The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Oral Bacteria Associated with Oral Cancer
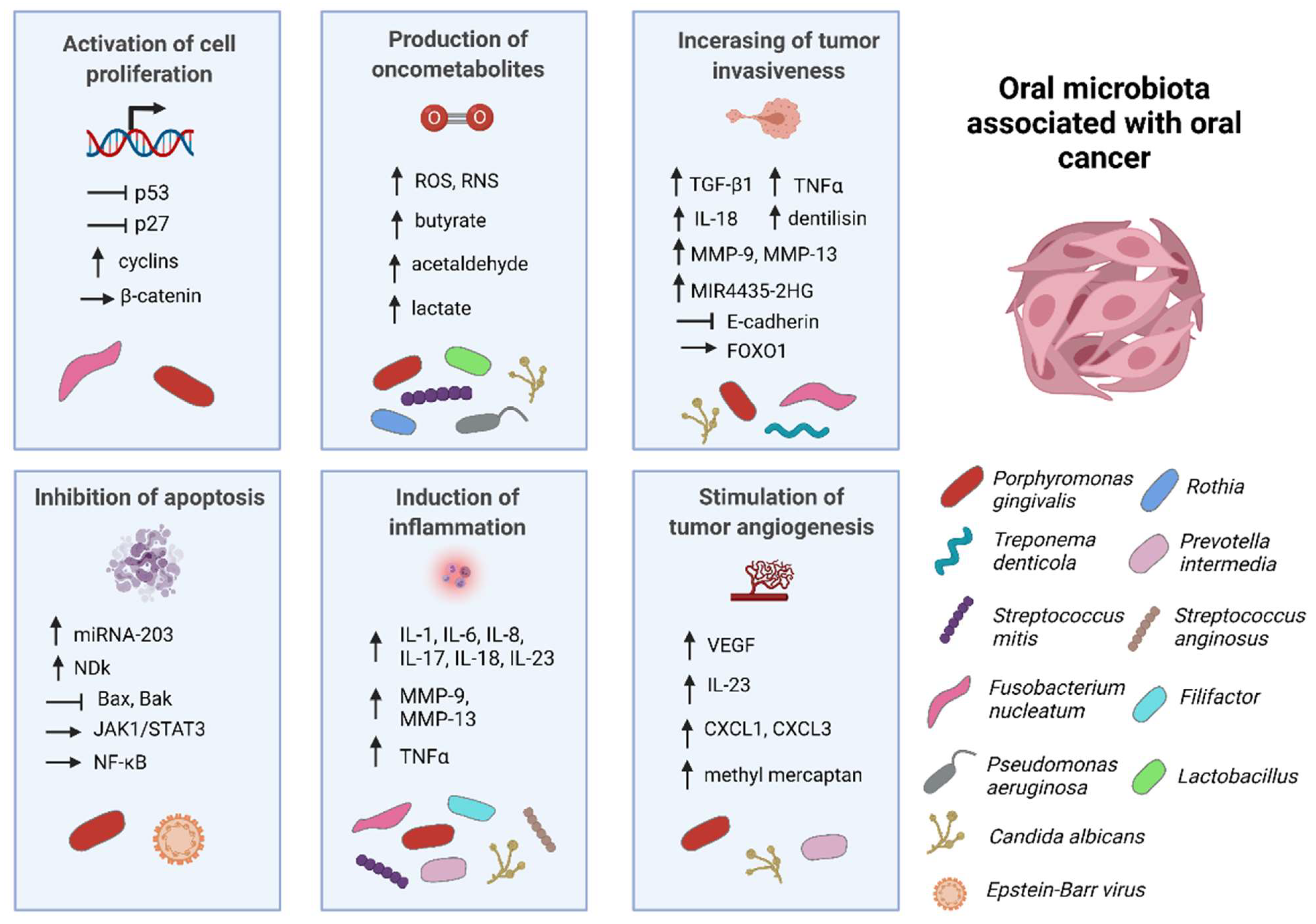
2.1. The Role of Porphyromonas gingivalis in Oral Cancer
2.1.1. Inhibition of Cell Apoptosis
2.1.2. Activation of Cell Proliferation
2.1.3. Induction of Chronic Inflammation
2.1.4. Production of Oncometabolites
2.2. The Role of Fusobacterium nucleatum in Oral Cancer
2.2.1. Secretion of IL-1β Due to NLRP3 Inflammasome Activation
2.2.2. Metalloproteinase Overexpression Due to p38 Activation
2.2.3. Ku70/p53 Signal Pathway-Dependent DNA Damage
2.2.4. Acceleration of the Cell Cycle through Downregulation of p27
2.2.5. Induction of Epithelial–Mesenchymal Transition
2.3. Role of Prevotella sp. in Oral Cancer
2.4. Role of Streptococcus sp. in Oral Cancer
2.4.1. Streptococcus anginosus
2.4.2. Streptococcus mitis
2.4.3. Streptococcus gordonii
2.5. Role of Lactobacillus spp. in Oral Cancer
3. Fungi Associated with Oral Cancer
3.1. The Role of Candida spp. in Oral Cancer
Candida albicans
4. Viruses Associated with Oral Cancer
4.1. The Role of Human Papillomavirus in Oral Cancer
4.2. The Role of Epstein–Barr Virus in Oral Cancer
4.3. The Role of Human Cytomegalovirus in Oral Cancer
4.4. Role of Herpes Simplex in Oral Cancer
5. Findings and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Perera, M.; Al-Hebshi, N.; Speicher, D.; Perera, I.; Johnson, N. Emerging role of bacteria in oral carcinogenesis: A review with special reference to perio-pathogenic bacteria. J. Oral Microbiol. 2016, 8, 32762. [Google Scholar] [CrossRef] [PubMed]
- Kakabadze, M.; Paresishvili, T.; Karalashvili, L.; Chakhunashvili, D.; Kakabadze, Z. Oral microbiota and oral cancer: Review. Oncol. Rev. 2020, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.; Elimairi, I.; Stanton, C.; Ross, R.; Ryan, C. The role of the microbiome in oral squamous cell carcinoma with insight into the microbiome–treatment axis. Int. J. Mol. Sci. 2020, 21, 8061. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Shearston, K.; Nguyen, A.P.; Kujan, O. Oral carcinogenesis and malignant transformation. In Premalignant Conditions of the Oral Cavity; Springer: Singapore, 2019; pp. 27–66. [Google Scholar] [CrossRef]
- Nowicki, S.; Gottlieb, E. Oncometabolites: Tailoring our genes. FEBS J. 2015, 282, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The oral microbiome in health and its implication in oral and systemic diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Z.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]
- Nagy, K.N.; Sonkodi, I.; Szöke, I.; Nagy, E.; Newman, H.N. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Katz, J.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.J.; Crean, S.J.; Lewis, M.A.O.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. Viable bacteria present within oral squamous cell carcinoma tissue. J. Clin. Microbiol. 2006, 44, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The oral microbiota may have influence on oral cancer. Front. Cell Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of oral bacteria in the development of oral squamous cell carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef]
- Ge, W.; Hu, H.; Cai, W.; Xu, J.; Hu, W.; Weng, X.; Qin, X.; Huang, Y.; Han, W.; Hu, Y.; et al. High-risk stage III colon cancer patients identified by a novel five-gene mutational signature are characterized by upregulation of IL-23A and gut bacterial translocation of the tumor microenvironment. Int. J. Cancer 2020, 146, 2027–2035. [Google Scholar] [CrossRef]
- Fitzsimonds, Z.R.; Rodriguez-Hernandez, C.J.; Bagaitkar, J.; Lamont, R.J. From beyond the pale to the pale riders: The emerging association of bacteria with oral cancer. J. Dent. Res. 2020, 99, 604–612. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory bacteriome and oral squamous cell carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Henrich, B.; Rumming, M.; Sczyrba, A.; Velleuer, E.; Dietrich, R.; Gerlach, W.; Gombert, M.; Rahn, S.; Stoye, J.; Borkhardt, A.; et al. Mycoplasma Salivarium as a dominant coloniser of fanconi anaemia associated oral carcinoma. PLoS ONE 2014, 9, 92297. [Google Scholar] [CrossRef]
