Antiretroviral Therapy Dampens Mucosal CD4+ T Lamina Propria Lymphocytes Immune Activation in Long-Term Treated People Living with HIV-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Partecipants
2.2. Sample Collection
2.3. Specimen Processing
2.4. Cell Activation, Surface Marker and Intracellular Marker Staining
2.5. Virological Analysisg
2.6. Statistical Analysis
3. Results
3.1. Study Population
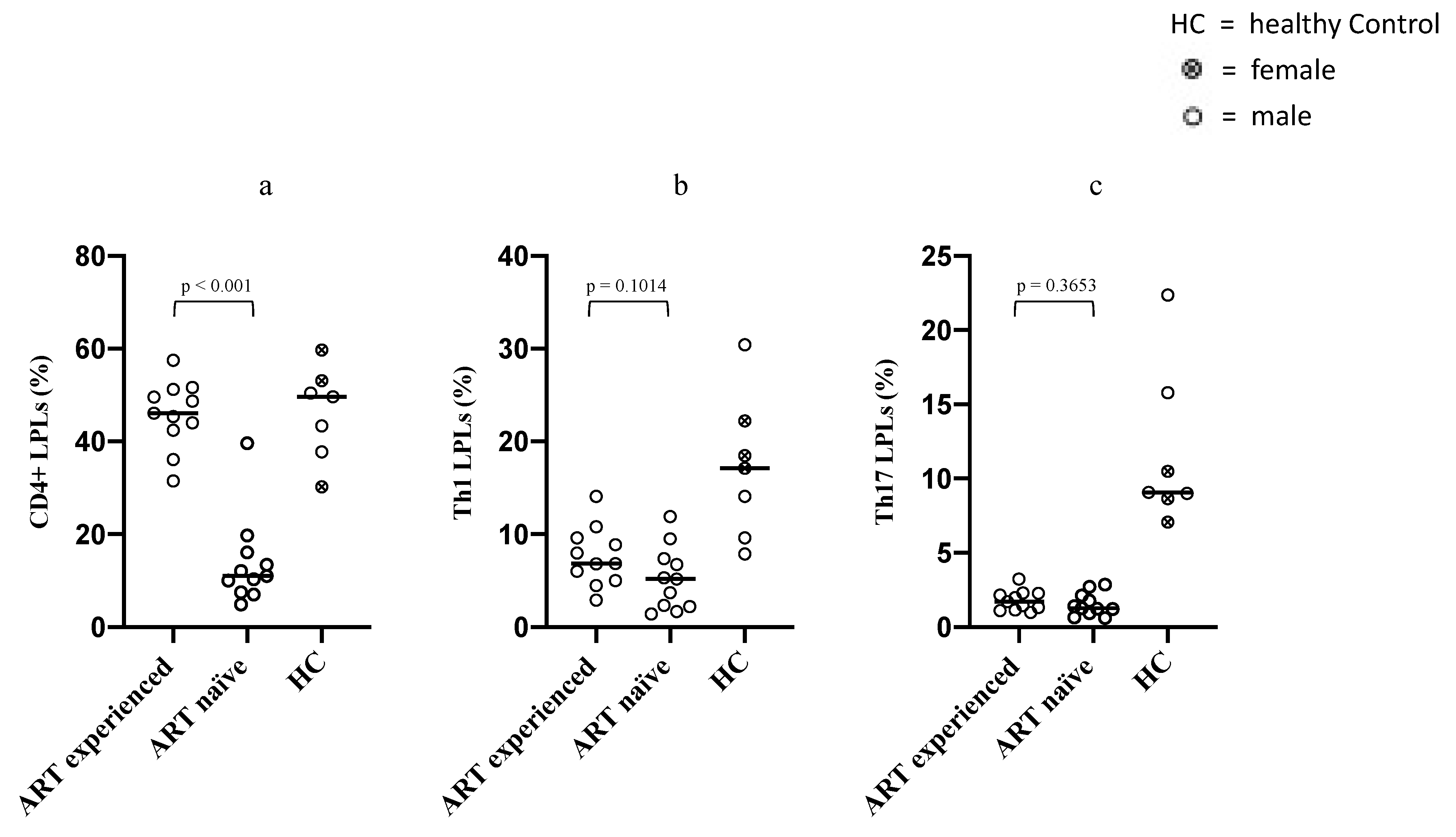
3.2. Differences in Intestinal Overall CD4+ LPLs, Th1 and Th17 LPLs between ART-Naïve and Long-Term ART-Treated PLWH
3.3. Differences in the Immune Activation Status of Intestinal CD4+ LPLs between ART-Naïve and Long-Term ART-Treated PLWH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okoye, A.A.; Picker, L.J. CD4(+) T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Douek, D.C. HIV disease: Fallout from a mucosal catastrophe? Nat. Immunol. 2006, 7, 235–239. [Google Scholar] [CrossRef]
- Haase, A.T. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 2005, 5, 783–792. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, F.; Cheroutre, H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev. Clin. Immunol. 2010, 6, 559–566. [Google Scholar] [CrossRef]
- Wacleche, V.S.; Landay, A.; Routy, J.P.; Ancuta, P. The Th17 Lineage: From Barrier Surfaces Homeostasis to Autoimmunity, Cancer, and HIV-1 Pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Estes, J.D.; Sun, X.; Ortiz, A.M.; Barber, J.S.; Harris, L.D.; Cervasi, B.; Yokomizo, L.K.; Pan, L.; Vinton, C.L.; et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012, 5, 646–657. [Google Scholar] [CrossRef]
- Nazli, A.; Chan, O.; Dobson-Belaire, W.N.; Ouellet, M.; Tremblay, M.J.; Gray-Owen, S.D.; Arsenault, A.L.; Kaushic, C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010, 6, e1000852. [Google Scholar] [CrossRef]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef]
- Sandler, N.G.; Douek, D.C. Microbial translocation in HIV infection: Causes, consequences and treatment opportunities. Nat. Rev. Microbiol. 2012, 10, 655–666. [Google Scholar] [CrossRef]
- Estes, J.D.; Harris, L.D.; Klatt, N.R.; Tabb, B.; Pittaluga, S.; Paiardini, M.; Barclay, G.R.; Smedley, J.; Pung, R.; Oliveira, K.M.; et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010, 6, e1001052. [Google Scholar] [CrossRef]
- Et Sabin, C.A.; Lundgren, J.D. The natural history of HIV infection. Curr. Opin. HIV AIDS 2013, 8, 311–317. [Google Scholar] [CrossRef]
- Suthar, A.B.; Granich, R.M.; Kato, M.; Nsanzimana, S.; Montaner, J.S.G.; Williams, B. Programmatic Implications of Acute and Early HIV Infection. J. Infect. Dis. 2015, 212, 1351–1360. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Paiardini, M.; Müller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Sokoya, T.; Steel, H.C.; Nieuwoudt, M.; Rossouw, T.M. HIV as a Cause of Immune Activation and Immunosenescence. Mediat. Inflamm. 2017, 2017, 6825493. [Google Scholar] [CrossRef]
