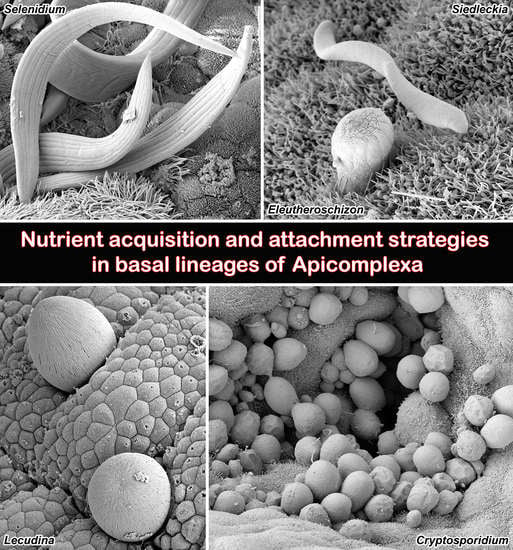
Nutrient Acquisition and Attachment Strategies in Basal Lineages: A Tough Nut to Crack in the Evolutionary Puzzle of Apicomplexa
Abstract
1. Introduction
2. Apical Complex Structure and Function
2.1. From Invading Apicomplexan Zoites to Vegetative Trophic Stages
2.2. From Myzocytosis to Full Invasive Capacity
3. The Niche and Feeding Strategies in Vegetative Stages
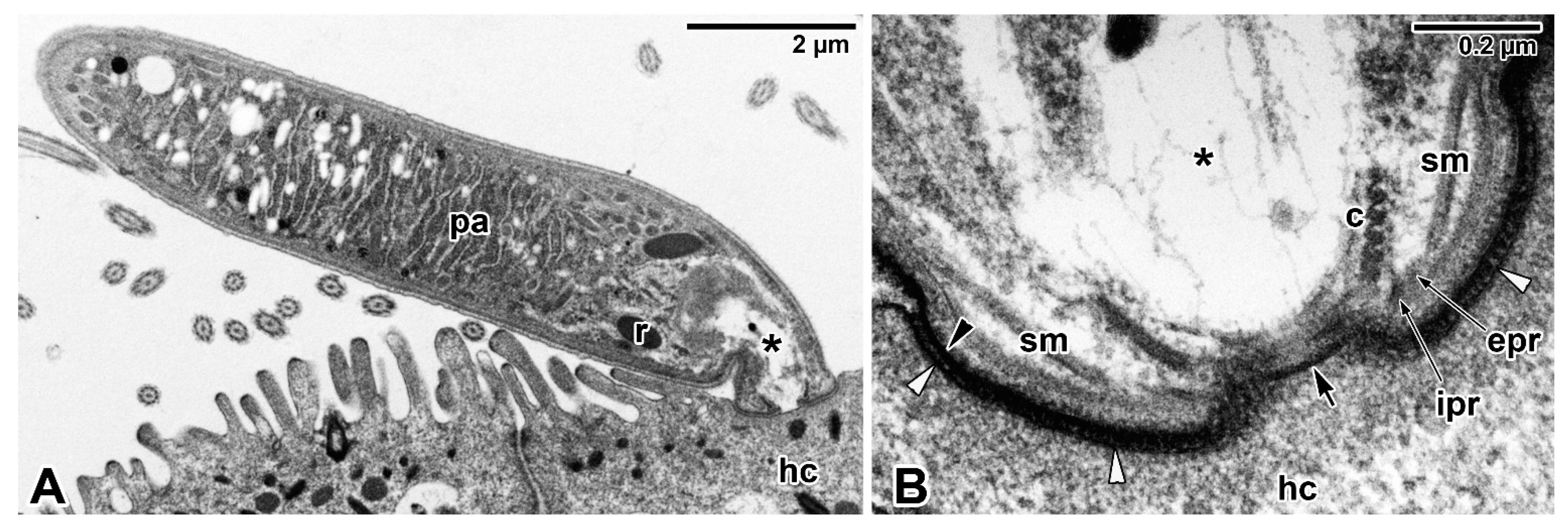
3.1. Epicellular Parasitism
3.2. Epicellular Parasitism in the Embrace of the Host Cell Membrane
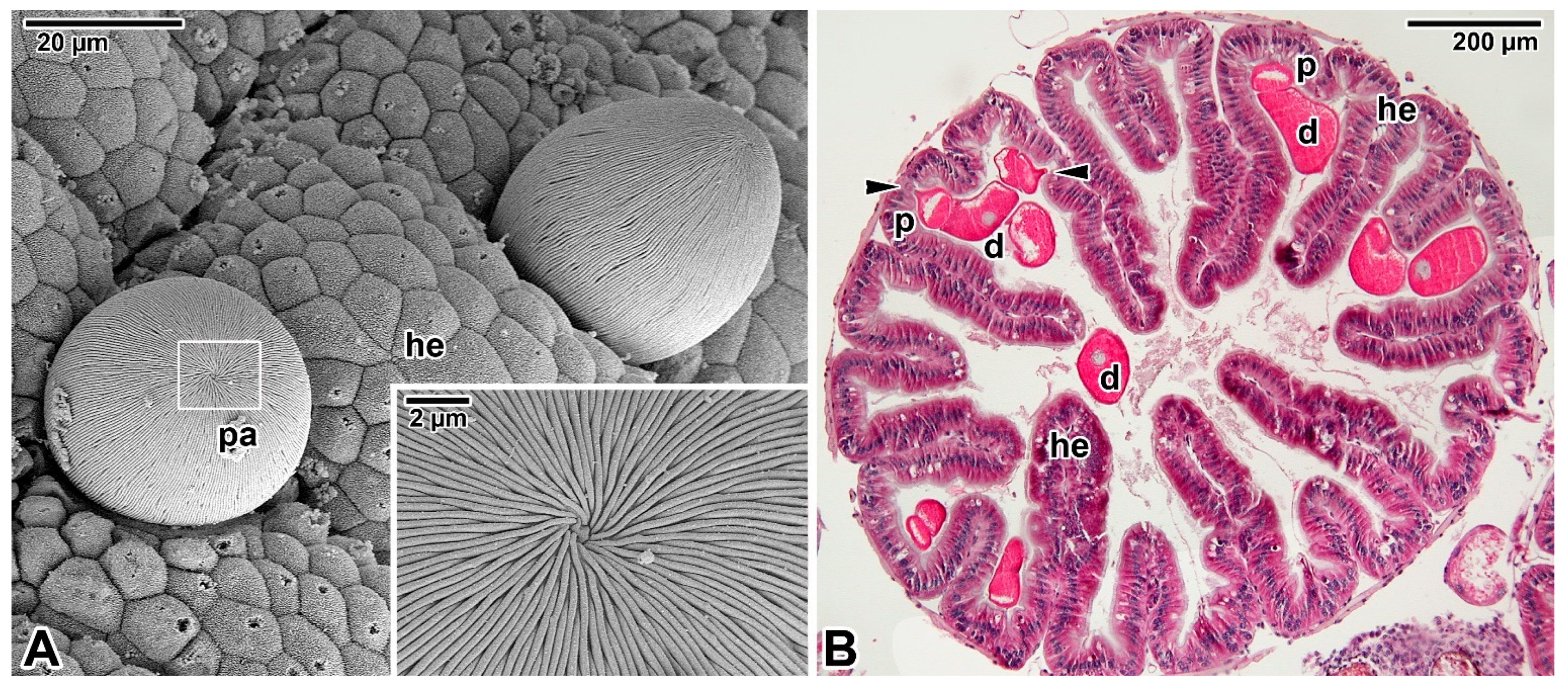
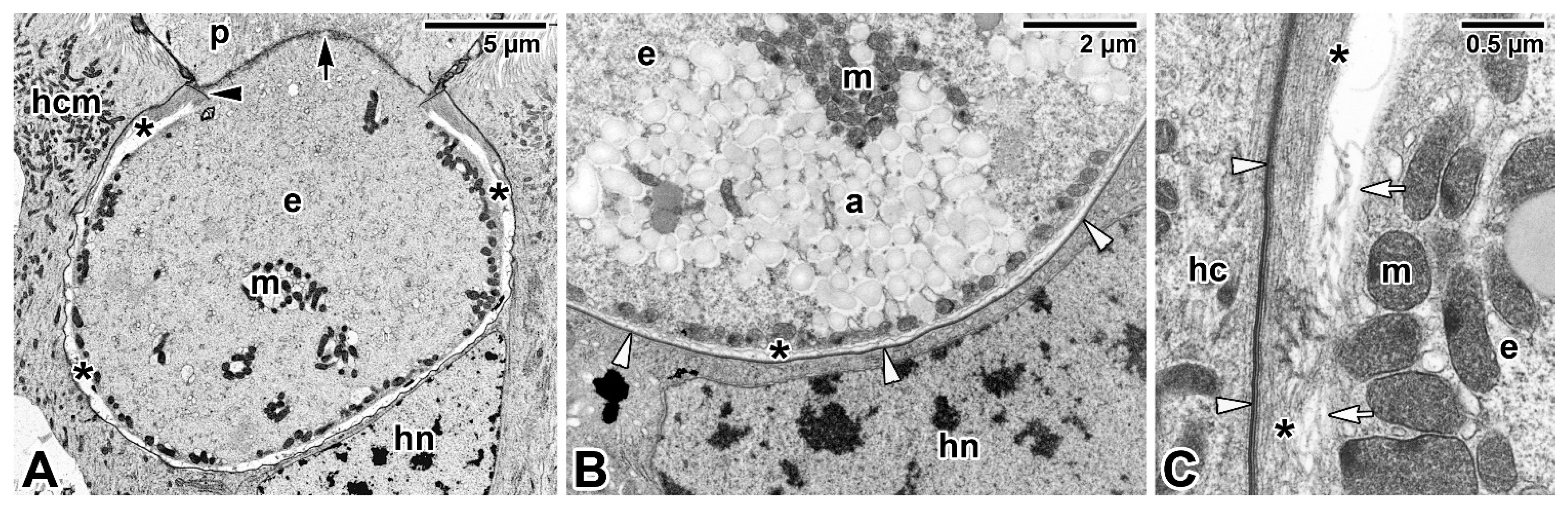
3.3. Intracellular Parasitism
4. The Storage Polysaccharide in Apicomplexan Parasites
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMA-1 | Apical membrane antigen 1 |
| CLSM | Confocal laser scanning microscopy |
| FE | Freeze-etching |
| FITC | Fluorescein isothiocyanate |
| GRA | Dense granule protein |
| HCPM | Host cell plasma membrane |
| IFA | Indirect immunofluorescent assay |
| IMC | Inner membrane complex |
| LM | Light microscopy |
| PPM | Parasite plasma membrane |
| PS | Parasitophorous sac |
| PV | Parasitophorous vacuole |
| PVM | Parasitophorous vacuole membrane |
| RON | Rhoptry neck protein |
| ROP | Rhoptry (bulb) protein |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TRITC | Tetramethylrhodamine isothiocyanate |
References
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukes, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.E.G. The evolutionary expansion of the Sporozoa. Int. J. Parasitol. 1994, 24, 1301–1316. [Google Scholar] [CrossRef]
- Levine, N.D.; Corliss, J.O.; Cox, F.E.G.; Deroux, G.; Grain, J.; Honigberg, B.M.; Leedale, G.F.; Loeblich, A.R.; Lom, I.J.; Lynn, D.; et al. A newly revised classification of the Protozoa. J. Protozool. 1980, 27, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Janouškovec, J.; Paskerova, G.G.; Miroliubova, T.S.; Mikhailov, K.V.; Birley, T.; Aleoshin, V.V.; Simdyanov, T.G. Apicomplexan-like parasites are polyphyletic and widely but selectively dependent on cryptic plastid organelles. Elife 2019, 8, e49662. [Google Scholar] [CrossRef]