- Khan, N.; Yılmaz, S.; Aksoy, S.; Uzel, A.; Tosun, Ç.; Kirmizibayrak, P.B.; Bedir, E. Polyethers isolated from the marine actinobacterium streptomyces cacaoi inhibit autophagy and induce apoptosis in cancer cells. Chem. Biol. Interact. 2019, 307, 167–178. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.R.M.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of oral dysbiosis with oral cancer development. Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Inoue, H.; Miyazaki, Y.; Kikuchi, K.; Sakashita, H.; Ochiai, K. Microorganisms and cancer of the oral cavity. Integr. Cancer Sci. Ther. 2016, 3, 510–515. [Google Scholar] [CrossRef][Green Version]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Amarnani, R.; Rapose, A. Colon cancer and enterococcus bacteremia co-affection: A dangerous alliance. J. Infect. Public Health 2017, 10, 681–684. [Google Scholar] [CrossRef]
- Maekawa, T.; Fukaya, R.; Takamatsu, S.; Itoyama, S.; Fukuoka, T.; Yamada, M.; Hata, T.; Nagaoka, S.; Kawamoto, K.; Eguchi, H.; et al. Possible involvement of enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun. 2018, 506, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Johnson, L.; Roberts, J.A.; Hung, S.C.; Lee, J.; Atanasova, K.R.; Huang, P.R.; Yilmaz, Ö.; Ojcius, D.M. Fusobacterium nucleatum infection of gingival epithelial cells leads to NLRP3 inflammasome-dependent secretion of IL-1β and the danger signals ASC and HMGB1. Cell Microbiol. 2016, 18, 970–981. [Google Scholar] [CrossRef]
- Uitto, V.J.; Baillie, D.; Wu, Q.; Gendron, R.; Grenier, D.; Putnins, E.E.; Kanervo, A.; Firth, J.D. Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect. Immun. 2005, 73, 1171–1179. [Google Scholar] [CrossRef]
- Geng, F.; Zhang, Y.; Lu, Z.; Zhang, S.; Pan, Y. Fusobacterium nucleatum caused DNA damage and promoted cell proliferation by the Ku70/P53 pathway in oral cancer cells. DNA Cell Biol. 2020, 39, 144–151. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the LncRNA MIR4435-2HG/MiR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Liu, Y.; Zhang, R.; Zhang, B.; Wang, T.; Zhu, X.; Mei, L.; Chen, H.; Zhang, H.; Ming, P.; et al. Homoharringtonine induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal pathway in gefitinib-resistant lung cancer cells. Sci. Rep. 2015, 5, 8477. [Google Scholar] [CrossRef]
- Karpiński, T.M. Role of oral microbiota in cancer development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Raj, A.T. Role of Mycoplasma in the initiation and progression of oral cancer. J. Int. Oral Health 2015, 7, i–ii. [Google Scholar]
- Yoshida, Y. Analysis of the butyrate-producing pathway in Porphyromonas gingivalis. Meth. Mol. Biol. 2021, 2210, 167–172. [Google Scholar] [CrossRef]
- Ramadan, A.; Land, W.G.; Paczesny, S. Editorial: Danger signals triggering immune response and inflammation. Front. Immunol. 2017, 8, 979. [Google Scholar] [CrossRef]
- Nisticò, P.; Bissell, M.J.; Radisky, D.C. Epithelial-mesenchymal transition: General principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Baraniya, D.; Jain, V.; Lucarelli, R.; Tam, V.; Vanderveer, L.; Puri, S.; Yang, M.; Al-Hebshi, N.N. Screening of health-associated oral bacteria for anticancer properties in vitro. Front. Cell Infect. Microbiol. 2020, 10, 575656. [Google Scholar] [CrossRef]
- Wang, L.; Ganly, I. The oral microbiome and oral cancer. Clin. Lab. Med. 2014, 34, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Mane, S.P.; Ji, X.; Li, Y.; Evans, C.; Crasta, O.R.; Morse, D.; Meagher, R.; Singh, A.; Saxena, D. Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol. Med. Microbiol. 2011, 61, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.S.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, K.-P.; et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef]
- Lee, W.-H.; Chen, H.-M.; Yang, S.-F.; Liang, C.; Peng, C.-Y.; Lin, F.-M.; Tsai, L.-L.; Wu, B.-C.; Hsin, C.-H.; Chuang, C.-Y.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef]
