Ruxolitinib Alleviates Uveitis Caused by Salmonella typhimurium Endotoxin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Induction of EIU and Ruxolitinib Treatments
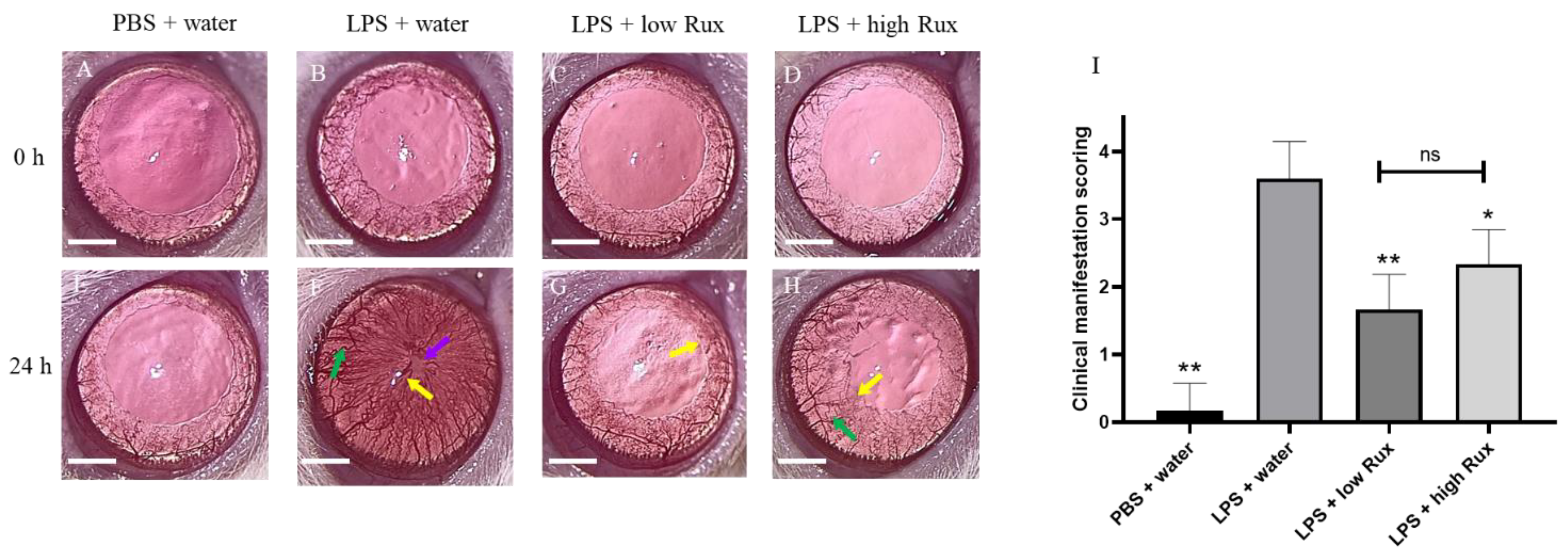
2.2. Clinical Scoring
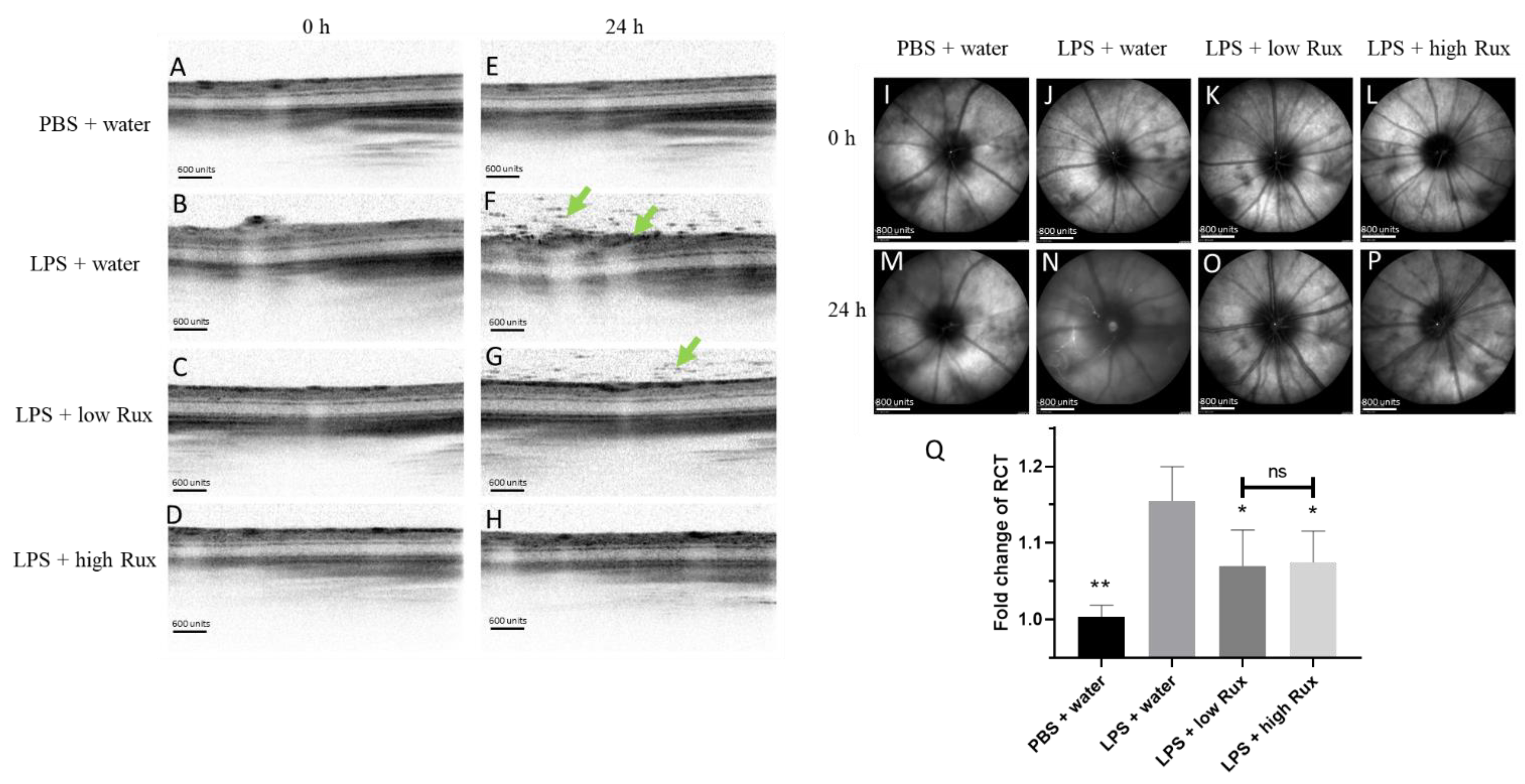
2.3. Retinal Examination by Confocal Scanning Laser Ophthalmoscope (CSLO) and Optical Coherence Tomography (OCT)
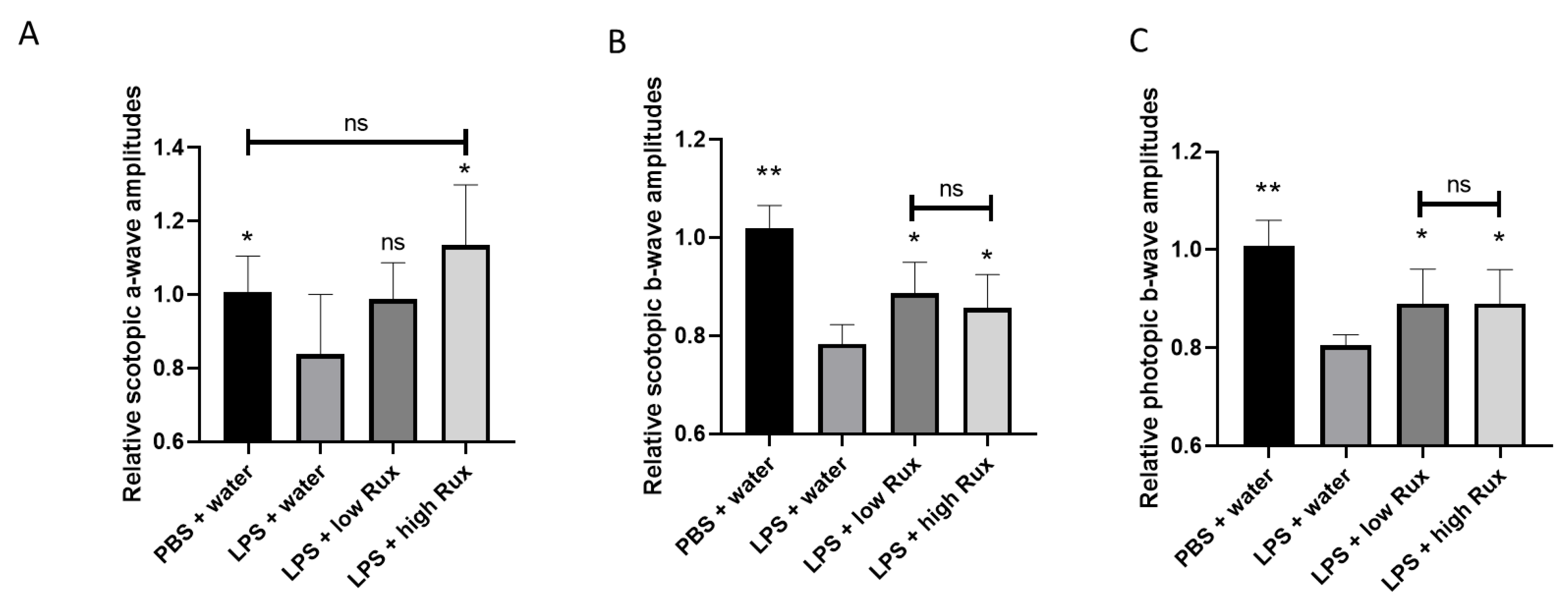
2.4. Electroretinography (ERG)
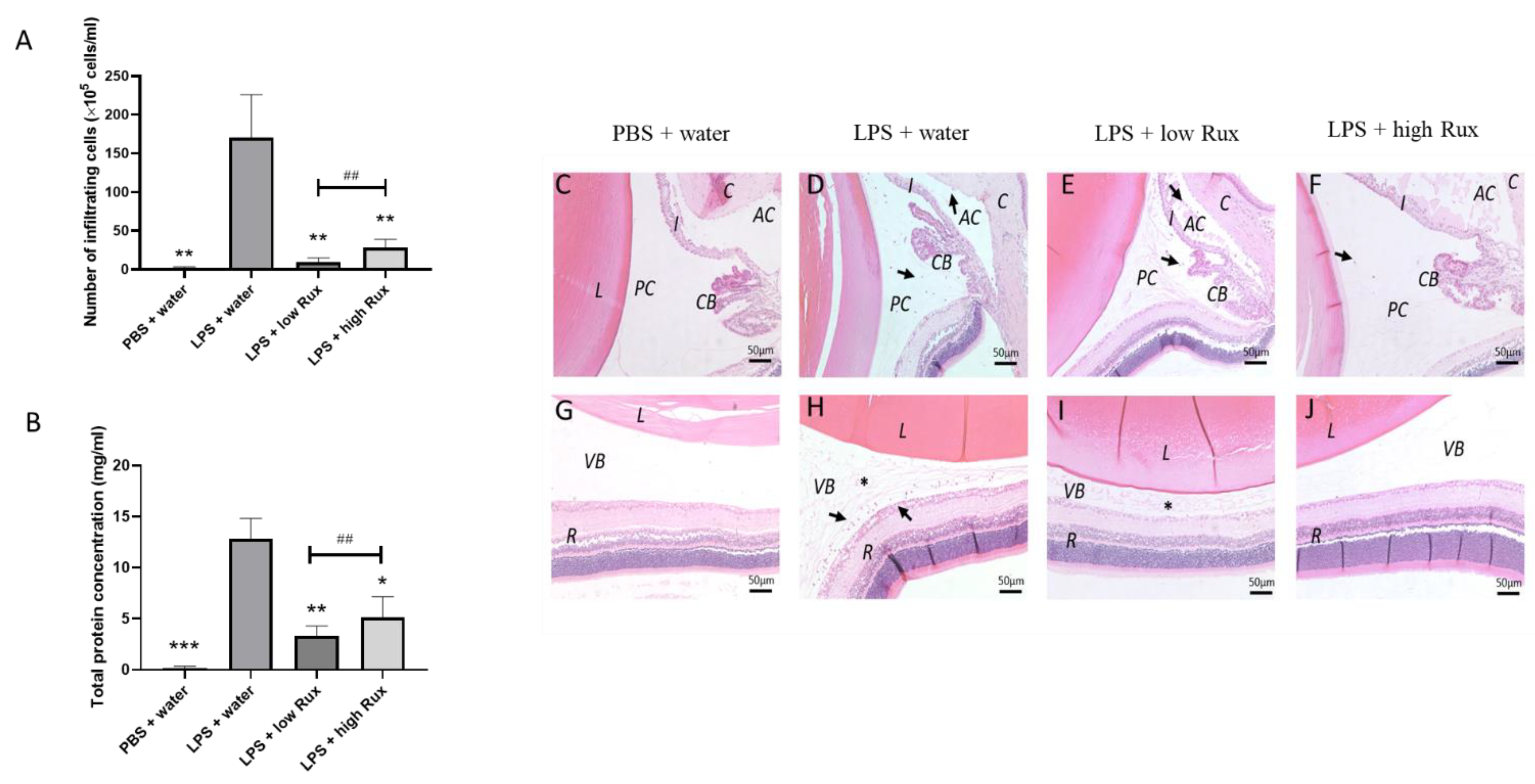
2.5. Cell Counting and Protein Quantification in Aqueous Humor
2.6. Histopathological Evaluation
2.7. Statistical Analysis
3. Results
3.1. Ruxolitinib Alleviated Clinical Manifestations of Inflammation in Eyes
3.2. Ruxolitinib Reduced Retinal–Choroidal Thickness and Infiltrating Cells in the Vitreous and Retina in EIU
3.3. Electroretinography
3.4. Ruxolitinib Alleviated Protein Secretion into Aqueous Humor and Infiltrating Cells in Anterior and Posterior Segments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill Gaston, J.S.; Lillicrap, M.S. Arthritis associated with enteric infection. Best Pract. Res. Clin. Rheumatol. 2003, 17, 219–239. [Google Scholar] [CrossRef]
- Lee, D.A.; Barker, S.M.; Su, W.P.; Allen, G.L.; Liesegang, T.J.; Ilstrup, D.M. The clinical diagnosis of Reiter’s syndrome. Ophthalmic and nonophthalmic aspects. Ophthalmology 1986, 93, 350–356. [Google Scholar] [CrossRef]
- Rothova, A.; Suttorp-van Schulten, M.S.; Frits Treffers, W.; Kijlstra, A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br. J. Ophthalmol. 