Current Epidemiological Characteristics of Imported Malaria, Vector Control Status and Malaria Elimination Prospects in the Gulf Cooperation Council (GCC) Countries
Abstract
1. Introduction
2. Status of Malaria Infection and Vector Control in Gulf Cooperation Council (GCC) Countries
2.1. Status of Vector Control in GCC Countries
2.2. Status of Malaria Infection in GCC Countries
2.2.1. Status of Malaria and Vector Control in Bahrain
2.2.2. Status of Imported Malaria in Kuwait
2.2.3. Status of Malaria and Vector Control in Oman
2.2.4. Status of Imported Malaria in Qatar
2.2.5. Status of Malaria and Vector Control in Saudi Arabia
2.2.6. Status of Imported Malaria in the United Arab Emirates (UAE)
3. Malaria Status among Countries Contributing Most of the Imported Malaria Cases in GCC Countries
3.1. Characterization of Malaria Cases from India
3.2. Characterization of Malaria Cases from Pakistan
3.3. Characterization of Malaria Cases from Afghanistan
3.4. Characterization of Malaria Cases from Bangladesh
3.5. Characterization of Malaria Cases from the Philippines
3.6. Characterization of Malaria Cases from Nigeria
3.7. Characterization of Malaria Cases from Ehiopia
3.8. Characterization of Malaria Cases from Sudan
4. Prospects for Malaria Elimination in GCC Countries
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herricks, J.R.; Hotez, P.J.; Wanga, V.; Coffeng, L.E.; Haagsma, J.A.; Basáñez, M.G.; Buckle, G.; Budke, C.M.; Carabin, H.; Fèvre, E.M.; et al. The global burden of disease study 2013: What does it mean for the NTDs? PLoS Negl. Trop. Dis. 2017, 11, e0005424:1–e0005424:21. [Google Scholar] [CrossRef]
- GBD. Chronic Respiratory Disease Collaborators, Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2019. Available online: https://www.who.int/malaria/publications/world-malaria-report-2019/en/ (accessed on 15 December 2020).
- Dayananda, K.K.; Achur, R.N.; Gowda, D.C. Epidemiology, drug resistance, and pathophysiology of Plasmodium vivax malaria. J. Vector Borne Dis. 2018, 55, 1–8. [Google Scholar] [PubMed]
- Twohig, K.A.; Pfeffer, D.A.; Baird, J.K.; Price, R.C.; Zimmerman, P.A.; Hay, S.I.; Gething, P.W.; Battle, K.E.; Howes, R.E. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl. Trop. Dis. 2019, 13, e0007140:1–e0007140:16. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2020. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 15 March 2021).
- World Health Organization. Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy: Status Report. Available online: https://apps.who.int/iris/handle/10665/274362 (accessed on 15 March 2021).
- Arya, A.; Kojom Foko, L.P.; Chaudhry, S.; Sharma, A.; Singh, V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions-India and sub-Saharan Africa. Int. J. Parasitol. Drugs Drug Resist. 2020, 15, 43–56. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016. Available online: https://www.who.int/malaria/publications/atoz/9789241514057/en/ (accessed on 18 March 2021).
- Arinaitwe, E.; Nankabirwa, J.I.; Krezanoski, P.; Rek, J.; Kamya, V.; Epstein, A.; Rosenthal, P.J.; Drakeley, C.; Kamya, M.R.; Dorsey, G.; et al. Association between recent overnight travel and use of long-lasting insecticidal nets in rural Uganda: A prospective cohort study in Tororo. Malar. J. 2020, 19, 405:1–405:10. [Google Scholar] [CrossRef]
- Martin, J.L.; Mosha, F.W.; Lukole, E.; Rowland, M.; Todd, J.; Charlwood, J.D.; Mosha, J.F.; Protopopoff, N. Personal protection with PBO-pyrethroid synergist-treated nets after 2 years of household use against pyrethroid-resistant Anopheles in Tanzania. Parasit. Vectors 2021, 14, 150:1–150:8. [Google Scholar] [CrossRef]
- Al-Awadhi, M.; Ahmad, S.; Iqbal, J. Current status and the epidemiology of malaria in the Middle East Region and beyond. Microorganisms 2021, 9, 338. [Google Scholar] [CrossRef]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030. Available online: https://www.who.int/malaria/publications/atoz/9789241564991/en/ (accessed on 20 March 2021).
- Murray, C.J.; Ortblad, K.F.; Guinovart, C.; Lim, S.S.; Wolock, T.M.; Roberts, D.A.; Dansereau, E.A.; Graetz, N.; Barber, R.M.; Brown, J.C.; et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 1005–1070. [Google Scholar] [CrossRef]
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Loutan, L. Malaria: Still a threat to travellers. Int. J. Antimicrob. Agents 2003, 21, 158–163. [Google Scholar] [CrossRef]
- Mischlinger, J.; Rönnberg, C.; Álvarez-Martínez, M.J.; Bühler, S.; Paul, M.; Schlagenhauf, P.; Petersen, E.; Ramharter, M. Imported malaria in countries where malaria is not endemic: A comparison of semi-immune and nonimmune travelers. Clin. Microbiol. Rev. 2020, 33, e00104–e00119. [Google Scholar] [CrossRef]
- Norman, F.F.; Comeche, B.; Chamorro, S.; Pérez-Molina, J.A.; López-Vélez, R. Update on the major imported protozoan infections in travelers and migrants. Future Microbiol. 2020, 15, 213–225. [Google Scholar] [CrossRef]
- Golassa, L.; Messele, A.; Amambua-Ngwa, A.; Swedberg, G. High prevalence and extended deletions in Plasmodium falciparum hrp2/3 genomic loci in Ethiopia. PLoS ONE 2020, 15, e0241807:1–e0241807:11. [Google Scholar] [CrossRef]
- Nyataya, J.; Waitumbi, J.; Mobegi, V.A.; Noreddin, A.; El Zowalaty, M.E. Plasmodium falciparum histidine-rich protein 2 and 3 gene deletions and their implications in malaria control. Diseases 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Khammanee, T.; Sawangjaroen, N.; Buncherd, H.; Tun, A.W.; Thanapongpichat, S. A LAMP-SNP assay detecting C580Y mutation in Pfkelch13 gene from clinically dried blood spot samples. Korean J. Parasitol. 2021, 59, 15–22. [Google Scholar] [CrossRef]
- Sherrard-Smith, E.; Hogan, A.B.; Hamlet, A.; Watson, O.J.; Whittaker, C.; Winskill, P.; Ali, F.; Mohammad, A.B.; Uhomoibhi, P.; Maikore, I.; et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat. Med. 2020, 26, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Bertozzi-Villa, A.; Rumisha, S.F.; Amratia, P.; Arambepola, R.; Battle, K.E.; Cameron, E.; Chestnutt, E.; Gibson, H.S.; Harris, J.; et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: A geospatial modelling analysis. Lancet Infect. Dis. 2021, 21, 59–69. [Google Scholar] [CrossRef]
- World Health Organization. Countries and Territories Certified Malaria-Free by WHO. Available online: https://www.who.int/malaria/areas/elimination/malaria-free-countries/en/ (accessed on 18 March 2021).
