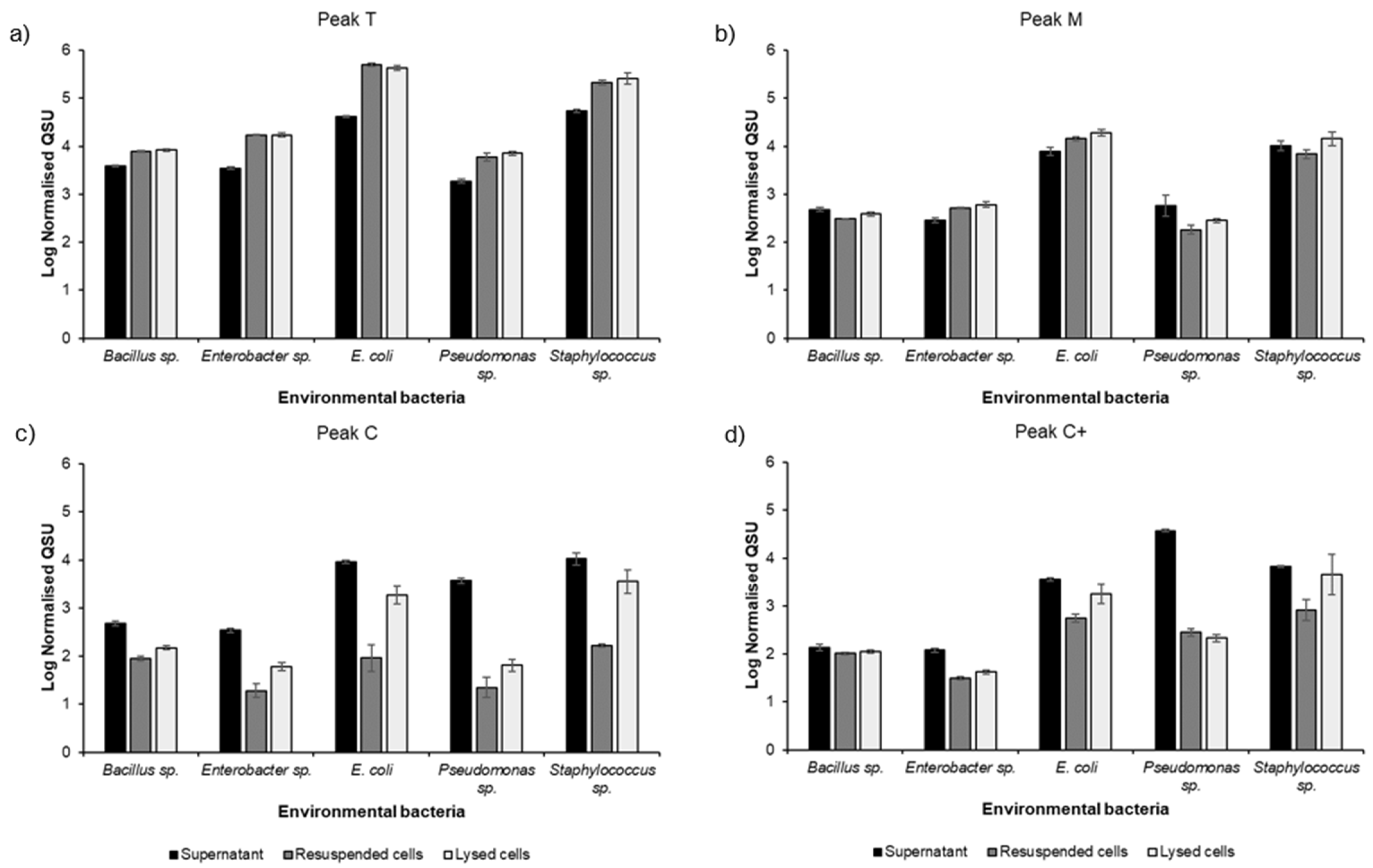
| Bacterial Isolates | Fluorescence Peaks (T, C, C+ & M) | ||
|---|---|---|---|
| Total Relative Fluorescence Quantum Yield (%) | % Extracellular | % Cellular | |
| E. coli | 100.00 | 11.46 | 88.54 |
| Staphylococcus sp. | 68.95 | 30.97 | 69.03 |
| Pseudomonas sp. | 9.25 | 85.91 | 14.09 |
| Enterobacter sp. | 4.04 | 19.21 | 80.79 |
| Bacillus sp. | 2.50 | 36.27 | 63.73 |
| Bacterial Isolates | ||||||
|---|---|---|---|---|---|---|
| Fluorescence Peaks | E. coli | Staphylococcus sp. | Pseudomonas sp. | Enterobacter sp. | Bacillus sp. | |
| Peak T | Relative contribution % | 92.71 | 88.12 | 16.77 | 93.66 | 87.69 |
| % Extracellular | 8.24 | 26.50 | 22.28 | 16.85 | 32.16 | |
| % Cellular | 91.76 | 73.50 | 77.72 | 83.15 | 67.84 | |
| Peak M | Relative contribution % | 4.52 | 5.69 | 1.71 | 3.86 | 6.14 |
| % Extracellular | 31.83 | 49.04 | 70.41 | 33.53 | 58.26 | |
| % Cellular | 68.17 | 50.96 | 29.59 | 66.47 | 41.74 | |
| Peak C | Relative contribution % | 1.88 | 3.45 | 7.40 | 1.76 | 4.36 |
| % Extracellular | 89.94 | 83.35 | 98.76 | 89.66 | 79.51 | |
| % Cellular | 10.06 | 16.65 | 1.24 | 10.34 | 20.49 | |
| Peak C+ | Relative contribution % | 0.89 | 2.74 | 74.12 | 0.72 | 1.81 |
| % Extracellular | 74.78 | 68.23 | 99.32 | 76.47 | 55.53 | |
| % Cellular | 25.22 | 31.77 | 0.68 | 23.53 | 44.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fox, B.G.; Thorn, R.M.S.; Reynolds, D.M. Laboratory In-Situ Production of Autochthonous and Allochthonous Fluorescent Organic Matter by Freshwater Bacteria. Microorganisms 2021, 9, 1623. https://doi.org/10.3390/microorganisms9081623
Fox BG, Thorn RMS, Reynolds DM. Laboratory In-Situ Production of Autochthonous and Allochthonous Fluorescent Organic Matter by Freshwater Bacteria. Microorganisms. 2021; 9(8):1623. https://doi.org/10.3390/microorganisms9081623
Chicago/Turabian StyleFox, Bethany G., Robin M. S. Thorn, and Darren M. Reynolds. 2021. "Laboratory In-Situ Production of Autochthonous and Allochthonous Fluorescent Organic Matter by Freshwater Bacteria" Microorganisms 9, no. 8: 1623. https://doi.org/10.3390/microorganisms9081623
APA StyleFox, B. G., Thorn, R. M. S., & Reynolds, D. M. (2021). Laboratory In-Situ Production of Autochthonous and Allochthonous Fluorescent Organic Matter by Freshwater Bacteria. Microorganisms, 9(8), 1623. https://doi.org/10.3390/microorganisms9081623