- Mathur, V.; Kolísko, M.; Hehenberger, E.; Irwin, N.A.T.; Leander, B.S.; Kristmundsson, A.; Freeman, M.A.; Keeling, P.J. Multiple independent origins of apicomplexan-like parasites. Curr. Biol. 2019, 29, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Janouškovec, J.; Tikhonenkov, D.V.; Burki, F.; Howe, A.T.; Kolísko, M.; Mylnikov, A.P.; Keeling, P.J. Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives. Proc. Natl. Acad. Sci. USA 2015, 112, 10200–10207. [Google Scholar] [CrossRef]
- Rueckert, S.; Betts, E.L.; Tsaousis, A.D. The symbiotic spectrum: Where do the gregarines fit? Trends Parasitol. 2019, 35, 687–694. [Google Scholar] [CrossRef]
- Carreno, R.A.; Martin, D.S.; Barta, J.R. Cryptosporidium is more closely related to the gregarines than to coccidia as shown by phylogenetic analysis of apicomplexan parasites inferred using small-subunit ribosomal RNA gene sequences. Parasitol. Res. 1999, 85, 899–904. [Google Scholar] [CrossRef]
- Barta, J.R.; Thompson, R.C.A. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol. 2006, 22, 463–468. [Google Scholar] [CrossRef]
- Leander, B.S.; Lloyd, S.A.J.; Marshall, W.; Landers, S.C. Phylogeny of marine gregarines (Apicomplexa)-Pterospora, Lithocystis and Lankesteria-and the origin(s) of coelomic parasitism. Protist 2006, 157, 45–60. [Google Scholar] [CrossRef]
- Valigurová, A.; Hofmannová, L.; Koudela, B.; Vávra, J. An ultrastructural comparison of the attachment sites between Gregarina steini and Cryptosporidium muris. J. Eukaryot. Microbiol. 2007, 54, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Valigurová, A. Sophisticated adaptations of Gregarina cuneata (Apicomplexa) feeding stages for epicellular parasitism. PLoS ONE 2012, 7, e42606. [Google Scholar] [CrossRef] [PubMed]
- Diakin, A.; Paskerova, G.G.; Simdyanov, T.G.; Aleoshin, V.V.; Valigurová, A. Morphology and molecular phylogeny of coelomic gregarines (Apicomplexa) with different types of motility: Urospora ovalis and U. travisiae from the polychaete Travisia forbesii. Protist 2016, 167, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Paskerova, G.G.; Miroliubova, T.S.; Diakin, A.; Kováčiková, M.; Valigurová, A.; Guillou, L.; Aleoshin, V.V.; Simdyanov, T.G. Fine structure and molecular phylogenetic position of two marine gregarines, Selenidium pygospionis sp. n. and S. pherusae sp. n., with notes on the phylogeny of Archigregarinida (Apicomplexa). Protist 2018, 169, 826–852. [Google Scholar] [CrossRef]
