Habitats Are More Important Than Seasons in Shaping Soil Bacterial Communities on the Qinghai-Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Measurements of Environmental Factors
2.3. Total DNA Extraction and PCR Amplification
2.4. Data Processing and Statistical Analyses
3. Results
3.1. Distribution of Environmental Variables
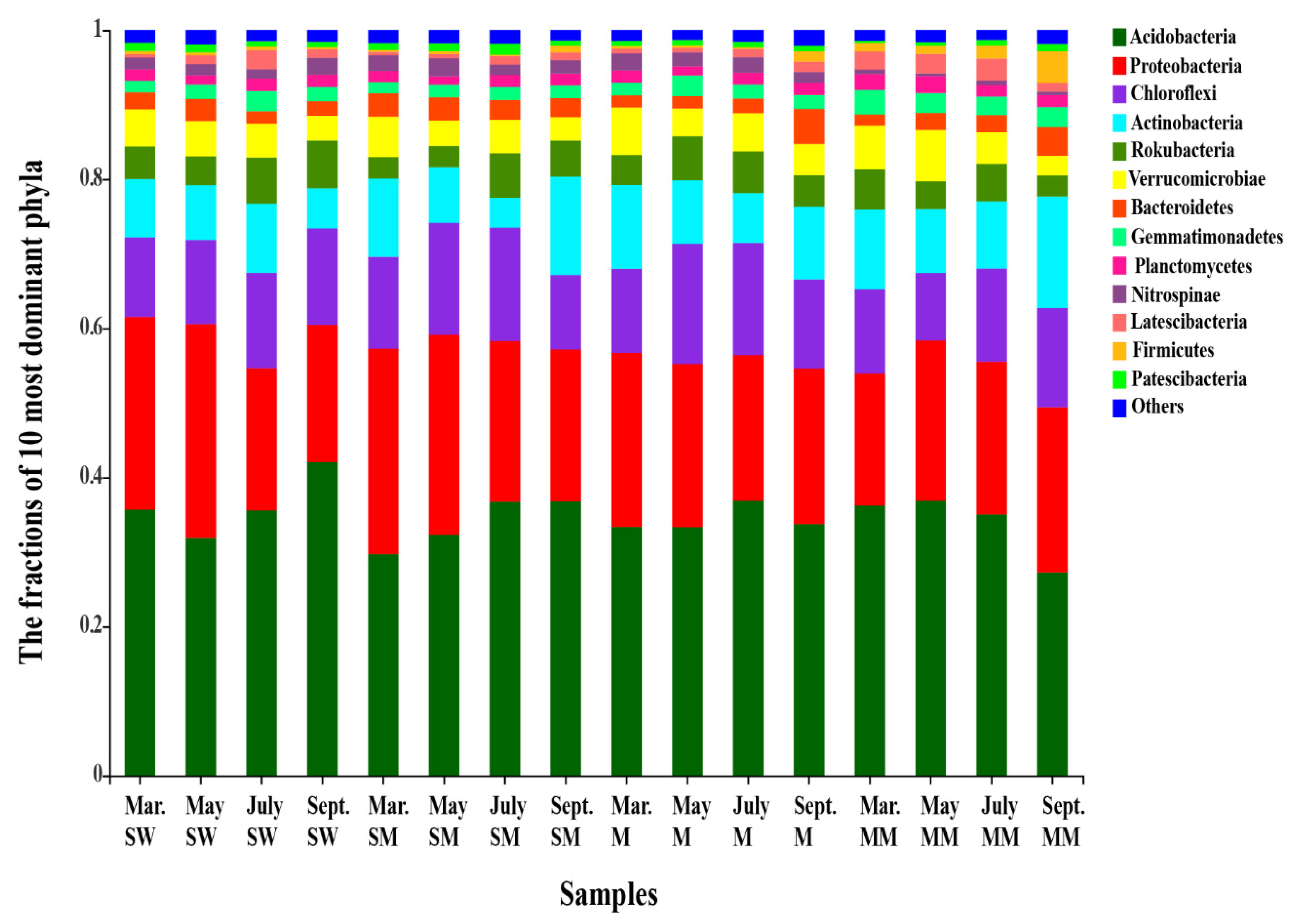
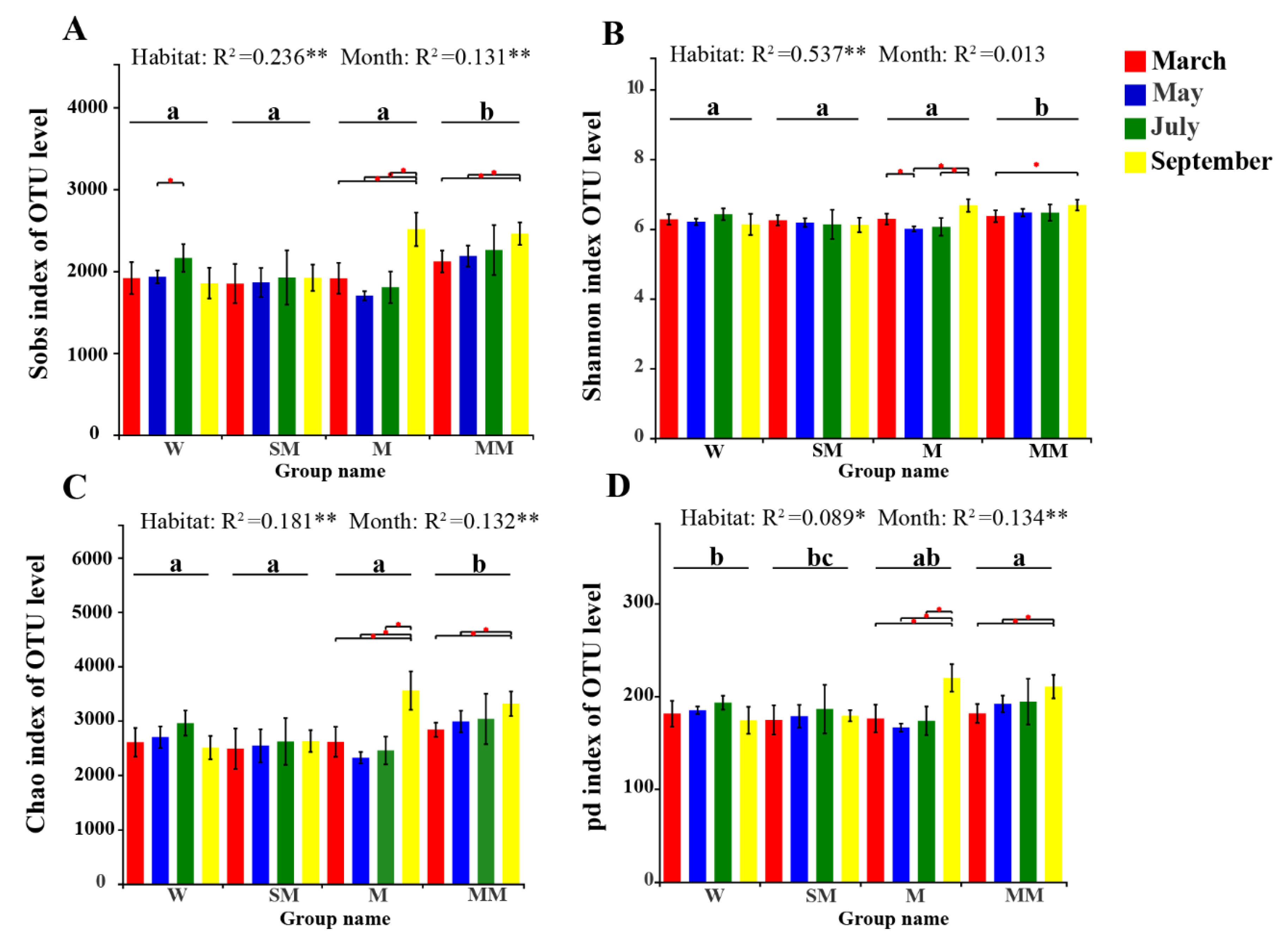
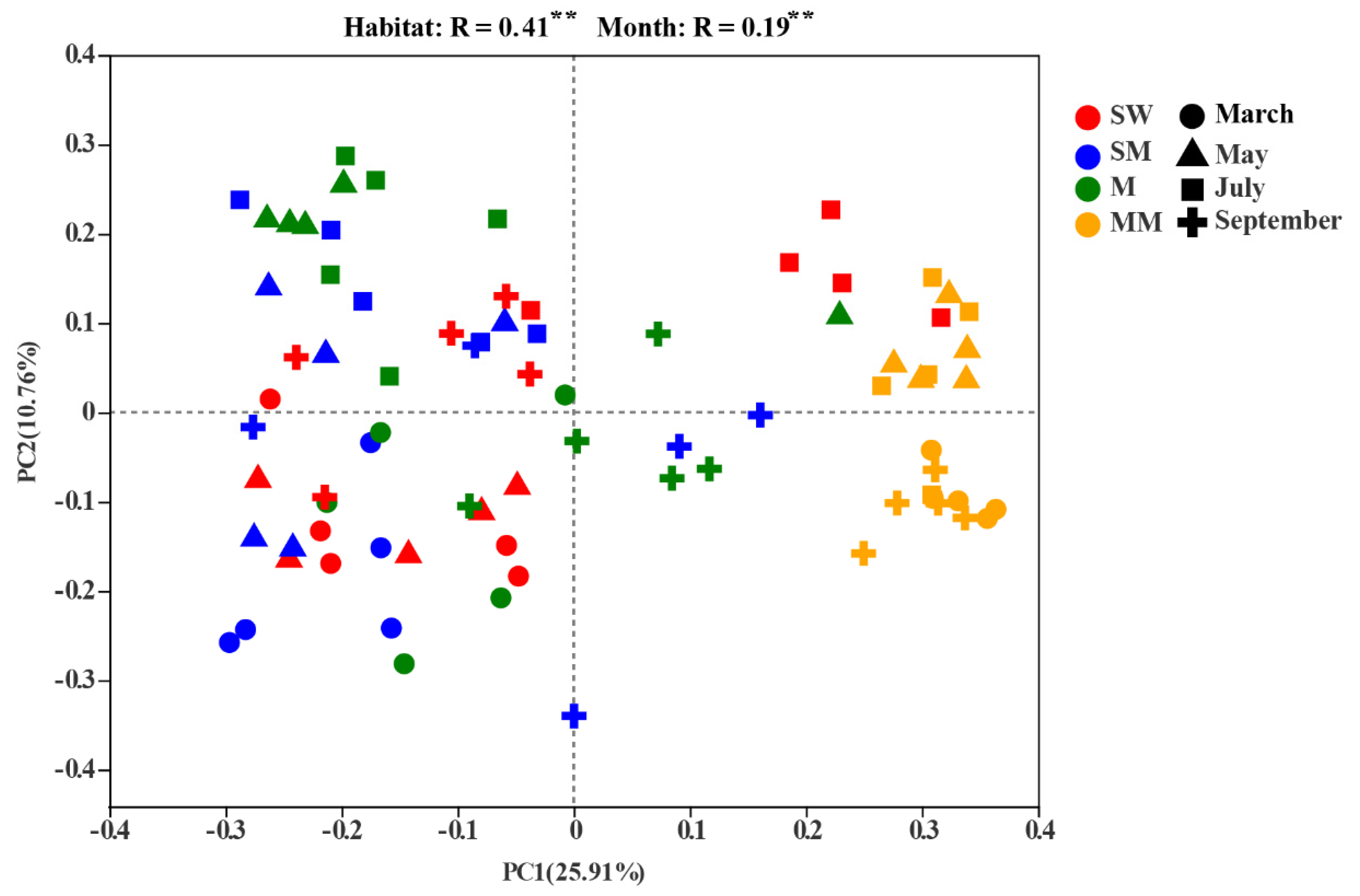
3.2. Soil Bacterial Diversities and Community Structure
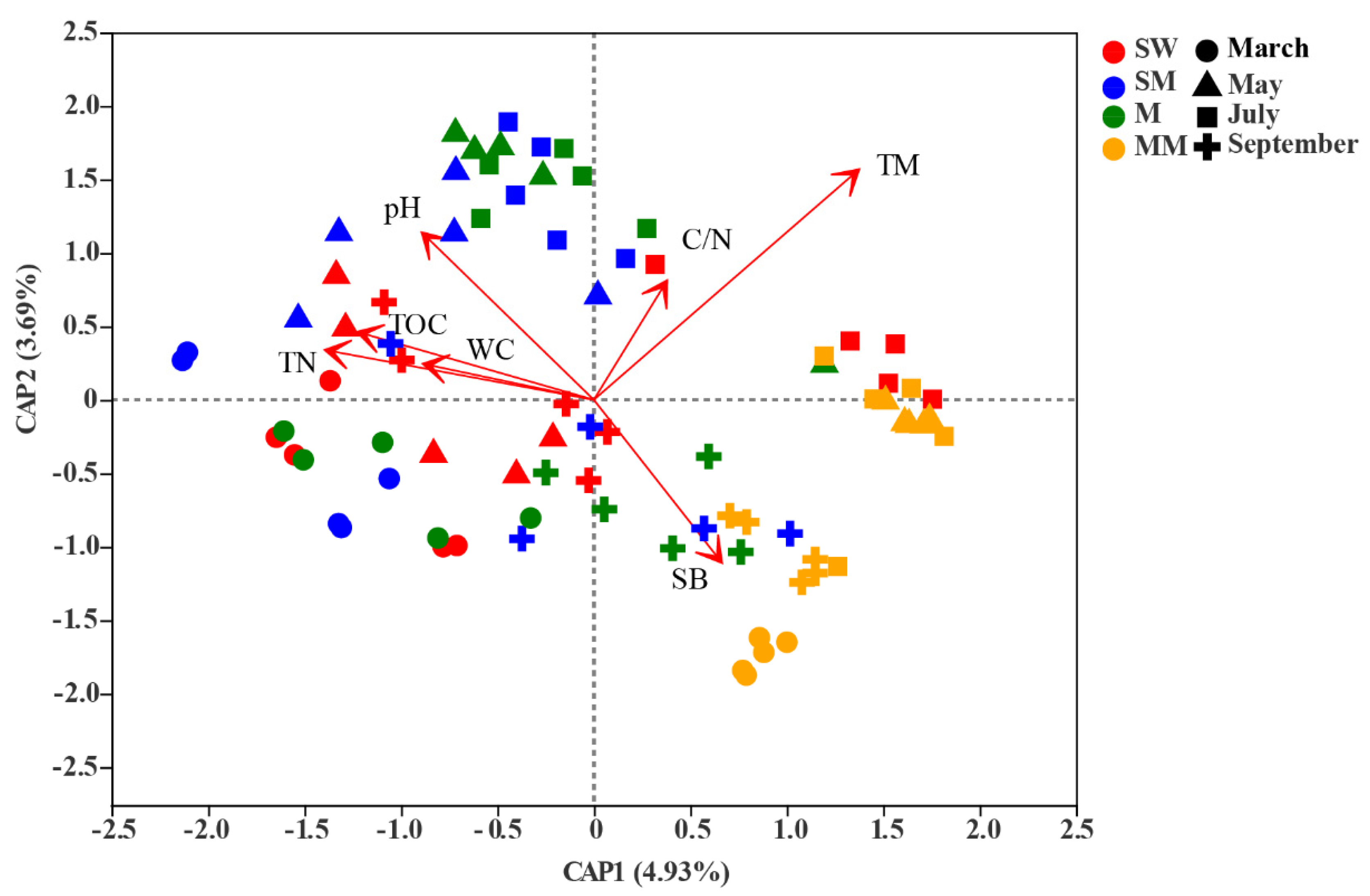
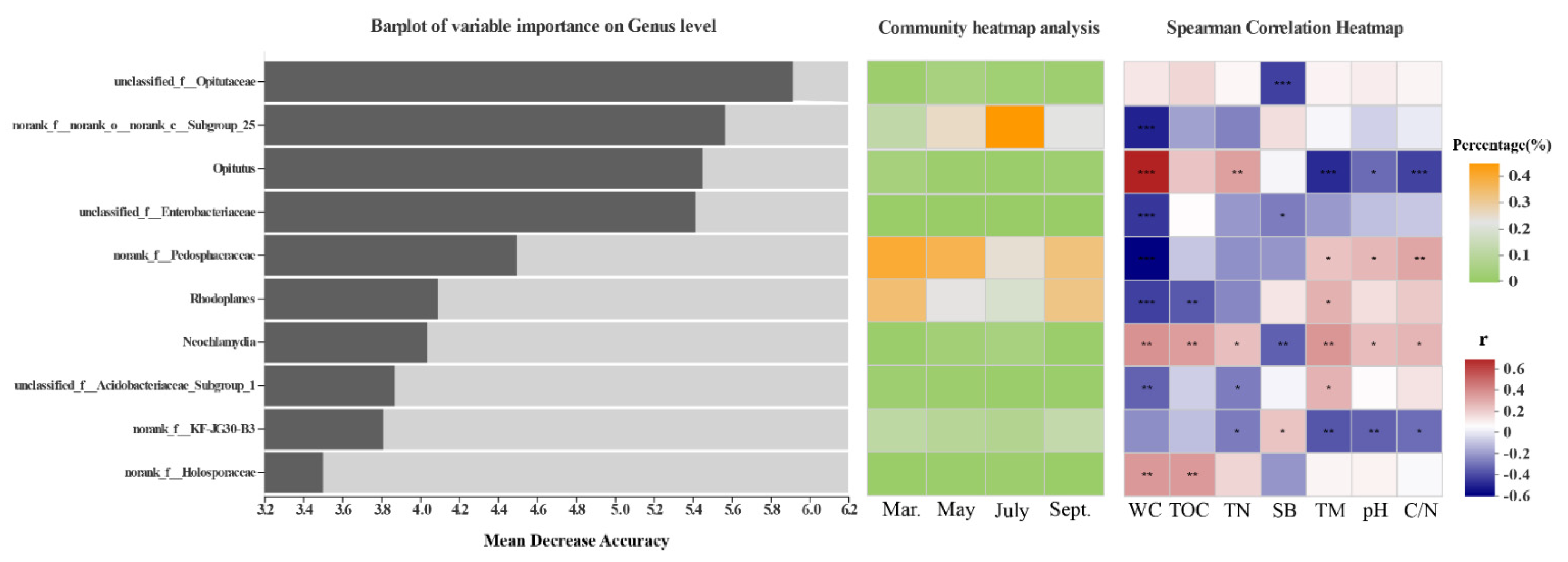
3.3. Effects of Environmental Factors on Bacterial Community Structure
3.4. Co-Occurrence Networks and Topological Properties of Bacteria in Different Months
4. Discussion
4.1. The Effect of Habitats Is Stronger Than Seasonal Changes on the Distribution of the Soil Bacterial Community
4.2. Environmental Variables Help Understand the Driving Effect of Habitats and Seasonal Shifts on Bacterial Communities
4.3. Bacterial Network Interactions in Different Sampling Months
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corneo, P.E.; Alberto, P.; Luca, C.; Marco, R.; Marco, C.; Cesare, G.; Ilaria, P. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. FEMS Microbiol. Ecol. 2013, 84, 588–602. [Google Scholar] [CrossRef]
- Coolen, M.; Giessen, J.; Zhu, E.Y.; Wuchter, C. Bioavailability of soil organic matter and microbial community dynamics upon permafrost thaw. Environ. Microbiol. 2011, 13, 2299–2314. [Google Scholar] [CrossRef]
- Jansson, J.; Tas, N. The microbial ecology of permafrost. Nat. Rev. Microbiol. 2014, 12, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Junpeng, R.; Jiabao, L.; Shiping, W.; Jiaxing, A.; Wentso, L. Responses of bacterial communities to simulated climate changes in alpine meadow soil of the Qinghai-Tibet Plateau. Appl. Environ. Microbiol. 2015, 81, 6070–6077. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, W.; Li, J.; Liu, J.; Li, B.; Wu, L.; Fang, C.; Zhang, Z. Effects of seasonal variations on soil microbial community composition of two typical zonal vegetation types in the Wuyi Mountains. J. Mt. Sci. 2016, 13, 1056–1065. [Google Scholar] [CrossRef]