- Hooper, S.J.; Crean, S.-J.; Fardy, M.J.; Lewis, M.A.O.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. A Molecular analysis of the bacteria present within oral squamous cell carcinoma. J. Med. Microbiol. 2007, 56, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Geng, F.; Shi, X.; Li, Y.; Zhang, X.; Zhao, X.; Pan, Y. The Prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl. Microbiol. Biotechnol. 2019, 103, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Fukuma, N.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. The performance of an oral microbiome biomarker panel in predicting oral cavity and oropharyngeal cancers. Front. Cell Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Chang, C.; Wang, H.; Liu, J.; Pan, C.; Zhang, D.; Li, X.; Pan, Y. Porphyromonas gingivalis infection promoted the proliferation of oral squamous cell carcinoma cells through the MiR-21/PDCD4/AP-1 negative signaling pathway. ACS Infect. Dis. 2019, 5, 1336–1347. [Google Scholar] [CrossRef]
- Sayehmiri, F.; Sayehmiri, K.; Asadollahi, K.; Soroush, S.; Bogdanovic, L.; Jalilian, F.A.; Emaneini, M.; Taherikalani, M. The prevalence rate of Porphyromonas gingivalis and its association with cancer: A systematic review and meta-analysis. Int. J. Immunopathol. Pharmacol. 2015, 28, 160–167. [Google Scholar] [CrossRef]
- Geng, F.; Liu, J.; Guo, Y.; Li, C.; Wang, H.; Wang, H.; Zhao, H.; Pan, Y. persistent exposure to Porphyromonas gingivalis promotes proliferative and invasion capabilities, and tumorigenic properties of human immortalized oral epithelial cells. Front. Cell Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Yilmaz, Ö.; Jungas, T.; Verbeke, P.; Ojcius, D.M. Activation of the phosphatidylinositol 3-Kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect. Immun. 2004, 72, 3743–3751. [Google Scholar] [CrossRef]
- Nakhjiri, S.F.; Park, Y.; Yilmaz, O.; Chung, W.O.; Watanabe, K.; El-Sabaeny, A.; Park, K.; Lamont, R.J. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol. Lett. 2001, 200, 145–149. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Ru, P.; Steele, R.; Hsueh, E.C.; Ray, R.B. Anti-MiR-203 upregulates SOCS3 expression in breast cancer cells and enhances cisplatin chemosensitivity. Genes Cancer 2011, 2, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Rao, X.; Zhang, K. Nucleoside diphosphate kinase (Ndk): A pleiotropic effector manipulating bacterial virulence and adaptive responses. Microbiol. Res. 2017, 205, 125–134. [Google Scholar] [CrossRef]
- Croker, B.A.; Kiu, H.; Nicholson, S.E. SOCS regulation of the JAK/STAT signalling pathway. Semin. Cell Dev. Biol. 2008, 19, 414–422. [Google Scholar] [CrossRef]
- Al-Rawi, N.H.; Al-Marzooq, F.; Al-Nuaimi, A.S.; Hachim, M.Y.; Hamoudi, R. Salivary microRNA 155, 146a/b and 203: A pilot study for potentially non-invasive diagnostic biomarkers of periodontitis and diabetes mellitus. PLoS ONE 2020, 15, e0237004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roberts, J.A.S.; Atanasova, K.R.; Chowdhury, N.; Yilmaz, Ö. A novel kinase function of a nucleoside-diphosphate-kinase homologue in Porphyromonas gingivalis is critical in subversion of host cell apoptosis by targeting heat-shock protein. Cell Microbiol. 2018, 20, e12825. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Andrieu, C.; Rocchi, P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kuboniwa, M.; Hasegawa, Y.; Mao, S.; Shizukuishi, S.; Amano, A.; Lamont, R.J.; Yilmaz, Ö.P. Gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes. Infect. 2008, 10, 122–128. [Google Scholar] [CrossRef]
- Zhou, Y.; Sztukowska, M.; Wang, Q.; Inaba, H.; Potempa, J.; Scott, D.A.; Wang, H.; Lamont, R.J. Noncanonical activation of β-catenin by Porphyromonas gingivalis. Infect. Immun. 2015, 83, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Roche, J. The epithelial-to-mesenchymal transition in cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yilmaz, Ö. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral Microbiol. 2019, 11, 1563410. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Hatori, M.; Kurihara, Y.; Kinugasa, Y.; Shirota, T.; Shintai, S. Inhibition of TGF-Β1 suppresses motility and invasiveness of oral squamous cell carcinoma cell lines via modulation of integrins and down-regulation of matrix-metalloproteinases. Oncol. Rep. 2009, 21, 205–210. [Google Scholar] [CrossRef]