- Valigurová, A.; Paskerova, G.G.; Diakin, A.; Kováčiková, M.; Simdyanov, T.G. Protococcidian Eleutheroschizon duboscqi, an unusual apicomplexan interconnecting gregarines and cryptosporidia. PLoS ONE 2015, 10, e0125063. [Google Scholar] [CrossRef] [PubMed]
- Schrével, J.; Valigurová, A.; Prensier, G.; Chambouvet, A.; Florent, I.; Guillou, L. Ultrastructure of Selenidium pendula, the type species of archigregarines, and phylogenetic relations to other marine Apicomplexa. Protist 2016, 167, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Simdyanov, T.G.; Paskerova, G.G.; Valigurová, A.; Diakin, A.; Kováčiková, M.; Schrével, J.; Guillou, L.; Dobrovolskij, A.A.; Aleoshin, V.V. First ultrastructural and molecular phylogenetic evidence from the blastogregarines, an early branching lineage of plesiomorphic Apicomplexa. Protist 2018, 169, 697–726. [Google Scholar] [CrossRef] [PubMed]
- Boisard, J.; Florent, I. Why the –omic future of Apicomplexa should include gregarines. Biol. Cell 2020, 112, 173–185. [Google Scholar] [CrossRef]
- Portman, N.; Šlapeta, J. The flagellar contribution to the apical complex: A new tool for the eukaryotic Swiss Army knife? Trends Parasitol. 2014, 30, 58–64. [Google Scholar] [CrossRef]
- Graindorge, A.; Frénal, K.; Jacot, D.; Salamun, J.; Marq, J.B.; Soldati-Favre, D. The conoid associated motor MyoH is indispensable for Toxoplasma gondii entry and exit from host cells. PLoS Pathog. 2016, 12, e1005388. [Google Scholar] [CrossRef]
- Tardieux, I.; Baum, J. Reassessing the mechanics of parasite motility and host-cell invasion. J. Cell Biol. 2016, 214, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, M.J.; Duraisingh, M.T. Evolution of apicomplexan secretory organelles. Int. J. Parasitol. 2012, 42, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Swapna, L.S.; Parkinson, J. Genomics of apicomplexan parasites. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, G.; Biesaga, M.; Touquet, B.; Lagal, V.; Balland, M.; Dufour, A.; Hakimi, M.A.; Tardieux, I. Toxoplasma parasite twisting motion mechanically induces host cell membrane fission to complete invasion within a protective vacuole. Cell Host Microbe 2018, 24, 81–96. [Google Scholar] [CrossRef]