- Averill, C.; Cates, L.L.; Dietze, M.C.; Bhatnagar, J.M. Spatial vs. temporal controls over soil fungal community similarity at continental and global scales. ISME J. 2019, 13, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Jurburg, S.D.; Natalda, L.; Tiago, R.; Morais, P.V. Bacterial communities in soil become sensitive to drought under intensive grazing. Sci. Total Environ. 2018, 15, 1638–1646. [Google Scholar] [CrossRef]
- Wessen, E.; Nyberg, K.; Jansson, J.K.; Hallin, S. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl. Soil Ecol. 2010, 45, 193–200. [Google Scholar] [CrossRef]
- Chu, H.; Neufeld, J.D.; Walker, V.K.; Grogan, P. The influence of vegetation type on the dominant soil bacteria, archaea, and fungi in a low arctic tundra landscape. Soil Sci. Soc. Am. J. 2011, 75, 1756. [Google Scholar] [CrossRef]
- Frank-Fahle, B.A.; Yergeau, E.; Greer, C.W.; Lantuit, H.; Wagner, D. Microbial functional potential and community composition in permafrost-affected soils of the NW Canadian arctic. PLoS ONE 2014, 9, e84761. [Google Scholar] [CrossRef]
- Aimeric, B.; Thomas, Z.L.; Gareth, K.P. Arctic soil microbial diversity in a changing world. Res. Microbiol. 2015, 166, 796–813. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Ponette, Q.; Rapp, M. Relationships between forest tree species, stand production and stand nutrient amount. Ann. For. Sci. 2000, 57, 313–324. [Google Scholar] [CrossRef]
- Oleg, V.; Menyailo, B.A.; Hungate, W. Tree species mediated soil chemical changes in a Siberian artificial afforestation experiment. Plant Soil 2002, 242, 171–182. [Google Scholar] [CrossRef]
- Frouz, J.; Pizl, V.; Cienciala, E.; Kalcik, J. Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 2009, 94, 111–121. [Google Scholar] [CrossRef]
- Orwin, K.; Buckland, S.; Johnson, D.; Turner, B.; Smart, S.; Oakley, S.; Bardgett, R.; Lancaster, C. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef]
- Larose, C.; Berger, S.; Ferrari, C.; Navarro, E.; Dommergue, A.; Schneider, D.; Vogel, T.M. Microbial sequences retrieved from environmental samples from seasonal Arctic snow and meltwater from Svalbard, Norway. Extremophiles 2010, 14, 205–212. [Google Scholar] [CrossRef]
- Kumar, M.; Männistö, M.K.; van Elsas, J.D.; Nissinen, R.M. Plants impact structure and function of bacterial communities in Arctic soils. Plant Soil 2016, 399, 319–332. [Google Scholar] [CrossRef]