- Ameena, M.; Rathy, R. Evaluation of tumor necrosis factor: Alpha in the saliva of oral cancer, leukoplakia, and healthy controls—A comparative study. J. Int. Oral. Health 2019, 11, 92. [Google Scholar] [CrossRef]
- Takeuchi, H.; Hirano, T.; Whitmore, S.E.; Morisaki, I.; Amano, A.; Lamont, R.J. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-ΚB RelA/P65. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef]
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, J.S.M.; Syu, S.H.; Wong, T.S.; Chan, J.Y.W.; Tang, Y.C.; Yang, Z.P.; Yang, W.C.; Chen, C.T.; Lu, S.C.; et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J. Cell Physiol. 2015, 230, 875–884. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Li, J.; Ban, S.; Duan, C.; Liu, W. Interleukin-18 expression in oral squamous cell carcinoma: Its role in tumor cell migration and invasion, and growth of tumor cell xenografts. FEBS Open Bio 2018, 8, 1953–1963. [Google Scholar] [CrossRef]
- Lamaa, A.; Le Bras, M.; Skuli, N.; Britton, S.; Frit, P.; Calsou, P.; Prats, H.; Cammas, A.; Millevoi, S. A novel cytoprotective function for the DNA repair protein Ku in regulating P53 MRNA translation and function. EMBO Rep. 2016, 17, 508–518. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kitamura, N.; Yoshida, K.; Yamamoto, T.; Ozaki, K.; Kudo, Y. Involvement of Fusobacterium species in oral cancer progression: A literature review including other types of cancer. Int. J. Mol. Sci. 2020, 21, 6207. [Google Scholar] [CrossRef]
- Rai, A.K.; Panda, M.; Das, A.K.; Rahman, T.; Das, R.; Das, K.; Sarma, A.; Kataki, A.C.; Chattopadhyay, I. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch. Microbiol. 2021, 203, 137–152. [Google Scholar] [CrossRef]
- Sasaki, M.; Yamaura, C.; Ohara-Nemoto, Y.; Tajika, S.; Kodama, Y.; Ohya, T.; Harada, R.; Kimura, S. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005, 11, 151–156. [Google Scholar] [CrossRef]
- Zaki, A.N.M.; Kadum, A.D.; Mousa, N.K.; Kareem, A.S.A.; Obaid, B.H. Cancer infection and its relationship with streptococcus mitis increasing numbers in human mouth. Int. J. Sci. Eng. Res. 2019, 5, 88–91. [Google Scholar]
- Park, O.-J.; Kwon, Y.; Park, C.; So, Y.J.; Park, T.H.; Jeong, S.; Im, J.; Yun, C.-H.; Han, S.H. Streptococcus gordonii: Pathogenesis and host response to its cell wall components. Microorganisms 2020, 8, 1852. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.C.; Collins, C.C.; Gout, P.W.; Wang, Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J. Pathol. 2013, 230, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Panduwawala, C.P.; Samaranayake, L.P. Biodiversity of the human oral mycobiome in health and disease. Oral Dis. 2019, 25, 363–371. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Shen, S.; Hou, Y.; Chen, Y.; Wang, T. The mycobiota of the human body: A spark can start a prairie fire. Gut Microbes 2020, 11, 655–679. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Dvořák, A.; Folwarski, M.; Daca, A.; Przewłócka, K.; Makarewicz, W. Fungal gut microbiota dysbiosis and its role in colorectal, oral, and pancreatic carcinogenesis. Cancers 2020, 12, 1326. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. A dysbiotic mycobiome dominated by candida albicans is identified within oral squamous-cell carcinomas. J. Oral Microbiol. 2017, 9. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K.; Kumar, V.N. A comparative study of Candida species diversity among patients with oral squamous cell carcinoma and oral potentially malignant disorders. BMC Res. Notes 2020, 13, 488. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wang, H.; Retuerto, M.; Zhang, H.; Burkey, B.; Ghannoum, M.A.; Eng, C. Bacteriome and mycobiome associations in oral tongue cancer. Oncotarget 2017, 8, 97273–97289. [Google Scholar] [CrossRef] [PubMed]
- Collette, J.R.; Zhou, H.; Lorenz, M.C. Candida albicans suppresses nitric oxide generation from macrophages via a secreted molecule. PLoS ONE 2014, 9, e96203. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-De-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Barbieri, A.; Bimonte, S.; Palla, M.; Zappavigna, S.; Caraglia, M.; Ascierto, P.A.; Ciliberto, G.; Arra, C. Interleukin 18: Friend or foe in cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2013, 1836, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P. Essential Microbiology for Dentistry, 4th ed.; Churchill Livingstone: London, UK, 2011. [Google Scholar]