1996, 80, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.R.; Huang, J.C.; Tao, Y.; Kaburaki, T.; Lee, C.S.; Lin, T.C.; Hsu, C.C.; Chiou, S.H.; Hwang, D.K. Noninfectious uveitis in the Asia-Pacific region. Eye 2019, 33, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.J.; Chu, K.O.; Yip, Y.W.; Li, W.Y.; Yang, Y.P.; Chan, K.P.; Ren, J.L.; Chan, S.O.; Pang, C.P. Green tea extract treatment alleviates ocular inflammation in a rat model of endotoxin-induced uveitis. PLoS ONE 2014, 9, e103995. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Ma, J.; Gu, R.; Ding, X.; Lei, B.; Wang, X.; Zhuang, H.; Xu, G. Lipocalin 2 Suppresses Ocular Inflammation by Inhibiting the Activation of NF-kappabeta Pathway in Endotoxin-Induced Uveitis. Cell. Physiol. Biochem. 2018, 46, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, T.; Honjo, M.; Yamagishi, R.; Aihara, M. The Anti-Inflammatory Effect of Ripasudil (K-115), a Rho Kinase (ROCK) Inhibitor, on Endotoxin-Induced Uveitis in Rats. Invest. Ophthalmol. Vis. Sci. 2017, 58, 5584–5593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Q.; Liu, H.L.; Wang, G.B.; Wu, P.F.; Yan, T.; Xie, J.; Tang, Y.; Sun, L.K.; Li, C. Effect of artesunate on endotoxin-induced uveitis in rats. Invest. Ophthalmol. Vis. Sci. 2011, 52, 916–919. [Google Scholar] [CrossRef]
- Smith, J.R.; Hart, P.H.; Williams, K.A. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol. Cell Biol. 1998, 76, 497–512. [Google Scholar] [CrossRef]
- Ren, J.L.; Yu, Q.X.; Ma, D.; Liang, W.C.; Leung, P.Y.; Ng, T.K.; Chu, W.K.; Schally, A.V.; Pang, C.P.; Chan, S.O. Growth hormone-releasing hormone receptor mediates cytokine production in ciliary and iris epithelial cells during LPS-induced ocular inflammation. Exp. Eye Res. 2019, 181, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.C.; Ren, J.L.; Yu, Q.X.; Li, J.; Ng, T.K.; Chu, W.K.; Qin, Y.J.; Chu, K.O.; Schally, A.V.; Pang, C.P.; et al. Signaling mechanisms of growth hormone-releasing hormone receptor in LPS-induced acute ocular inflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 6067–6074. [Google Scholar] [CrossRef]
- Pouvreau, I.; Zech, J.C.; Thillaye-Goldenberg, B.; Naud, M.C.; Van Rooijen, N.; de Kozak, Y. Effect of macrophage depletion by liposomes containing dichloromethylene-diphosphonate on endotoxin-induced uveitis. J. Neuroimmunol. 1998, 86, 171–181. [Google Scholar] [CrossRef]
- Wu, T.T.; Chen, T.L.; Chen, R.M. Lipopolysaccharide triggers macrophage activation of inflammatory cytokine expression, chemotaxis, phagocytosis, and oxidative ability via a toll-like receptor 4-dependent pathway: Validated by RNA interference. Toxicol. Lett. 2009, 191, 195–202. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, P.G.; Crewe, J. Endotoxin-induced uveitis. Kinetics and phenotype of the inflammatory cell infiltrate and the response of the resident tissue macrophages and dendritic cells in the iris and ciliary body. Invest. Ophthalmol. Vis. Sci. 1995, 36, 1949–1959. [Google Scholar]
- Chu, C.J.; Gardner, P.J.; Copland, D.A.; Liyanage, S.E.; Gonzalez-Cordero, A.; Kleine Holthaus, S.M.; Luhmann, U.F.; Smith, A.J.; Ali, R.R.; Dick, A.D. Multimodal analysis of ocular inflammation using the endotoxin-induced uveitis mouse model. Dis. Models Mech. 2016, 9, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, S.; Letko, E.; Qamruddin, S.; Baltatzis, S.; Foster, C.S. Long-term progression, prognosis, and treatment of patients with recurrent ocular manifestations of Reiter’s syndrome. Ophthalmology 2003, 110, 1764–1769. [Google Scholar] [CrossRef]