- Mannisto, M.; Tiirola, M.; Haggblom, M. Bacterial communities in Arctic fields of Finnish Lapland are stable but highly pH-dependent. FEMS Microbiol. Ecol. 2007, 59, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ehrenfeld, J. Relationships among plants, soils and microbial communities along a hydrological gradient in the New Jersey Pinelands, USA. Ann. Bot. 2009, 105, 185–196. [Google Scholar] [CrossRef]
- Campisano, A.; Albanese, D.; Yousaf, S.; Pancher, M.; Donati, C.; Pertot, I. Temperature drives the assembly of endophytic communities’ seasonal succession. Environ. Microbiol. 2017, 19, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Taketani, R.; Lanconi, M.; Nessner Kavamura, V.; Durrer, A.; Andreote, F.; Melo, I. Dry season constrains bacterial phylogenetic diversity in a semi-arid rhizosphere system. Microb. Ecol. 2017, 73, 153–161. [Google Scholar] [CrossRef]
- Carini, P.; Delgado-Baquerizo, M.; Hinckley, E.; Holland-Oritz, H.; Fierer, N. Effects of spatial variability and relic DNA removal on the detection of temporal dynamics in soil microbial communities. MBio 2020, 11, e02776. [Google Scholar] [CrossRef]
- Pratt, B.; Riesen, R.; Johnston, C.G. PLFA analyses of microbial communities associated with PAH-contaminated riverbank sediment. Microb. Ecol. 2012, 64, 680–691. [Google Scholar] [CrossRef]
- Kaoping, Z.; Delgado-Baquerizo, M.; Chu, H. Space is more important than season when shaping soil microbial communities at a large spatial scale. mSystems 2020, 5, e00783-19. [Google Scholar] [CrossRef]
- Peay, K.G.; Tedersoo, L.; Bahram, M. Local-scale biogeography and spatiotemporal variability in communities of mycorrhizal fungi. New Phytol. 2015, 205. [Google Scholar] [CrossRef]
- Barberan, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, Z.; Liu, X.; Zhang, X.H. Microbial assembly, interaction, functioning, activity and diversification: A review derived from community compositional data. Mar. Life Sci. Technol. 2019, 1, 122–128. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, G.; Yang, J.; Chen, W.; Lin, Y. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef]