- Hakimi, M.A.; Olias, P.; Sibley, L.D. Toxoplasma effectors targeting host signaling and transcription. Clin. Microbiol. Rev. 2017, 30, 615–645. [Google Scholar] [CrossRef]
- Boucher, L.E.; Bosch, J. The apicomplexan glideosome and adhesins—Structures and function. J. Struct. Biol. 2015, 190, 93–114. [Google Scholar] [CrossRef]
- Templeton, T.J.; Pain, A. Diversity of extracellular proteins during the transition from the ‘proto-apicomplexan’ alveolates to the apicomplexan obligate parasites. Parasitology 2016, 143, 1–17. [Google Scholar] [CrossRef]
- Bartošová-Sojková, P.; Oppenheim, R.D.; Soldati-Favre, D.; Lukeš, J. Epicellular apicomplexans: Parasites “on the way in”. PLoS Pathog. 2015, 11, e1005080. [Google Scholar] [CrossRef] [PubMed]
- Dogga, S.K.; Bartošová-Sojková, P.; Lukeš, J.; Soldati-Favre, D. Phylogeny, morphology, and metabolic and invasive capabilities of epicellular fish coccidium Goussia janae. Protist 2015, 166, 659–676. [Google Scholar] [CrossRef]
- Simdyanov, T.G.; Kuvardina, O.N. Fine structure and putative feeding mechanism of the archigregarine Selenidium orientale (Apicomplexa: Gregarinomorpha). Eur. J. Protistol. 2007, 43, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T.; Chao, E.E. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). Eur. J. Protistol. 2004, 40, 185–212. [Google Scholar] [CrossRef]
- Leander, B.S. Marine gregarines: Evolutionary prelude to the apicomplexan radiation? Trends Parasitol. 2008, 24, 60–67. [Google Scholar] [CrossRef]
- Dos Santos Pacheco, N.; Tosetti, N.; Koreny, L.; Waller, R.F.; Soldati-Favre, D. Evolution, composition, assembly, and function of the conoid in Apicomplexa. Trends Parasitol. 2020, 36, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Leander, B.S.; Keeling, P.J. Morphostasis in alveolate evolution. Trends Ecol. Evol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Okamoto, N.; Keeling, P.J. The 3D structure of the apical complex and association with the flagellar apparatus revealed by serial TEM tomography in Psammosa pacifica, a distant relative of the Apicomplexa. PLoS ONE 2014, 9, e84653. [Google Scholar] [CrossRef] [PubMed]