- Fischel, J.D.; Lipton, J. Acute anterior uveitis in juvenile Reiter’s syndrome. Clin. Rheumatol. 1996, 15, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic side effects of eye drops: A pharmacokinetic perspective. Clin. Ophthalmol. 2016, 10, 2433–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Aggarwal, K.; Gupta, V. Infectious uveitis: An Asian perspective. Eye 2019, 33, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar] [CrossRef]
- Rodig, S.J.; Meraz, M.A.; White, J.M.; Lampe, P.A.; Riley, J.K.; Arthur, C.D.; King, K.L.; Sheehan, K.C.; Yin, L.; Pennica, D.; et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998, 93, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Jamilloux, Y.; El Jammal, T.; Vuitton, L.; Gerfaud-Valentin, M.; Kerever, S.; Seve, P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2019, 18, 102390. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology 2019, 58, 1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vainchenker, W.; Leroy, E.; Gilles, L.; Marty, C.; Plo, I.; Constantinescu, S.N. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Research 2018, 7, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilling, A.D.; Nedza, F.M.; Emm, T.; Diamond, S.; McKeever, E.; Punwani, N.; Williams, W.; Arvanitis, A.; Galya, L.G.; Li, M.; et al. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab. Dispos. 2010, 38, 2023–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoerl, S.; Mathew, N.R.; Bscheider, M.; Schmitt-Graeff, A.; Chen, S.; Mueller, T.; Verbeek, M.; Fischer, J.; Otten, V.; Schmickl, M.; et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 2014, 123, 3832–3842. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.M.; Thillaye, B.; de Kozak, Y. Retino-choroidal changes in endotoxin-induced uveitis in the rat. Ophthalmic Res. 1992, 24, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Z.W.; Weinreb, R.N.; Xu, G.; Lindsey, J.D.; Ye, C.; Yung, W.H.; Pang, C.P.; Lam, D.S.; Leung, C.K. Tracking retinal microgliosis in models of retinal ganglion cell damage. Invest. Ophthalmol. Vis. Sci. 2012, 53, 6254–6262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, J.; Yip, Y.W.Y.; Zhang, X.; Chu, K.O.; Ng, T.K.; Chan, S.O.; Pang, C.P.; Chu, W.K. Quantitative Characterization of Autoimmune Uveoretinitis in an Experimental Mouse Model. Invest. Ophthalmol. Vis. Sci. 2017, 58, 4193–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Normando, E.M.; Nizari, S.; Lara, D.; Cordeiro, M.F. Tracking longitudinal retinal changes in experimental ocular hypertension using the cSLO and spectral domain-OCT. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6504–6513. [Google Scholar] [CrossRef]
- Yeung, I.Y.; Popp, N.A.; Chan, C.C. The role of sex in uveitis and ocular inflammation. Int. Ophthalmol. Clin. 2015, 55, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, Y.S.; Yu, C.R.; Egwuagu, C.E. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 2008, 180, 6070–6076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miserocchi, E.; Giuffre, C.; Cornalba, M.; Pontikaki, I.; Cimaz, R. JAK inhibitors in refractory juvenile idiopathic arthritis-associated uveitis. Clin. Rheumatol. 2020, 39, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Bauermann, P.; Heiligenhaus, A.; Heinz, C. Effect of Janus Kinase Inhibitor Treatment on Anterior Uveitis and Associated Macular Edema in an Adult Patient with Juvenile Idiopathic Arthritis. Ocul. Immunol. Inflamm. 