- Wu, Q.; Hou, Y.; Yun, H.; Liu, Y. Changes in active-layer thickness and near-surface permafrost between 2002 and 2012 in alpine ecosystems, Qinghai-Xizang (Tibet) Plateau, China. Glob. Planet. Chang. 2014, 124, 149–155. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, X.; Du, G. Role of soil seed bank along a disturbance gradient in an alpine meadow on the Tibet plateau. Flora 2010, 205, 128–134. [Google Scholar] [CrossRef]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.; Schuur, E.; Ping, C.-L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.; Koven, C.; et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef]
- Ping, C.L.; Jastrow, J.; Jorgenson, M.; Shur, Y. Permafrost soils and carbon cycling. Soil 2015, 1, 147–171. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, G.; Liu, G.; Chen, T.; Qin, Y.; Zhang, B.; Sun, L. Response of soil bacterial community structure to permafrost degradation in the upstream regions of the Shule River Basin, Qinghai-Tibet Plateau. Geomicrobiol. J. 2016, 34, 300–308. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, X.; Du, G.; Media, B. Soil seed bank dynamics in alpine wetland succession on the Tibetan Plateau. Plant Soil 2011, 346, 19–28. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Munyaka, P.; Eissa, N.; Bernstein, C.; Khafipour, E.; Ghia, J.E. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: A role of the gut microbiota. PLoS ONE 2015, 10, e0142536. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Mitter, E.K.; Freitas, J.; Germida, J.J. Bacterial root microbiome of plants growing in oil sands reclamation covers. Front. Microbiol. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Mack, I.; Cuntz, U.; Gramer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016, 6, 26752. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.D.; Li, S.; Leng, X.H. Soil bacterial community structure and co-occurrence pattern during vegetation restoration in karst rocky desertification area. Front. Microbiol. 2017, 8, 2377. [Google Scholar] [CrossRef]