- Füssy, Z.; Oborník, M. Reductive Evolution of apicomplexan parasites from phototrophic ancestors. In Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolution, Methods and Concepts; Pontarotti, P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 217–236. [Google Scholar]
- Salomaki, E.D.; Terpis, K.X.; Rueckert, S.; Kotyk, M.; Varadínová, Z.K.; Čepička, I.; Lane, C.E.; Kolisko, M. Gregarine single-cell transcriptomics reveals differential mitochondrial remodeling and adaptation in apicomplexans. BMC Biol. 2021, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.P.; Otto, T.D.; Aslett, M.; Armstrong, S.D.; Bringaud, F.; Schlacht, A.; Hartley, C.; Sanders, M.; Wastling, J.M.; Dacks, J.B.; et al. Kinetoplastid phylogenomics reveals the evolutionary innovations associated with the origins of parasitism. Curr. Biol. 2016, 26, 161–172. [Google Scholar] [CrossRef]
- Leander, B.S.; Esson, H.J.; Breglia, S.A. Macroevolution of complex cytoskeletal systems in euglenids. BioEssays 2007, 29, 987–1000. [Google Scholar] [CrossRef]
- Burns, J.A.; Pittis, A.A.; Kim, E. Gene-based predictive models of trophic modes suggest Asgard archaea are not phagocytotic. Nat. Ecol. Evol. 2018, 2, 697–704. [Google Scholar] [CrossRef]
- Dyson, J.; Grahame, J.; Evennett, P.J. The mucron of the gregarine Digyalum oweni (Protozoa: Apicomplexa), parasitic in Littorina species (Mollusca: Gastropoda). J. Nat. Hist. 1993, 27, 557–564. [Google Scholar] [CrossRef]
- Dyson, J.; Grahame, J.; Evennett, P.J. The apical complex of the gregarine Digyalum oweni (Protozoa: Apicomplexa). J. Nat. Hist. 1994, 28, 1–7. [Google Scholar] [CrossRef]
- Simdyanov, T.G.; Guillou, L.; Diakin, A.Y.; Mikhailov, K.V.; Schrével, J.; Aleoshin, V.V. A new view on the morphology and phylogeny of eugregarines suggested by the evidence from the gregarine Ancora sagittata (Leuckart, 1860) Labbé, 1899 (Apicomplexa: Eugregarinida). PeerJ 2017, 5, e3354. [Google Scholar] [CrossRef] [PubMed]
- Valigurová, A.; Vaškovicová, N.; Diakin, A.; Paskerova, G.G.; Simdyanov, T.G.; Kováčiková, M. Motility in blastogregarines (Apicomplexa): Native and drug-induced organisation of Siedleckia nematoides cytoskeletal elements. PLoS ONE 2017, 12, e0179709. [Google Scholar] [CrossRef] [PubMed]