- Wang, J.; Gao, Y.; Zhao, F. Phage-bacteria interaction network in human oral microbiome. Environ. Microbiol. 2016, 18, 2143–2158. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef]
- Edlund, A.; Santiago-Rodriguez, T.M.; Boehm, T.K.; Pride, D.T. Bacteriophage and their potential roles in the human oral cavity. J. Oral Microbiol. 2015, 7. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Aghazadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Windon, M.J.; Hernandez, T.; Miles, B.; Ha, P.K.; Ryan, W.R.; Zante, A.V.; Eisele, D.W.; D’Souza, G.; Fakhry, C.; et al. HPV-positive squamous cell carcinoma of the larynx, oral cavity, and hypopharynx. Am. J. Surg. Pathol. 2020, 44, 691–702. [Google Scholar] [CrossRef]
- Yang, L.-Q.; Xiao, X.; Li, C.-X.; Wu, W.-Y.; Shen, X.-M.; Zhou, Z.-T.; Fan, Y.; Shi, L.-J. Human papillomavirus genotypes and P16 expression in oral leukoplakia and squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 1022–1028. [Google Scholar]
- Shillitoe, E.J. The microbiome of oral cancer. Crit. Rev. Oncog. 2018, 23, 153–160. [Google Scholar] [CrossRef]
- She, Y.; Nong, X.; Zhang, M.; Wang, M. Epstein-Barr virus infection and oral squamous cell carcinoma risk: A meta-analysis. PLoS ONE 2017, 12, e0186860. [Google Scholar] [CrossRef]
- Fauzi, F.H.; Hamzan, N.I.; Rahman, N.A.; Mohamad, I.; Suraiya, S.; Kallarakkal, T.G.; Mohamad, S. Detection of human papillomavirus types 16 and 18 in oral squamous cell carcinoma samples in malaysia. Arch. Orofac. Sci. 2019, 14, 21–29. [Google Scholar]
- Kaminagakura, E.; Villa, L.L.; Andreoli, M.A.; Sobrinho, J.S.; Vartanian, J.G.; Soares, F.A.; Nishimoto, I.N.; Rocha, R.; Kowalski, L.P. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int. J. Cancer 2012, 130, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Al-Malkey, M.; Abass, A.; Jabbar, F.; Ismail, M. Detection of human papilloma virus in oral squamous cell carcinoma. Int. J. Curr. Res. 2015, 7, 23707–23711. [Google Scholar]
- Kojima, A.; Maeda, H.; Sugita, Y.; Tanaka, S.; Kameyama, Y. Human papillomavirus type 38 infection in oral squamous cell carcinomas. Oral Oncol. 2002, 38, 591–596. [Google Scholar] [CrossRef]
- Khovidhunkit, S.P.; Buajeeb, W.; Sanguansin, S.; Poomsawat, S.; Weerapradist, W. Detection of human papillomavirus in oral squamous cell carcinoma, leukoplakia and lichen planus in Thai patients. Asian Pac. J. Cancer Prev. 2008, 9, 771–775. [Google Scholar]
- Heawchaiyaphum, C.; Iizasa, H.; Ekalaksananan, T.; Burassakarn, A.; Kiyono, T.; Kanehiro, Y.; Yoshiyama, H.; Pientong, C. Epstein-Barr virus infection of oral squamous cells. Microorganisms 2020, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-Y.; Lu, M.-C.; Tzeng, C.-C.; Huang, J.-Y.; Chang, H.-W.; Chen, R.-S.; Liu, S.-Y.; Liu, S.-T.; Shieh, B.; Li, C. Detection of EBV infection and gene expression in oral cancer from patients in Taiwan by microarray analysis. J. Biomed. Biotechnol. 2009, 2009, 904589. [Google Scholar] [CrossRef]
- Prathyusha, M.; Kattappagari, K.; Chowdary, D.; Shekar, P.; Alivelu, D.; Reddy, B.R. A study on association of epstein barr virus in oral squamous cell carcinoma using polymerase chain reaction technique. J. Dr. NTR Univ. Health Sci. 2019, 8, 233. [Google Scholar] [CrossRef]
- Saravani, S.; Kadeh, H.; Miri-Moghaddam, E.; Zekri, A.; Sanadgol, N.; Gholami, A. Human cytomegalovirus in oral squamous cell carcinoma in southeast of Iran. Jundishapur J. Microbiol. 2015, 8, e21838. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R.; Elhag, W. Molecular detection of herpes simplex virus types [1 and 2] in oral squamous cell carcinoma (OSCC) at Khartoum. J. Adv. Med. Med. Res. 2018, 26, 1–6. [Google Scholar] [CrossRef]