- Lederman, M.M.; Funderburg, N.T.; Sekaly, R.P.; Klatt, N.R.; Hunt, P.W. Residual immune dysregulation syndrome in treated HIV infection. Adv. Immunol. 2013, 119, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Chege, D.; Sheth, P.M.; Kain, T.; Kim, C.J.; Kovacs, C.; Loutfy, M.; Halpenny, R.; Kandel, G.; Chun, T.-W.; Ostrowski, M.; et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS 2011, 25, 741–749. [Google Scholar] [CrossRef]
- Mavigner, M.; Cazabat, M.; Dubois, M.; L’Faqihi, F.-E.; Requena, M.; Pasquier, C.; Klopp, P.; Amar, J.; Alric, L.; Barange, K.; et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J. Clin. Investig. 2012, 122, 62–69. [Google Scholar] [CrossRef]
- Kim, C.J.; McKinnon, L.R.; Kovacs, C.; Kandel, G.; Huibner, S.; Chege, D.; Shahabi, K.; Benko, E.; Loutfy, M.; Ostrowski, M.; et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J. Immunol. 2013, 191, 2164–2173. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, H.; Wang, H.; Li, X.; Lu, X.; Liu, L.; Zhou, B.; Chen, Z. Imbalances of gut-homing CD4+ T-cell subsets in HIV-1-infected Chinese patients. J. Acquir. Immune Defic. Syndr. 2013, 64, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef]
- Yukl, S.A.; Shergill, A.K.; Girling, V.; Li, Q.; Killian, M.; Epling, L.; Li, P.; Kaiser, P.; Haase, A.; Havlir, D.V.; et al. Site-specific differences in T cell frequencies and phenotypes in the blood and gut of HIV-uninfected and ART-treated HIV+ adults. PLoS ONE 2015, 10, e0121290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehandru, S.; Poles, M.; Tenner-Racz, K.; Jean-Pierre, P.; Manuelli, V.; Lopez, P.; Shet, A.; Low, A.; Mohri, H.; Boden, D.; et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006, 3, e484. [Google Scholar] [CrossRef]
- Sheth, P.M.; Chege, D.; Shin, L.Y.Y.; Huibner, S.; Yue, F.Y.; Loutfy, M.; Halpenny, R.; Persad, D.; Kovacs, C.; Chun, T.W.; et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. 2008, 1, 382–388. [Google Scholar] [CrossRef]
- D’Ettorre, G.; Baroncelli, S.; Micci, L.; Ceccarelli, G.; Andreotti, M.; Sharma, P.; Fanello, G.; Fiocca, F.; Cavallari, E.N.; Giustini, N.; et al. Reconstitution of intestinal CD4 and Th17 T cells in antiretroviral therapy suppressed HIV-infected subjects: Implication for residual immune activation from the results of a clinical trial. PLoS ONE 2014, 9, e109791. [Google Scholar] [CrossRef][Green Version]
- D’Ettorre, G.; Borrazzo, C.; Pinacchio, C.; Santinelli, L.; Cavallari, E.N.; Statzu, M.; Fanello, G.; Ceccarelli, G.; Antonelli, G.; Vullo, V.; et al. Increased IL-17 and/or IFN-γ producing T-cell subsets in gut mucosa of long-term-treated HIV-1-infected women. AIDS 2019, 33, 627–636. [Google Scholar] [CrossRef]
- Brucklacher-Waldert, V.; Carr, E.; Linterman, M.; Veldhoen, M. Cellular Plasticity of CD4+ T Cells in the Intestine. Front. Immunol. 2014, 5, 488. [Google Scholar] [CrossRef]
- Mudd, J.C.; Brenchley, J.M. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. J. Infect. Dis. 2016, 214 (Suppl. 2), S58–S66. [Google Scholar] [CrossRef]
- Hunt, P.W. Th17, gut, and HIV: Therapeutic implications. Curr. Opin. HIV AIDS 2010, 5, 189–193. [Google Scholar] [CrossRef]