- Snow, R.W.; Amratia, P.; Zamani, G.; Mundia, C.W.; Noor, A.M.; Memish, Z.A.; Al Zahrani, M.H.; Al Jasari, A.; Fikri, M.; Atta, H. The malaria transition on the Arabian Peninsula: Progress toward a malaria-free region between 1960–2010. Adv. Parasitol. 2013, 82, 205–251. [Google Scholar]
- Hay, S.I.; Sinka, M.E.; Okara, R.M.; Kabaria, C.W.; Mbithi, P.M.; Tago, C.C.; Benz, D.; Gething, P.W.; Howes, R.E.; Patil, A.P. Developing global maps of the dominant Anopheles vectors of human malaria. PLoS Med. 2010, 7, e1000209:1–e1000209:6. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasit. Vectors 2012, 5, 69:1–69:11. [Google Scholar] [CrossRef]
- Mahmood, R.A. History of eradication of malaria in Bahrain. J. Bahrain Med. Soc. 1992, 4, 118–119. [Google Scholar]
- Ismaeel, A.Y.; Senok, A.C.; Jassim Al-Khaja, K.A.; Botta, G.A. Status of malaria in the Kingdom of Bahrain: A 10-year review. J. Travel Med. 2004, 11, 97–101. [Google Scholar] [CrossRef]
- Hassan, K.S. World Malaria Day: Our story with malaria in Oman. Sultan Qaboos Univ. Med. J. 2017, 17, e133–e134. [Google Scholar] [CrossRef]
- Simon, B.; Sow, F.; Al Mukhaini, S.K.; Al-Abri, S.; Ali, O.A.; Bonnot, G.; Bienvenu, A.L.; Petersen, E.; Picot, S. An outbreak of locally acquired Plasmodium vivax malaria among migrant workers in Oman. Parasite 2017, 24, 25:1–25:10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ministry of Health, Oman. Annual Report 2019. Available online: www.moh.gov.om (accessed on 1 March 2021).
- Al-Kuwari, M.G. Epidemiology of imported malaria in Qatar. J. Travel Med. 2009, 16, 119–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alahmed, A.M.; Munawar, K.; Khalil, S.M.; Harbach, R.E. Assessment and an updated list of the mosquitoes of Saudi Arabia. Parasit. Vectors 2019, 12, 356:1–356:9. [Google Scholar] [CrossRef]
- Hawash, Y.; Ismail, K.; Alsharif, K.; Alsanie, W. Malaria prevalence in a low transmission area, Jazan District of southwestern Saudi Arabia. Korean J. Parasitol. 2019, 57, 233–242. [Google Scholar] [CrossRef]
- Nilles, E.J.; Alosert, M.; Mohtasham, M.A.; Saif, M.; Sulaiman, L.; Seliem, R.M.; Kotlyar, S.; Dziura, J.D.; Al-Najjar, F.J. Epidemiological and clinical characteristics of imported malaria in the United Arab Emirates. J. Travel Med. 2014, 21, 201–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. The Work of WHO in the Eastern Mediterranean Region: Annual Report of the Regional Director 2017. Available online: https://applications.emro.who.int/docs/RD_Annual_Rep_2018_20507_EN.pdf (accessed on 12 March 2021).