- Miller, C.S.; Johnstone, B.M. Human papillomavirus as a risk factor for oral squamous cell carcinoma: A meta-analysis, 1982–1997. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Yakin, M.; Seo, B.; Hussaini, H.; Rich, A.; Hunter, K. Human papillomavirus and oral and oropharyngeal carcinoma: The essentials. Aust. Dent. J. 2019, 64, 11–18. [Google Scholar] [CrossRef]
- Tomaić, V. Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers 2016, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, S. Role of human papillomavirus in oral squamous cell carcinoma and oral potentially malignant disorders: A review of the literature. Indian J. Dent. 2015, 6, 91. [Google Scholar] [CrossRef]
- Wołącewicz, M.; Becht, R.; Grywalska, E.; Niedźwiedzka-Rystwej, P. Herpesviruses in head and neck cancers. Viruses 2020, 12, 172. [Google Scholar] [CrossRef]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; Ibrahim, S.O.; Larsson, P.-A.; Sand, L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res. 2012, 32, 571–580. [Google Scholar]
- Meurman, J.H. Infectious and dietary risk factors of oral cancer. Oral Oncol. 2010, 46, 411–413. [Google Scholar] [CrossRef]
- Al-Hebshi, N.N.; Borgnakke, W.S.; Johnson, N.W. The microbiome of oral squamous cell carcinomas: A functional perspective. Curr. Oral Health Rep. 2019, 6, 145–160. [Google Scholar] [CrossRef]
- Allaband, C.; McDonald, D.; Vázquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, analyzing, and interpreting gut microbiome data for clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Galvin, S.; Healy, C.M.; Moran, G.P. The microbiome of potentially malignant oral leukoplakia exhibits enrichment for Fusobacterium, Leptotrichia, Campylobacter, and Rothia species. Front. Microbiol. 2017, 8, 2391. [Google Scholar] [CrossRef] [PubMed]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression innan oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, D.; Gonzalez-Gonzalez, M.; Sykut, J.; Koziel, J.; Ciaston, I.; Adamowicz, K.; Bras, G.; Zawrotniak, M.; Karkowska-Kuleta, J.; Satala, D.; et al. Candida albicans shields the periodontal killer Porphyromonas gingivalis from recognition by the host immune system and supports the bacterial infection of gingival tissue. Int. J. Mol. Sci. 2020, 21, 1984. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.D.; Xie, Z.; Sobue, T.; Thompson, A.; Biyikoglu, B.; Ricker, A.; Ikonomou, L.; Dongari-Bagtzoglou, A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 2012, 80, 620–632. [Google Scholar] [CrossRef]
- Shigeishi, H.; Sugiyama, M.; Ohta, K. Relationship between the prevalence of oral human papillomavirus DNA and periodontal disease (Review). Biomed. Rep. 2021, 14, 40. [Google Scholar] [CrossRef]
- Glasspoole, C.; Louise, D. The Role of Periodontal Bacteria and Epigenetic Modifications on Human Papillomavirus Pathogenicity. Ph.D. Thesis, University of North Carolina, Chapel Hill, NC, USA, 2019. [Google Scholar] [CrossRef]
- Núñez-Acurio, D.; Bravo, D.; Aguayo, F. Epstein-Barr virus-oral bacterial link in the development of oral squamous cell carcinoma. Pathogens 2020, 9, 1059. [Google Scholar] [CrossRef]
- Broderick, N.A. A common origin for immunity and digestion. Front. Immunol. 2015, 6, 72. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.R.; Antia, R. Why we don’t get sick: The within-host population dynamics of bacterial infections. Science 2001, 292, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.R.; Baquero, F.; Ankomah, P.P.; McCall, I.C. Phagocytes, antibiotics, and self-limiting bacterial infections. Trends Microbiol. 2017, 25, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Venutolo, G.; Tanyà, M.P.; Carri, M.D.; Landolfi, A.; Fasano, A. COVID-19 and the gastrointestinal tract: Source of infection or merely a target of the inflammatory process following SARS-CoV-2 infection? World J. Gastroenterol. 2021, 27, 1406–1418. [Google Scholar] [CrossRef]
- Howell, M.C.; Green, R.; McGill, A.R.; Dutta, R.; Mohapatra, S.; Mohapatra, S.S. SARS-CoV-2-induced gut microbiome dysbiosis: Implications for colorectal cancer. Cancers 2021, 13, 2676. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Garbarino, J.; Sturley, S.L. Saturated with fat: New perspectives on lipotoxicity. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 110–116. [Google Scholar] [CrossRef]