2019, 27, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Lee, C.H.; Pei, S.N. Pulmonary tuberculosis reactivation following ruxolitinib treatment in a patient with primary myelofibrosis. Leuk. Lymphoma 2015, 56, 1528–1529. [Google Scholar] [CrossRef] [PubMed]
- Hopman, R.K.; Lawrence, S.J.; Oh, S.T. Disseminated tuberculosis associated with ruxolitinib. Leukemia 2014, 28, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Feenstra, J.; Georghiou, P.R. Pneumocystis jiroveci pneumonitis complicating ruxolitinib therapy. BMJ Case Rep. 2014, 2014, bcr2014204950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, R.A.; Reichel, E.; Oshry, L.J. Bilateral toxoplasmosis retinitis associated with ruxolitinib. N. Engl. J. Med. 2013, 369, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, B.; Joshipura, D.; Saraiya, A.; Abdat, R.; Ashkar, H.; Turkowski, Y.; Sheth, V.; Huang, V.; Au, S.C.; Kachuk, C.; et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J. Am. Acad. Dermatol. 2017, 76, 1054–1060.e1051. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Sadrai, Z.; Hua, J.; Kodati, S.; Huang, J.F.; Chauhan, S.K.; Dana, R. Effects of topical Janus kinase inhibition on ocular surface inflammation and immunity. Cornea 2014, 33, 177–183. [Google Scholar] [CrossRef]
- Li, L.; He, X.; Wang, X.; Sun, Y.; Wu, G.; Fang, H.; Wang, C.; Luo, P.; Xia, Z. Ruxolitinib protects lipopolysaccharide (LPS)-induced sepsis through inhibition of nitric oxide production in mice. Ann. Transl. Med. 2020, 8, 546. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Meylan, F.; O’Shea, J.J.; Gadina, M. JAK inhibitors: Ten years after. Eur. J. Immunol. 2021, 51, 1615–1627. [Google Scholar] [CrossRef]