- Thompson, L.; Sanders, J.; Mcdonald, D.; Amir, A.; Ladau, J.; Locey, K.; Prill, R.; Tripathi, A.; Gibbons, S.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-Gonzalez, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Hampton-Marcell, J.; O’Brien, S.L.; Meyer, F.; Gilbert, J.A. Spatial scale drives patterns in soil bacterial diversity. Environ. Microbiol. 2016, 18, 2039–2051. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Hanson, C.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Martiny, J.B.H. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef]
- Nemergut, D.; Schmidt, S.; Fukami, T.; O’Neill, S.; Bilinski, T.; Stanish, L.; Knelman, J.; Darcy, J.; Lynch, R.; Wickey, P.; et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, Y.; Yang, K.; Wang, S.; Zeng, H.; Baumann, F.; Kuehn, P.; Scholten, T.; He, J.S. Soil respiration in Tibetan alpine grasslands: Belowground biomass and soil moisture, but not soil temperature, best explain the large-scale patterns. PLoS ONE 2012, 7, e34968. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.; Franzluebbers, A.; Schmidt, T.; Coleman, D.; Whitman, W. Land-Use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184. [Google Scholar] [CrossRef]
- Shi, Y.; Xiang, X.; Shen, C.; Chu, H.; Neufeld, J.D.; Walker, V.K.; Grogan, P. Vegetation-associated impacts on arctic tundra bacterial and microeukaryotic communities. Appl. Environ. Microbiol. 2015, 81, 492–501. [Google Scholar] [CrossRef]
- Hui, L.; Ye, D.; Wang, X.; Settles, M.L.; Wang, J.; Hao, Z.; Zhou, L.; Ping, D.; Jiang, Y.; Ma, Z.S. Soil bacterial communities of different natural forest types in Northeast China. Plant Soil 2014, 383, 203–216. [Google Scholar] [CrossRef]
- Yergeau, E.; Bokhorst, S.; Kang, S.; Zhou, J.; Greer, C.; Aerts, R.; Kowalchuk, G. Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J. 2011, 6, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Arocha, H.; Canales-del-Castillo, R.; Eguiarte, L.; Souza, V.; De la Torre, S. High diversity and suggested endemicity of culturable Actinobacteria in an extremely oligotrophic desert oasis. PeerJ 2017, 5, e3247. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lennon, J.T. The generation and maintenance of diversity in microbial communities. Am. J. Bot. 2011, 98, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Zifcakova, L.; Vetrovsky, T.; Lombard, V.; Henrissat, B.; Howe, A.; Baldrian, P. Feed in summer, rest in winter: Microbial carbon utilization in forest topsoil. Microbiome 2017, 5, 122. [Google Scholar] [CrossRef]