- Whisner, C.M.; Aktipis, C.A. The Role of the microbiome in cancer initiation and progression: How microbes and cancer cells utilize excess energy and promote one another’s growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef]
- Andrews, M.C.; Reuben, A.; Gopalakrishnan, V.; Wargo, J.A. Concepts collide: Genomic, immune, and microbial influences on the tumor microenvironment and response to cancer therapy. Front. Immunol. 2018, 9, 946. [Google Scholar] [CrossRef]
- Woo, B.H.; Kim, D.J.; Choi, J.I.; Kim, S.J.; Park, B.S.; Song, J.M.; Lee, J.H.; Park, H.R. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget 2017, 8, 46981–46992. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y. Microbiome harbored within tumors: A new chance to revisit our understanding of cancer pathogenesis and treatment. Signal. Transduct. Target. Ther. 2020, 5, 136. [Google Scholar] [CrossRef] [PubMed]
| Genus/Species | Possible Mechanisms of Association with Oral Cancer |
|---|---|
| Acetobacter syzygii | Possesses anticancer activity promoting the induction of apoptosis in oral cancer cells [22] |
| Actinobacillus | Upregulation of CCL20 in cancer cells [23] |
| Aggregatibacter | Production of proinflammatory cytokines [4]; production of hydrogen sulfide and methyl mercaptan inducing inflammation, cell proliferation, and tumor angiogenesis [9] |
| Capnocytophaga | Stimulation of inflammation [4] |
| Catonella | Induction of chronic inflammation [15] |
| Eikenella corrodens | Elevated production of IL-1, IL-6, IL-8, and TNF-α [24] |
| Enterococcus | Increase in genomic instability linked to superoxide production [25]; maintaining chronic inflammation [26] |
| Filifactor | Production of proinflammatory cytokines; activation of oncogenes; enhances tumor progression by promoting colonization by other pathogens [9] |
| Fusobacterium nucleatum | Secretion of IL-1β through activation of NLRP3 inflammasome [27]; p38 activation leading to increased production of MMP-13 and MMP-9 [28]; Ku70/p53 signaling pathway-dependent DNA damage. [29]; acceleration of cell cycle through p27 downregulation; induction of epithelial–mesenchymal transition through lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 pathway [30]; activation of oncogenes cyclin D1 and myc through β-catenin pathway [31] |
| Gemella | IL-23 upregulation [17] |
| Lactobacillus | Some species produce lactate; L. fermentum produces hydrogen peroxide [32]; |
| L. planarum induces cancer cell apoptosis via upregulation of PTEN and downregulation of MAPK pathway [16] | |
| Mycoplasma salivarium | p53 inhibition; activation of NF-κB signal pathway [33] |
| Parvimonas | Inflammation induction [16] |
| Porphyromonas gingivalis | Stimulation of Jak1/Stat3 signaling pathway through upregulation of proinflammatory cytokines [27]; upregulation of miRNA-203 [34]; production of nucleoside diphosphate kinases [35]; stimulation of cell proliferation through upregulation of cyclins and p53 inhibition [28]; induction of epithelial–mesenchymal transition through overexpression of β-catenin; chronic inflammation induction through IL-8, IL-6, TGF-β1, and TNF-α expression [36]; production of reactive oxygen species, butyrate, and acetaldehyde [37] |
| Prevotella intermedia | Production of virulent factors (lipopolysaccharides, peptidoglycans, lipoteichoic acid [9]; IL-1, IL-6, IL-17, IL-23, and TNF-α expression [22]; secretion of proteases [15]; production of hydrogen sulfide, methyl mercaptan, and acetaldehyde [4] |
| Propionibacterium | Production of IL-6 and IL-8 [4] |
| Pseudomonas aeruginosa | Induction of inflammation through NF-κB pathway activation [9]; DNA break induction leading to chromosomal instability; secretion of LasI factor leading to downregulation of E-cadherin expression [9]; endotoxins such as LPS or flagella contribute to the induction of inflammation [16] |
| Rothia | Acetaldehyde production [9] |
| Streptococcus anginosus | Production of proinflammatory cytokines; nitric oxide and cyclooxygenase-2 production [9]; acetaldehyde production [38] |
| Streptococcus aureus | Upregulation of COX-2 transcription; production of prostaglandins PGE2; induction of cyclin D1 overexpression [16] |