- Shibl, A.; Senok, A.; Memish, Z. Infectious diseases in the Arabian Peninsula and Egypt. Clin. Microbiol. Infect. 2012, 18, 1068–1080. [Google Scholar] [CrossRef]
- Mokaddas, E.; Ahmad, S.; Samir, I. Secular trends in susceptibility patterns of Mycobacterium tuberculosis isolates in Kuwait, 1996–2005. Int. J. Tuberc. Lung Dis. 2008, 12, 319–325. [Google Scholar] [PubMed]
- Ahmad, S.; Al-Mutairi, N.M.; Mokaddas, E. Variations in the occurrence of specific rpoB mutations in rifampicin-resistant Mycobacterium tuberculosis strains isolated from patients of different ethnic groups in Kuwait. Indian J. Med. Res. 2012, 135, 756–762. [Google Scholar] [PubMed]
- Al-Mutairi, N.M.; Ahmad, S.; Mokaddas, E. Molecular characterization of multidrug-resistant Mycobacterium tuberculosis (MDR-TB) isolates identifies local transmission of infection in Kuwait, a country with a low incidence of TB and MDR-TB. Eur. J. Med. Res. 2019, 24, 38:1–38:13. [Google Scholar] [CrossRef]
- Al-Awadhi, M.; Iqbal, J.; Ahmad, S. Cysticercosis, a potential public health concern in Kuwait: A new diagnostic method to screen Taenia solium taeniasis carriers in the expatriate population. Med. Princ. Pract. 2020, 29, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.M.I.; Amarasekara, S.; Wickremasinghe, R.; Fernando, D.; Udagama, P. Prevention of re-establishment of malaria: Historical perspective and future prospects. Malar. J. 2020, 19, 452:1–452:16. [Google Scholar] [CrossRef]
- Iqbal, J.; Hira, P.R.; Al-Ali, F.; Sher, A. Imported malaria in Kuwait (1985–2000). J. Travel Med. 2003, 10, 324–329. [Google Scholar] [CrossRef]
- Iqbal, J.; Al-Awadhi, M.; Ahmad, S. Decreasing trend of imported malaria cases but increasing influx of mixed P. falciparum and P. vivax infections in malaria-free Kuwait. PLoS ONE 2020, 15, e0243617:1–e0243617:12. [Google Scholar] [CrossRef]
- Farag, E.; Bansal, D.; Chehab, M.A.H.; Al-Dahshan, A.; Bala, M.; Ganesan, N.; Al Abdulla, Y.A.; Al Thani, M.; Sultan, A.A.; Al-Romaihi, H. Epidemiology of malaria in the State of Qatar, 2008–2015. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018050:1–e2018050:5. [Google Scholar] [CrossRef]
- Al-Rumhi, A.; Al-Hashami, Z.; Al-Hamidhi, S.; Gadalla, A.; Naeem, R.; Ranford-Cartwright, L.; Pain, A.; Sultan, A.A.; Babiker, H.A. Influx of diverse, drug resistant and transmissible Plasmodium falciparum into a malaria-free setting in Qatar. BMC Infect. Dis. 2020, 20, 413:1–413:10. [Google Scholar] [CrossRef] [PubMed]
- El Hassan, I.M.; Sahly, A.; Alzahrani, M.H.; Alhakeem, R.F.; Alhelal, M.; Alhogail, A.; Alsheikh, A.A.; Assiri, A.M.; ElGamri, T.B.; Faragalla, I.A.; et al. Progress toward malaria elimination in Jazan Province, Kingdom of Saudi Arabia: 2000–2014. Malar. J. 2015, 14, 444:1–444:10. [Google Scholar] [CrossRef]
- Memish, Z.A.; Alzahrani, M.; Alhakeem, R.F.; Bamgboye, E.A.; Smadi, H.N. Toward malaria eradication in Saudi Arabia: Evidence from 4-year surveillance in Makkah. Ann. Saudi Med. 2014, 34, 153–158. [Google Scholar] [CrossRef]
- Alshahrani, A.M.; Abdelgader, T.M.; Saeed, I.; Al-Akhshami, A.; Al-Ghamdi, M.; Al-Zahrani, M.H.; Snow, R.W. Risk associated with malaria infection in Tihama Qahtan, Aseer Region, Kingdom of Saudi Arabia: 2006–2007. Malar. Control. Elimin. 2019, 5, 144:1–144:11. [Google Scholar]
- Soliman, R.H.; Garcia-Aranda, P.; Elzagawy, S.M.; Hussein, B.E.; Mayah, W.W.; Martin Ramirez, A. Imported and autochthonous malaria in West Saudi Arabia: Results from a reference hospital. Malar. J. 2018, 17, 286:1–286:7. [Google Scholar] [CrossRef] [PubMed]
- The Public Authority for Civil Information, Kuwait. Statistical Reports 2019. Available online: https://www.paci.gov.kw/Default.aspx (accessed on 20 December 2020).
- Iqbal, J.; Sher, A.; Hira, P.R.; Al-Anezi, A.A. Risk of introduction of drug-resistant malaria in a non-endemic country, Kuwait: A real threat? Med. Princ. Pract. 2000, 9, 125–130. [Google Scholar] [CrossRef]
- National Centre for Statistics and Information, Sultanate of Oman. Population Statistics 2020. Available online: https://data.gov.om/data/#topic=Population (accessed on 5 March 2021).
- Abdelraheem, M.H.; Bansal, D.; Idris, M.A.; Mukhtar, M.M.; Hamid, M.M.A.; Imam, Z.S.; Getachew, S.; Sehgal, R.; Kaur, H.; Gadalla, A.H.; et al. Genetic diversity and transmissibility of imported Plasmodium vivax in Qatar and three countries of origin. Sci. Rep. 2018, 8, 8870:1–8870:9. [Google Scholar] [CrossRef] [PubMed]
- Aabdien, M.; Selim, N.; Himatt, S.; Hmissi, S.; Merenkov, Z.; AlKubaisi, N.; Abdel-Rahman, M.E.; Abdelmola, A.; Khelfa, S.; Farag, E.; et al. Prevalence and trends of transfusion transmissible infections among blood donors in the State of Qatar, 2013–2017. BMC Infect. Dis. 2020, 20, 617:1–617:9. [Google Scholar] [CrossRef] [PubMed]
- General Authority for Statistics, Kingdom of Saudi Arabia. Population in Saudi Arabia by Gender, Age, Nationality (Saudi/Non-Saudi)-Mid 2016 A.D. Available online: https://www.stats.gov.sa/en/5305 (accessed on 5 March 2021).