- Dijkstra, P.; Thomas, S.; Heinrich, P.; Koch, G.; Schwartz, E.; Hungate, B. Effect of temperature on metabolic activity of intact microbial communities: Evidence for altered metabolic pathway activity but not for increased maintenance respiration and reduced carbon use efficiency. Soil Biol. Biochem. 2011, 43, 2023–2031. [Google Scholar] [CrossRef]
- Sun, H.; Peng, F.; Zhang, H.; Xue, X.; Gibbons, S.; Jack, G.; Chu, H. Characterizing changes in soil bacterial community structure in response to short-term warming. FEMS Microbiol. Ecol. 2014, 89, 281–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Q.; Liu, S.; Zhou, H.; Ganjurjav, H.; Wang, X. Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2016, 562, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Ray, M.; Rao, N.; Saisree, L.; Jagannadham, M.; Kumar, G.; Reddy, G.; Bhargava, P. Sphingobacterium antarcticus sp. nov., a Psychrotrophic Bacterium from the Soils of Schirmacher Oasis, Antarctica. Int. J. Syst. Bacteriol. 1992, 42, 102–106. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhao, L.; Xu, S.J., Jr.; Liu, Y.Z.; Liu, H.Y.; Cheng, G.D. Soil moisture effect on bacterial and fungal community in Beilu River (Tibetan Plateau) permafrost soils with different vegetation types. J. Appl. Microbiol. 2013, 114, 1054–1065. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.; Franzluebbers, A.; Sanderlin, J.; Reeves, J.; Jenkins, M.; Endale, D.; Coleman, D.; Whitman, W. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843. [Google Scholar] [CrossRef]
- Bartram, A.K.; Jiang, X.; Lynch, M.D.J.; Masella, A.P.; Nicol, G.W.; Jonathan, D.; Neufeld, J.D. Exploring links between pH and bacterial community composition in soils from the Craibstone Experimental Farm. FEMS Microbiol. Ecol. 2014, 87, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.W.; Convey, P.; Pearce, D.A.; Tan, I. Assessment of soil bacterial communities on Alexander Island (in the maritime and continental Antarctic transitional zone). Polar Biol. 2012, 35, 387–399. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; Quadros, P.; Davis-Richardson, A.; Camargo, F.; Clark, I.; Mcgrath, S.; Hirsch, P.; Triplett, E. Soil pH determines microbial diversity and composition in the park grass experiment. Microb. Ecol. 2014, 69, 395–406. [Google Scholar] [CrossRef]