| Streptococcus gordonii | Suppression of epithelial–mesenchymal transition through decreasing ZEB2 expression; acetaldehyde production [36] |
| Streptococcus mitis | Suppression of OSCC cell proliferation in vitro [37]; prevents colonization by virulent microorganisms [4]; acetaldehyde production [38] |
| Streptococcus salivarius | Acetaldehyde production [9] |
| Streptomyces | Induction of cancer cell apoptosis [21] |
| Tannerella | Proinflammatory cytokine production [4] |
| Treponema denticola | Dentilisin overexpression associated with increased tumor invasiveness [18] |
| Genus/Species | Abundance in OSCC Case Samples Relative to Control/Case Samples | Case Samples from OSCC Patients | Control/Case Samples | Number of Participants | Reference |
|---|---|---|---|---|---|
| Aspergillus tamarii | Decreased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Alternaria | Decreased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Candida albicans | Increased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Candida etchellsii | Increased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Candida famata | Increased | Saliva | Saliva | 97 OSCC patients, 200 OPMD patients, 200 healthy individuals | [83] |
| Cladosporium halotolerans | Decreased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Emericella | Decreased | Tongue cancer tissue | Normal tissue | 39 OSCC patients | [84] |
| Gibberella | Increased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Hannaella | Increased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Malassezia restricta | Decreased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Pichia anomala | Decreased | Saliva | Saliva | 97 OSCC patients, 200 OPMD patients, 200 healthy individuals | [83] |
| Rhodotorula mucilaginosa | Increased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Trametes | Decreased | OSCC tissue | FEP | 25 OSCC patients, 27 FEP patients | [82] |
| Virus Type | Samples from OSCC Patients | Conclusion | Number of Participants 1 | Reference |
|---|---|---|---|---|
| HPV | Saliva and OSCC tissue | HPV 16 positivity rate was 15.4% (saliva), HPV 18 positivity rate was 1.6% (tissue) | 135 samples (13 saliva, 59 blood, 63 OSCC tissues) | [97] |
| Paraffin-embedded OSCC tissue | HPV 16 positivity rate was 19.2% | 114 OSCC patients | [98] | |
| Saliva | 46% of patients had positive HPV-DNA | 35 OSCC patients, 20 healthy individuals | [99] | |
| FFPE OSCC tissue | 66% of cases were positive for HPV 38 DNA | 53 OSCC patients | [100] | |
| Samples of OSCC, oral leukoplakia, oral lichen planus | The frequency of HPV positivity was 1.54% | 32 OSCC patients, 17 patients with oral leukoplakia, 16 patients with oral lichen planus | [101] | |
| EBV | FFPE OSCC tissue | The prevalence of EBV in OSCC was 41.2% | 165 OSCC patients | [102] |
| OSCC tissue | Microarray analysis found 82.5% EBV prevalent rate | 57 OSCC patients | [103] | |
| OSCC tissue | 20% of cases were positive for EBV | 20 OSCC patients, 20 controls | [104] | |
| HCMV | FFPE OSCC tissue | 6.3% of cases were positive for HCMV | 48 OSCC patients | [105] |
| HSV | FFPE OSCC tissue | HSV-1 was detected in 22% of cases, HSV-2 in 8% of cases | 40 OSCC patients, 10 patients with benign tumor | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyhnalova, T.; Danek, Z.; Gachova, D.; Linhartova, P.B. The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma. Microorganisms 2021, 9, 1549. https://doi.org/10.3390/microorganisms9081549
Vyhnalova T, Danek Z, Gachova D, Linhartova PB. The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma. Microorganisms. 2021; 9(8):1549. https://doi.org/10.3390/microorganisms9081549
Chicago/Turabian StyleVyhnalova, Tereza, Zdenek Danek, Daniela Gachova, and Petra Borilova Linhartova. 2021. "The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma" Microorganisms 9, no. 8: 1549. https://doi.org/10.3390/microorganisms9081549
APA StyleVyhnalova, T., Danek, Z., Gachova, D., & Linhartova, P. B. (2021). The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma. Microorganisms, 9(8), 1549. https://doi.org/10.3390/microorganisms9081549