- Koizumi, K.; Poulaki, V.; Doehmen, S.; Welsandt, G.; Radetzky, S.; Lappas, A.; Kociok, N.; Kirchhof, B.; Joussen, A.M. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2184–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekici, F.; Karaca, E.E.; Korkmaz, S.; Yuksel, O.; Gulbahar, O.; Alper, M.; Ercan, S.; Or, M. Effect of the Toll-like receptor 4 antagonist eritoran on retinochoroidal inflammatory damage in a rat model of endotoxin-induced inflammation. Mediat. Inflamm. 2014, 2014, 643525. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Yip, Y.W.Y.; Ng, H.K.; Ho, B.M.; He, J.-N.; Chan, S.O.; Pang, C.P.; Chu, W.K. Ruxolitinib Alleviates Uveitis Caused by Salmonella typhimurium Endotoxin. Microorganisms 2021, 9, 1481. https://doi.org/10.3390/microorganisms9071481
Du L, Yip YWY, Ng HK, Ho BM, He J-N, Chan SO, Pang CP, Chu WK. Ruxolitinib Alleviates Uveitis Caused by Salmonella typhimurium Endotoxin. Microorganisms. 2021; 9(7):1481. https://doi.org/10.3390/microorganisms9071481
Chicago/Turabian StyleDu, Lin, Yolanda Wong Ying Yip, Him Kwan Ng, Bo Man Ho, Jing-Na He, Sun On Chan, Chi Pui Pang, and Wai Kit Chu. 2021. "Ruxolitinib Alleviates Uveitis Caused by Salmonella typhimurium Endotoxin" Microorganisms 9, no. 7: 1481. https://doi.org/10.3390/microorganisms9071481
APA StyleDu, L., Yip, Y. W. Y., Ng, H. K., Ho, B. M., He, J.-N., Chan, S. O., Pang, C. P., & Chu, W. K. (2021). Ruxolitinib Alleviates Uveitis Caused by Salmonella typhimurium Endotoxin. Microorganisms, 9(7), 1481. https://doi.org/10.3390/microorganisms9071481