- Coleman, M.; Al-Zahrani, M.H.; Coleman, M.; Hemingway, J.; Omar, A.; Stanton, M.C.; Thomsen, E.K.; Alsheikh, A.A.; Alhakeem, R.F.; McCall, P.J.; et al. A country on the verge of malaria elimination--the Kingdom of Saudi Arabia. PLoS ONE 2014, 9, e105980:1–e105980:8. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.M.; Abdelgader, T.M.; Saeed, I.; Al-Akhshami, A.; Al-Ghamdi, M.; Al-Zahrani, M.H.; El Hassan, I.; Kyalo, D.; Snow, R.W. The changing malaria landscape in Aseer region, Kingdom of Saudi Arabia: 2000–2015. Malar. J. 2016, 15, 538:1–538:11. [Google Scholar] [CrossRef]
- Al Zahrani, M.H.; Omar, A.I.; Abdoon, A.M.O.; Ibrahim, A.A.; Alhogail, A.; Elmubarak, M.; Elamin, Y.E.; AlHelal, M.A.; Alshahrani, A.M.; Abdelgader, T.M.; et al. Cross-border movement, economic development and malaria elimination in the Kingdom of Saudi Arabia. BMC Med. 2018, 16, 98:1–98:9. [Google Scholar] [CrossRef]
- Bin Dajem, S.M. Molecular investigation of mixed malaria infections in southwest Saudi Arabia. Saudi Med. J. 2015, 36, 248–251. [Google Scholar] [CrossRef]
- Dafalla, O.M.; Alzahrani, M.; Sahli, A.; Al Helal, M.A.; Alhazmi, M.M.; Noureldin, E.M.; Mohamed, W.S.; Hamid, T.B.; Abdelhaleem, A.A.; Hobani, Y.A.; et al. Kelch 13-propeller polymorphisms in Plasmodium falciparum from Jazan region, southwest Saudi Arabia. Malar. J. 2020, 19, 397:1–397:9. [Google Scholar] [CrossRef]
- Madkhali, A.M.; Al-Mekhlafi, H.M.; Atroosh, W.M.; Ghzwani, A.H.; Zain, K.A.; Abdulhaq, A.A.; Ghailan, K.Y.; Anwar, A.A.; Eisa, Z.M. Increased prevalence of pfdhfr and pfdhps mutations associated with sulfadoxine-pyrimethamine resistance in Plasmodium falciparum isolates from Jazan Region, Southwestern Saudi Arabia: Important implications for malaria treatment policy. Malar. J. 2020, 19, 446. [Google Scholar] [CrossRef]
- Abu Dhabi & Dubai Population Statistics. Available online: https://www.scad.gov.ae/en/pages/statistics.aspx?topicid=24& (accessed on 18 March 2021).
- Available online: https://www.dsc.gov.ae/en-us/Themes/Pages/Population-and-Vital-Statistics.aspx?Theme=42 (accessed on 18 March 2021).
- Singh, V.; Mishra, N.; Awasthi, G.; Dash, A.P.; Das, A. Why is it important to study malaria epidemiology in India? Trends Parasitol. 2009, 25, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Kumar, D.; Yavasoglu, S.I.; Manning, S.; Hanafi-Bojd, A.A.; Ghasemzadeh, H.; Sikder, I.; Kumar, D.; Murmu, N.; Haque, U. Malaria epidemics in India: Role of climatic condition and control measures. Sci. Total Environ. 2020, 712, e136368. [Google Scholar] [CrossRef] [PubMed]
- Haanshuus, C.G.; Chandy, S.; Manoharan, A.; Vivek, R.; Mathai, D.; Xena, D.; Singh, A.; Langeland, N.; Blomberg, B.; Vasanthan, G.; et al. A high malaria prevalence identified by PCR among patients with acute undifferentiated fever in India. PLoS ONE 2016, 11, e0158816:1–e0158816:13. [Google Scholar] [CrossRef]