- Koranda, M.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S.; Richter, A. Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biol. Biochem. 2013, 60, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Barboza, A.; Pylro, V.S.; Jacques, R.; Gubiani, P.I.; Quadros, F.F.; Trindade, J.; Triplett, E.W.; Roesch, L. Seasonal dynamics alter taxonomical and functional microbial profiles in Pampa biome soils under natural grasslands. PeerJ 2018, 6, e4991. [Google Scholar] [CrossRef]
- Schachter, J.; Caldwell, H.D. Chlamydiae. Annu. Rev. Microbiol. 1980, 34, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Buchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.; Gilbert, J.; Xu, J. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2015, 10, 1891–1901. [Google Scholar] [CrossRef]
- Kielak, A.; Pijl, A.S.; Veen, J.; Kowalchuk, G.A. Phylogenetic diversity of Acidobacteria in a former agricultural soil. ISME J. 2009, 3, 378–382. [Google Scholar] [CrossRef]
- Rawat, S.R.; Mannisto, M.K.; Bromberg, Y.; Haggblom, M.M. Comparative genomic and physiological analysis provides insights into the role of Acidobacteria in organic carbon utilization in Arctic tundra soils. FEMS Microbiol. Ecol. 2012, 82, 341–355. [Google Scholar] [CrossRef]
- Kulichevskaya, I.S.; Suzina, N.E.; Liesack, W.; Dedysh, S.N. Bryobacter aggregatus gen. nov. sp. nov. a peat-inhabiting, aerobic chemo-organotroph from subdivision 3 of the acidobacteria. Int. J. Syst. Evol. Micr. 2010, 60, 301. [Google Scholar] [CrossRef]
- Cuesta, G.; Soler, A.; Alonso, J.L.; Ruvira, M.A.; Lucena, T.; Arahal, D.R.; Goodfellow, M. Pseudonocardia hispaniensis sp. nov., a novel actinomycete isolated from industrial wastewater activated sludge. Antonie Van Leeuwenhoek 2013, 103, 135–142. [Google Scholar] [CrossRef]
- Kaewkla, O.; Franco, C.M. Pseudonocardia adelaidensis sp. nov., an endophytic actinobacterium isolated from the surface-sterilized stem of a grey box tree (Eucalyptus microcarpa). Int. J. Syst. Evol. Micr. 2010, 60, 2818–2822. [Google Scholar] [CrossRef][Green Version]


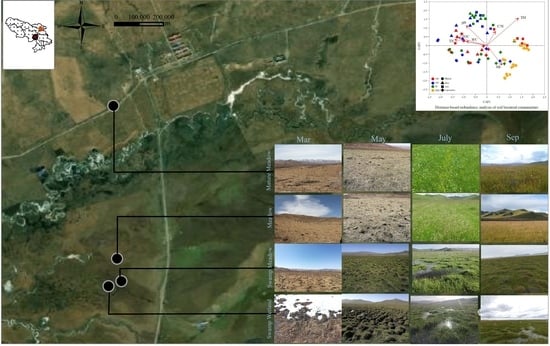
| Habitats | Altitude | Latitude & Longitude | Dominant Plant | Description (Ma et al., 2011) |