- D’Ettorre, G.; Rossi, G.; Scagnolari, C.; Andreotti, M.; Giustini, N.; Serafino, S.; Schietroma, I.; Scheri, G.C.; Fard, S.N.; Trinchieri, V.; et al. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun. Inflamm. Dis. 2017, 5, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Pinacchio, C.; Scheri, G.C.; Statzu, M.; Santinelli, L.; Ceccarelli, G.; Innocenti, G.P.; Vullo, V.; Antonelli, G.; Brenchley, J.M.; D’Ettorre, G.; et al. Type I/II Interferon in HIV-1-Infected Patients: Expression in Gut Mucosa and in Peripheral Blood Mononuclear Cells and Its Modification upon Probiotic Supplementation. J. Immunol. Res. 2018, 2018, 1738676. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.; Lee, E.J.; Kotter, C.V.; Austin, G.; Dong, Z.; Hecht, D.K.; Gianella, S.; Siewe, B.; Smith, D.; Landay, A.L.; et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014, 7, 983–994. [Google Scholar] [CrossRef]
- Dillon, S.M.; Kibbie, J.; Lee, E.J.; Guo, K.; Santiago, M.L.; Austin, G.; Gianella, S.; Landay, A.L.; Donovan, A.M.; Frank, D.N.; et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS 2017, 31, 511–521. [Google Scholar] [CrossRef] [PubMed]

| ART-Naïve (n = 11) | ART Experienced (n = 11) | |
|---|---|---|
| parameter (unit) | median (IQR 1) | median (IQR 1) |
| Age (years) | 46 (39–49) | 48 (32–56) |
| Viral load at enrollment (copies/mL) | 37,862 (7906–55,784) | <37 |
| Blood CD4+ T cells count at enrollment (cells/µL) | 412 (277–612) | 773 (708–1350) |
| Nadir 2 blood CD4+ T cells (cells/µL) | 363 (310–505) | 360 (337–485) |
| ART exposure time (years) | - | 11 (9–20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzaro, A.; Innocenti, G.P.; Santinelli, L.; Pinacchio, C.; De Girolamo, G.; Vassalini, P.; Fanello, G.; Mastroianni, C.M.; Ceccarelli, G.; d’Ettorre, G. Antiretroviral Therapy Dampens Mucosal CD4+ T Lamina Propria Lymphocytes Immune Activation in Long-Term Treated People Living with HIV-1. Microorganisms 2021, 9, 1624. https://doi.org/10.3390/microorganisms9081624
Lazzaro A, Innocenti GP, Santinelli L, Pinacchio C, De Girolamo G, Vassalini P, Fanello G, Mastroianni CM, Ceccarelli G, d’Ettorre G. Antiretroviral Therapy Dampens Mucosal CD4+ T Lamina Propria Lymphocytes Immune Activation in Long-Term Treated People Living with HIV-1. Microorganisms. 2021; 9(8):1624. https://doi.org/10.3390/microorganisms9081624
Chicago/Turabian StyleLazzaro, Alessandro, Giuseppe Pietro Innocenti, Letizia Santinelli, Claudia Pinacchio, Gabriella De Girolamo, Paolo Vassalini, Gianfranco Fanello, Claudio Maria Mastroianni, Giancarlo Ceccarelli, and Gabriella d’Ettorre. 2021. "Antiretroviral Therapy Dampens Mucosal CD4+ T Lamina Propria Lymphocytes Immune Activation in Long-Term Treated People Living with HIV-1" Microorganisms 9, no. 8: 1624. https://doi.org/10.3390/microorganisms9081624
APA StyleLazzaro, A., Innocenti, G. P., Santinelli, L., Pinacchio, C., De Girolamo, G., Vassalini, P., Fanello, G., Mastroianni, C. M., Ceccarelli, G., & d’Ettorre, G. (2021). Antiretroviral Therapy Dampens Mucosal CD4+ T Lamina Propria Lymphocytes Immune Activation in Long-Term Treated People Living with HIV-1. Microorganisms, 9(8), 1624. https://doi.org/10.3390/microorganisms9081624