- Dayanand, K.K.; Kishore, P.; Chandrashekar, V.; Achur, R.N.; Ghosh, S.K.; Kakkilaya, S.B.; Kumari, S.N.; Tiwari, S.; Boloor, A.; Devi, R.; et al. Malaria severity in Mangaluru City in the southwestern coastal region of India. Am. J. Trop. Med. Hyg. 2019, 100, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Punnath, K.; Dayanand, K.K.; Chandrashekar, V.N.; Achur, R.N.; Kakkilaya, S.B.; Ghosh, S.K.; Mukhi, B.; Midya, V.; Kumari, S.N.; Gowda, D.C. Clinical features and haematological parameters among malaria patients in Mangaluru city area in the southwestern coastal region of India. Parasitol. Res. 2020, 119, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Siwal, N.; Singh, U.S.; Dash, M.; Kar, S.; Rani, S.; Rawal, C.; Singh, R.; Anvikar, A.R.; Pande, V.; Das, A. Malaria diagnosis by PCR revealed differential distribution of mono and mixed species infections by Plasmodium falciparum and P. vivax in India. PLoS ONE 2018, 13, e0193046:1–e0193046:14. [Google Scholar]
- Gupta, B.; Gupta, P.; Sharma, A.; Singh, V.; Dash, A.P.; Das, A. High proportion of mixed-species Plasmodium infections in India revealed by PCR diagnostic assay. Trop. Med. Int. Health 2010, 15, 819–824. [Google Scholar] [CrossRef]
- Hossain, M.S.; Commons, R.J.; Douglas, N.M.; Thriemer, K.; Alemayehu, B.H.; Amaratunga, C.; Anvikar, A.R.; Ashley, E.A.; Asih, P.B.S.; Carrara, V.I.; et al. The risk of Plasmodium vivax parasitaemia after P. falciparum malaria: An individual patient data meta-analysis from the World Wide Antimalarial Resistance Network. PLoS Med. 2020, 17, e1003393:1–e1003393:26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gahlawat, S.K.; Singh, V. Comparative analysis of Plasmodium falciparum dihydrofolate-reductase gene sequences from different regions of India. Heliyon 2020, 6, e03715:1–e03715:4. [Google Scholar] [CrossRef]
- Zomuanpuii, R.; Hmar, C.L.; Lallawmzuala, K.; Hlimpuia, L.; Balabaskaran Nina, P.; Senthil Kumar, N. Epidemiology of malaria and chloroquine resistance in Mizoram, northeastern India, a malaria-endemic region bordering Myanmar. Malar. J. 2020, 19, 95:1–95:11. [Google Scholar] [CrossRef]
- Matlani, M.; Kumar, A.; Singh, V. Assessing the in vitro sensitivity with associated drug resistance polymorphisms in Plasmodium vivax clinical isolates from Delhi, India. Exp. Parasitol. 2020, 220, e108047. [Google Scholar] [CrossRef]
- Kaur, H.; Sehgal, R.; Kumar, A.; Bharti, P.K.; Bansal, D.; Mohapatra, P.K.; Mahanta, J.; Sultan, A.A. Distribution pattern of amino acid mutations in chloroquine and antifolate drug resistance associated genes in complicated and uncomplicated Plasmodium vivax isolates from Chandigarh, North India. BMC Infect. Dis. 2020, 20, 671:1–671:9. [Google Scholar] [CrossRef]
- Joy, S.; Mukhi, B.; Ghosh, S.K.; Achur, R.N.; Gowda, D.C.; Surolia, N. Drug resistance genes: Pvcrt-o and pvmdr-1 polymorphism in patients from malaria endemic South Western Coastal Region of India. Malar. J. 2018, 17, 40:1–40:5. [Google Scholar] [CrossRef]
- Das, S.; Manna, S.; Saha, B.; Hati, A.K.; Roy, S. Novel pfkelch13 Gene polymorphism associated with artemisinin resistance in eastern India. Clin. Infect. Dis. 2019, 69, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Khan, N.H.; Wahid, S.; Ullah, Z.; Kausar, A.; Ali, N. Malaria epidemiology and comparative reliability of diagnostic tools in Bannu; an endemic malaria focus in south of Khyber Pakhtunkhwa, Pakistan. Pathog. Glob. Health 2019, 113, 75–85. [Google Scholar] [CrossRef]
- Karim, A.M.; Hussain, I.; Malik, S.K.; Lee, J.H.; Cho, I.H.; Kim, Y.B.; Lee, S.H. Epidemiology and clinical burden of malaria in the war-torn area, Orakzai Agency in Pakistan. PLoS Negl. Trop. Dis. 2016, 10, e0004399:1–e0004399:12. [Google Scholar] [CrossRef]