|---|---|---|---|---|
| SW | 3512 m | 33°39′ N 101°32′ E | Festuca sinensis Keng ex S. L. Lu Kobresia royleana (Nees) Bocklr. Potentilla anserina L. | The dominant species are typical wetland plants grazed by yak and Tibetan sheep. |
| SM | 3522 m | 33°39′ N 101°52′ E | Ranunculus japonicus Thunb. Carex tristachya Thunb. Leontopodium nanum (Hook. f. et Thoms.) Hand.-Mazz. Festuca sinensis Keng ex S. L. Lu | The habitat is drier than SW. Water is distributed on the soil surface in some parts of this habitat only in the rainy season. The swamp meadow has been lightly grazed by yak and Tibetan sheep. |
| M | 3540 m | 33°39′ N 101°53′ E | Pleurospermum camtschaticum Hoffm. Carex tristachya Thunb. Sanguisorba filiformis (Hook. f.) Hand.-Mazz. Festuca sinensis Keng ex S. L. Lu | The habitat is drier than SM, without water on the soil surface at any time. It has already become meadow with some drought-tolerant vegetation. |
| MM | 3572 m | 34°40′ N 102°52′ E | Artemisia mongolica (Fisch. ex Bess.) Nakai Elymus nutans Griseb. Anemone obtusiloba D. Don Pedicularis szetschuanica Maxim. | It is a mature alpine meadow and soil moisture content is the lowest among four habitats. The mature meadow is under low disturbance of grazing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, M.; Wang, J.; Lin, Y. Habitats Are More Important Than Seasons in Shaping Soil Bacterial Communities on the Qinghai-Tibetan Plateau. Microorganisms 2021, 9, 1595. https://doi.org/10.3390/microorganisms9081595
Wang R, Wang M, Wang J, Lin Y. Habitats Are More Important Than Seasons in Shaping Soil Bacterial Communities on the Qinghai-Tibetan Plateau. Microorganisms. 2021; 9(8):1595. https://doi.org/10.3390/microorganisms9081595
Chicago/Turabian StyleWang, Rui, Miao Wang, Jing Wang, and Yinghua Lin. 2021. "Habitats Are More Important Than Seasons in Shaping Soil Bacterial Communities on the Qinghai-Tibetan Plateau" Microorganisms 9, no. 8: 1595. https://doi.org/10.3390/microorganisms9081595
APA StyleWang, R., Wang, M., Wang, J., & Lin, Y. (2021). Habitats Are More Important Than Seasons in Shaping Soil Bacterial Communities on the Qinghai-Tibetan Plateau. Microorganisms, 9(8), 1595. https://doi.org/10.3390/microorganisms9081595