- Qureshi, H.; Khan, M.I.; Ambachew, H.; Pan, H.F.; Ye, D.Q. Baseline survey for malaria prevalence in Khyber Pakhtunkhwa Province, Pakistan. East. Mediterr. Health J. 2020, 26, 453–460. [Google Scholar] [CrossRef]
- Khan, A.; Godil, F.J.; Naseem, R. Chloroquine-resistant Plasmodium vivax in Pakistan: An emerging threat. Lancet Glob. Health 2016, 4, e790:1. [Google Scholar] [CrossRef][Green Version]
- Waheed, A.A.; Ghanchi, N.K.; Rehman, K.A.; Raza, A.; Mahmood, S.F.; Beg, M.A. Vivax malaria and chloroquine resistance: A neglected disease as an emerging threat. Malar. J. 2015, 14, 146:1–146:3. [Google Scholar] [CrossRef][Green Version]
- Mosawi, S.H.; Dalimi, A.; Safi, N.; Ghaffarifar, F.; Sadraei, J. Evaluation of asymptomatic malaria status in eastern of Afghanistan using high resolution melting analysis. Iran. J. Parasitol. 2020, 15, 177–186. [Google Scholar] [CrossRef]
- Delam, H.; Shokrpour, N.; Nikbakht, H.A.; Hassanipour, S.; Safari, K.; Bazrafshan, M.R. Changing patterns in epidemiology of malaria between 2006 and 2018 in the south of Fars Province, southern Iran: The fall and rise of malaria. Ann. Glob. Health 2020, 86, 80:1–80:7. [Google Scholar]
- Haque, U.; Glass, G.E.; Haque, W.; Islam, N.; Roy, S.; Karim, J.; Noedl, H. Antimalarial drug resistance in Bangladesh, 1996–2012. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 745–752. [Google Scholar] [CrossRef]
- Fornace, K.M.; Herman, L.S.; Abidin, T.R.; Chua, T.H.; Daim, S.; Lorenzo, P.J.; Grignard, L.; Nuin, N.A.; Ying, L.T.; Grigg, M.J.; et al. Exposure and infection to Plasmodium knowlesi in case study communities in Northern Sabah, Malaysia and Palawan, The Philippines. PLoS Negl. Trop. Dis. 2018, 12, e0006432:1–e0006432:16. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; de Jesus Milanez, G.; Masangkay, F.R. Plasmodium spp. mixed infection leading to severe malaria: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11068:1–11068:12. [Google Scholar] [CrossRef]
- Bareng, A.P.; Espino, F.E.; Chaijaroenkul, W.; Na-Bangchang, K. Molecular monitoring of dihydrofolatereductase (dhfr) and dihydropteroatesynthetase (dhps) associated with sulfadoxine-pyrimethamine resistance in Plasmodium vivax isolates of Palawan, Philippines. Acta Trop. 2018, 180, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Amimo, F.; Lambert, B.; Magit, A.; Sacarlal, J.; Hashizume, M.; Shibuya, K. Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in Africa: A systematic analysis of national trends. BMJ Glob. Health 2020, 5, e003217:1–e003217:12. [Google Scholar] [CrossRef]
- Tola, M.; Ajibola, O.; Idowu, E.T.; Omidiji, O.; Awolola, S.T.; Amambua-Ngwa, A. Molecular detection of drug resistant polymorphisms in Plasmodium falciparum isolates from Southwest, Nigeria. BMC Res. Notes 2020, 13, 497:1–497:7. [Google Scholar] [CrossRef]
- Esayas, E.; Tufa, A.; Massebo, F.; Ahemed, A.; Ibrahim, I.; Dillu, D.; Bogale, E.A.; Yared, S.; Deribe, K. Malaria epidemiology and stratification of incidence in the malaria elimination setting in Harari Region, Eastern Ethiopia. Infect. Dis. Poverty 2020, 9, 160:1–160:12. [Google Scholar] [CrossRef]
- Gebrekidan, M.G.; Gebremedhin, G.B.; Gebregiorgis, Y.S.; Gezehegn, A.A.; Weldearegay, K.T. Artemether-lumefantrin treatment adherence among uncomplicated Plasmodium falciparum malaria patients, visiting public health facilities in AsgedeTsimbla district, Tigray, Ethiopia: A cross-sectional study. Antimicrob. Resist. Infect. Control. 2020, 9, 184:1–184:12. [Google Scholar] [CrossRef] [PubMed]
- Bakhiet, A.M.A.; Abdelraheem, M.H.; Kheir, A.; Omer, S.; Gismelseed, L.; Abdel-Muhsin, A.; Naiem, A.; Al Hosni, A.; Al Dhuhli, A.; Al Rubkhi, M.; et al. Evolution of Plasmodium falciparum drug resistance genes following artemisinin combination therapy in Sudan. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Hussien, M.; Abdel Hamid, M.M.; Elamin, E.A.; Hassan, A.O.; Elaagip, A.H.; Salama, A.H.A.; Abdelraheem, M.H.; Mohamed, A.O. Antimalarial drug resistance molecular makers of Plasmodium falciparum isolates from Sudan during 2015-2017. PLoS ONE 2020, 15, e0235401. [Google Scholar] [CrossRef]
- Enayati, A.; Hanafi-Bojd, A.A.; Sedaghat, M.M.; Zaim, M.; Hemingway, J. Evolution of insecticide resistance and its mechanisms in Anopheles stephensi in the WHO Eastern Mediterranean Region. Malar. J. 2020, 19, 258. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.V.; Drake, J.M.; Jones, L.; Murdock, C.C. Assessing temperature-dependent competition between two invasive mosquito species. Ecol. Appl. 2021, 26, e02334. [Google Scholar]
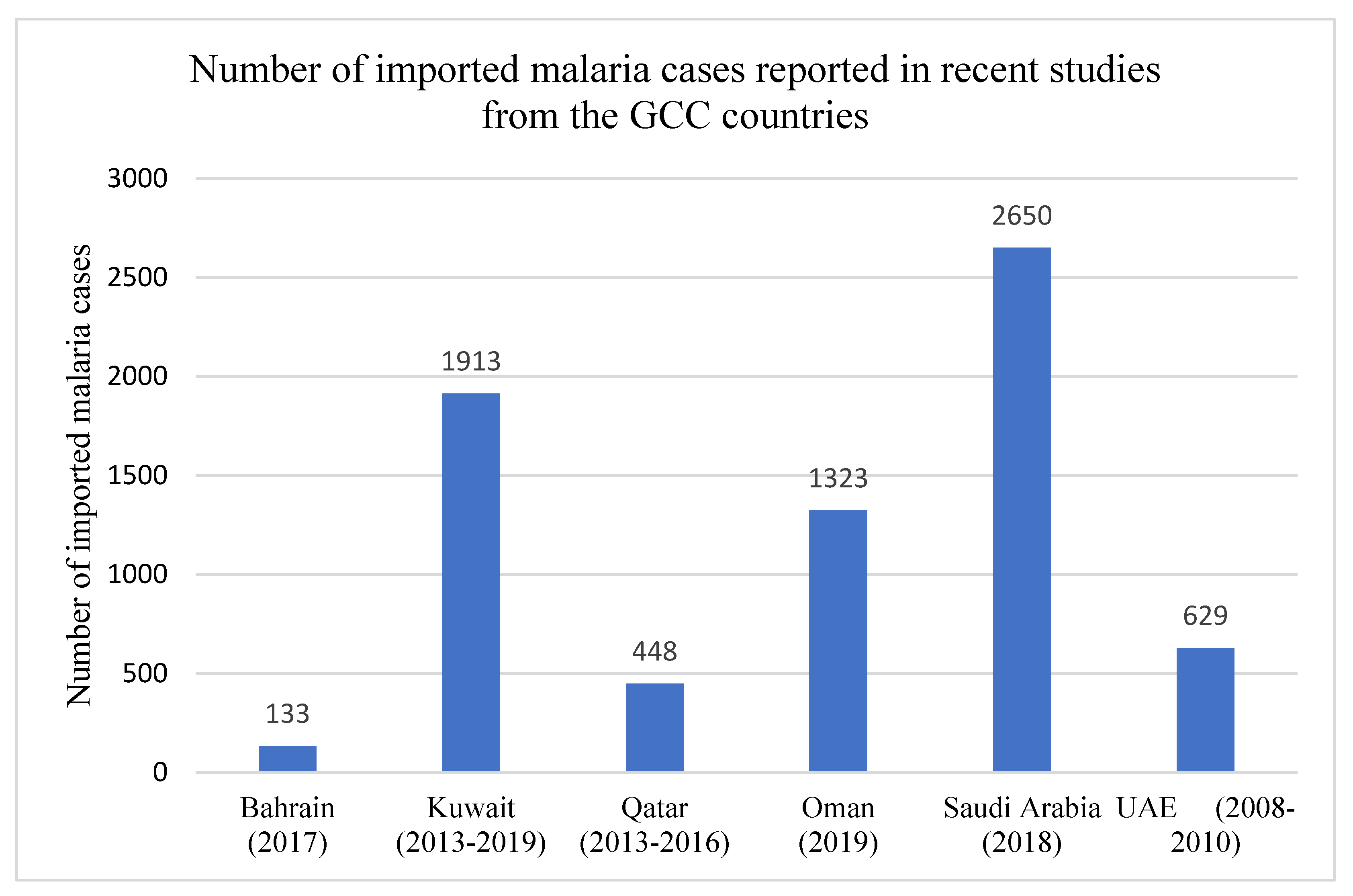


| Country | Major Vectors | Indigenous Malaria Status * | References Number |
|---|---|---|---|
| Bahrain | None | Malaria-free since 2012 | Mahmood RA, 1992 [28] Ismaeel et al., 2004 [29] |
| Kuwait | None # | Malaria-free since 1963 | |
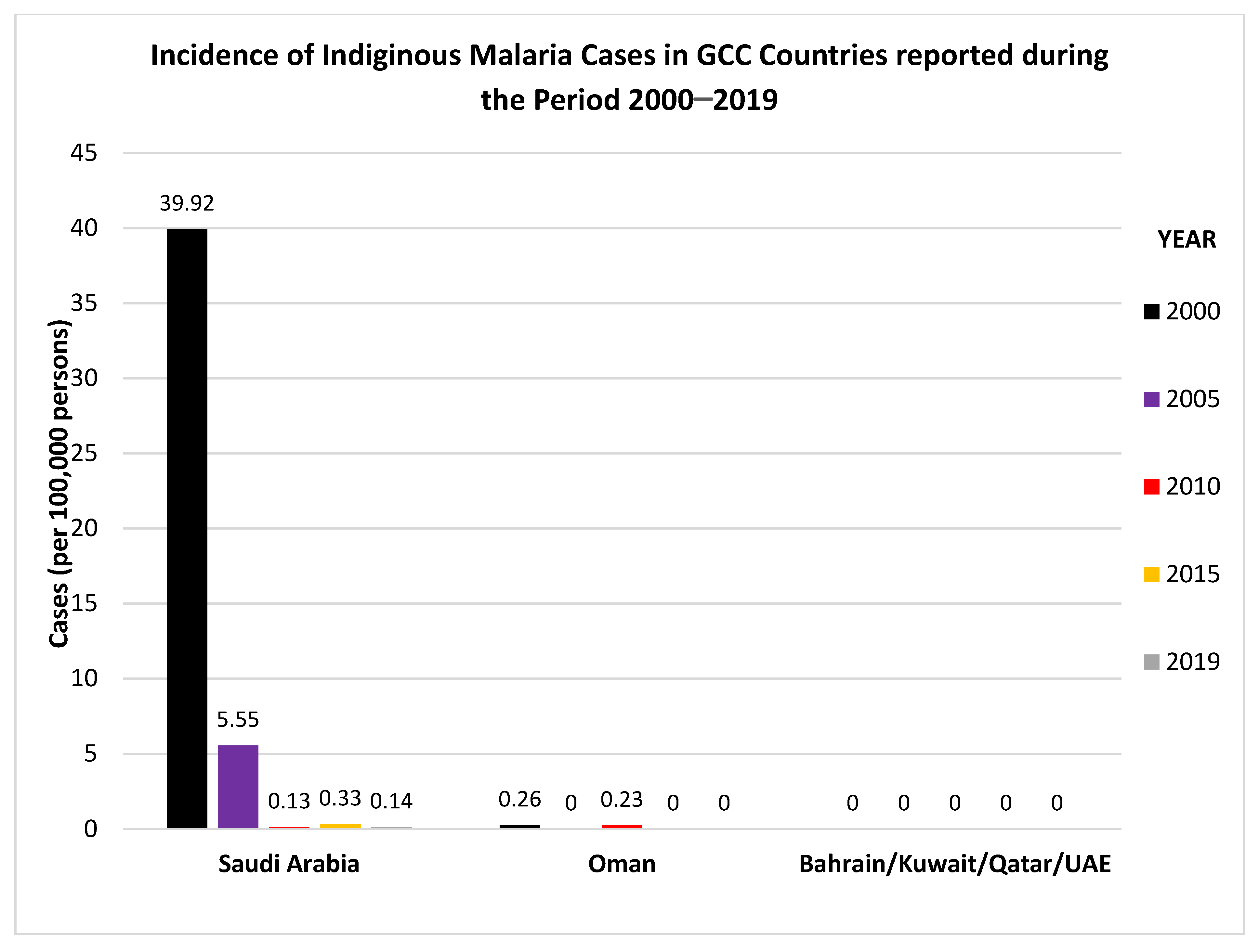
| Oman | Anopheles culicifacies, very low density | very low and unstable (3 indigenous malaria cases in 2016) | Hassan KS, 2017 [30] Simon et al., 2017 [31] MOH, Annual Report 2019 [32] |
| Qatar | An. stephensi, An. multicolor | Malaria-free since 2012 | Al-Kuwari MG, 2009 [33] |
| Saudi Arabia | An. Stephensi **, An. sergenti, An. gambiae, An. arabiensis, An. bwambae, An. coluzzii, An. merus | Low and unstable (mainly in the southeastern region) 61 cases (57 P. falciparum and 4 P. vivax) in 2018 | Alahmed, AM et al., 2019 [34] Hawash Y et al., 2019 [35] |
| United Arab Emirates | None | Malaria-free since 2007 | Nilles et al., 2014 [36] |
| Country | Duration of Study | No. of Indigenous and Imported Malaria Cases | No. of Imported Malaria Cases Detected among Nationals (Citizens) or Expatriates | Malaria Cases by Plasmodium spp. | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citizens | India | Pakistan | AFGN | BGD | Nigeria | Sudan | Other African Countries | Others | P. falciparum | P. vivax | Pf/Pv Mixed | Others | ||||
| Bahrain | 1992–2001 | 0 and 1572 | N. A. | 629 | 566 | N. A. | 31 | NA | 63 | N. A. | 283 | 220 | 1346 | 5 | 1 | Ismaeel et al., 2004 [29] |
| Bahrain | 2017 | 0 and 133 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | WHO/EMRO Annual Report 2017 [37] | ||||
| Kuwait | 1985–2000 | 0 and 6776 * | 39 | 3569 | 1057 | 1871 | 0 | 89 | 48 | N. A. | 133 | 1137 | 5207 | 395 | 0 | Iqbal et al., 2003 [44] |
| Kuwait | 2013–2018 | 0 and 1913 | 18 | 1012 | 390 | 94 | 5 | 16 | 48 | 275 | 55 | 361 | 124 | 1383 | 45 | Iqbal et al., 2020 [45] |
| Oman | 2014 | 53 and 1 | 1 | 14 | 6 | 0 | 32 | 0 | 0 | 0 | 1 | 0 | 54 | 0 | 0 | Simon et al., 2017 [31] |
| Oman | 2019 | 15 and 1323 | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | 1080 | 206 | 0 | 52 | MoH, Oman [32] |
| Qatar | 2004–2006 | 0 and 438 | N. A. | 210 | 128 | N. A. | N. A. | N. A. | 37 | N. A. | 63 | 60 | 175 | 0 | 203 | Al-Kuwari, 2009 [33] |
| Qatar a | 2008–2015 | 0 and 4092 | 14 | 812 | 772 | 0 | 0 | 200 | 0 | 0 | 14 | 404 | 2336 b | 0 | 229 b | Farag et al. 2018 [46] |
| Qatar | 2013–2016 | 0 and 448 | 1 | 168 | 108 | 0 | 0 | 16 | 74 | 8 | 73 | 118 | 318 | 12 | 0 | Al-Rumhi et al., 2020 [47] |
| Saudi Arabia | 2000–2014 | 5522 and 9930 | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | El Hassan et al., 2015 [48] |
| Saudi Arabia | 2008–2011 | 318 c | 16 | 37 | 108 | 5 | 3 | 53 | 12 | 7 | 77 | 204 | 103 | 0 | 11 | Memish et al. 2014 [49] |
| Saudi Arabia | 2012–2015 | 121 and 224 | 113 | 28 | 73 | 3 | 0 | 0 | 48 | 37 | 43 | 212 | 128 | 2 | 3 | Alshahrani et al., 2016 [50] |
| Saudi Arabia | 2016 | 4 and 22 | 4 | 4 | 5 | 0 | 0 | 2 | 10 | 1 | 0 | 13 | 13 | 0 | 0 | Soliman et al. 2018 [51] |
| Saudi Arabia | 2016–2018 | 5 and 25 | 11 | 2 | 2 | 0 | 0 | 0 | 4 | 0 | 11 | 23 | 5 | 2 | 0 | Hawash et al., 2019 [35] |
| Saudi Arabia | 2018 | 61 and 2650 | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | N. A. | 57 | 4 | 0 | 0 | WHO Malaria Report 2019 [3] |
| UAE | 2008–2010 | 0 and 629 | 0 | 338 | 228 | 0 | 0 | 14 ** | 14 ** | 14 ** | 21 | 122 | 493 | 14 | 0 | Nilles et al., 2014 [36] |
| GCC | Total Population in | Population of Citizens | Population of Expatriates from Different Countries in GCC Countries in 2019 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | 2019 (in Millions) | in 2019 (in Millions) | India | Pakistan | Afghanistan | Bangladesh | Philippines | Nigeria | Sudan | Ethiopia |
| Bahrain | 1.59 | 1.21 | 318,547 | 78,638 | 690 | 82,518 | 50,585 | 2054 | 7917 | 713 |
| Kuwait | 4.77 | 1.43 | 1,124,256 | 330,824 | 2826 | 370,844 | 192,143 | 4702 | 48,204 | 3806 |
| Qatar | 2.68 | 0.33 | 698,088 | 235,876 | 1602 | 263,086 | 168,461 | 4152 | 23,954 | 1700 |
| Oman | 4.7 | 2.6 | 1,325,444 | 240,965 | N. A. | 304,917 | 44,546 | N. A. | 19,155 | N. A. |
| Saudi Arabia | 34 | 23.2 | 2,440,489 | 1,447,071 | 469,324 | 1,246,052 | 628,894 | N. A. | 469,324 | 160,192 |
| UAE | 9.7 | 1.2 | 3,419,875 | 981,536 | 8071 | 1,079,013 | 556,407 | 15,465 | 131,254 | 10,886 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, J.; Ahmad, S.; Sher, A.; Al-Awadhi, M. Current Epidemiological Characteristics of Imported Malaria, Vector Control Status and Malaria Elimination Prospects in the Gulf Cooperation Council (GCC) Countries. Microorganisms 2021, 9, 1431. https://doi.org/10.3390/microorganisms9071431
Iqbal J, Ahmad S, Sher A, Al-Awadhi M. Current Epidemiological Characteristics of Imported Malaria, Vector Control Status and Malaria Elimination Prospects in the Gulf Cooperation Council (GCC) Countries. Microorganisms. 2021; 9(7):1431. https://doi.org/10.3390/microorganisms9071431
Chicago/Turabian StyleIqbal, Jamshaid, Suhail Ahmad, Ali Sher, and Mohammad Al-Awadhi. 2021. "Current Epidemiological Characteristics of Imported Malaria, Vector Control Status and Malaria Elimination Prospects in the Gulf Cooperation Council (GCC) Countries" Microorganisms 9, no. 7: 1431. https://doi.org/10.3390/microorganisms9071431
APA StyleIqbal, J., Ahmad, S., Sher, A., & Al-Awadhi, M. (2021). Current Epidemiological Characteristics of Imported Malaria, Vector Control Status and Malaria Elimination Prospects in the Gulf Cooperation Council (GCC) Countries. Microorganisms, 9(7), 1431. https://doi.org/10.3390/microorganisms9071431