- Leander, B.S. Ultrastructure of the archigregarine Selenidium vivax (Apicomplexa)—A dynamic parasite of sipunculid worms (host: Phascolosoma agassizii). Mar. Biol. Res. 2006, 2, 178–190. [Google Scholar] [CrossRef]
- Schrével, J.; Vivier, E. Étude de l’ultrastructure et du role de la région antérieure (mucron et épimérite) de grégarines parasites d’annélides polychètes. Protistologica 1966, 2, 17–28. [Google Scholar]
- Desportes, I.; Schrével, J. (Eds.) The gregarines: The early branching Apicomplexa (2 vols). In Treatise on Zoology-Anatomy, Taxonomy, Biology; Brill: Leiden, The Netherlands, 2013; p. 781. [Google Scholar]
- Kováčiková, M.; Simdyanov, T.G.; Diakin, A.; Valigurová, A. Structures related to attachment and motility in the marine eugregarine Cephaloidophora cf. communis (Apicomplexa). Eur. J. Protistol. 2017, 59, 1–13. [Google Scholar] [CrossRef]
- MacMillan, W.G. Gregarine attachment organelles-structure and permeability of an interspecific cell junction. Parasitology 1973, 66, 207–214. [Google Scholar] [CrossRef]
- Valigurová, A.; Koudela, B. Fine structure of trophozoites of the gregarine Leidyana ephestiae (Apicomplexa: Eugregarinida) parasitic in Ephestia kuehniella larvae (Lepidoptera). Eur. J. Protistol. 2005, 41, 209–218. [Google Scholar] [CrossRef]
- Valigurová, A.; Koudela, B. Morphological analysis of the cellular, interactions between the eugregarine Gregarina garnhami (Apicomplexa) and the epithelium of its host, the desert locust Schistocerca gregaria. Eur. J. Protistol. 2008, 44, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Valigurová, A.; Michalková, V.; Koudela, B. Eugregarine trophozoite detachment from the host epithelium via epimerite retraction: Fiction or fact? Int. J. Parasitol. 2009, 39, 1235–1242. [Google Scholar] [CrossRef]
- Cook, T.J.P.; Janovy, J.; Clopton, R.E. Epimerite-host epithelium relationships among eugregarines parasitizing the damselflies Enallagma civile and Ischnura verticalis. J. Parasitol. 2001, 87, 988–996. [Google Scholar] [CrossRef]
- Fowell, R.R. The fibrillar structures of protozoa, with special reference to schizogregarines of the genus Selenidium. J. R. Micr. Soc. 1936, 56, 12–28. [Google Scholar] [CrossRef]
- Schrével, J. Contribution a l’étude des Selenidiidae parasites d’annélides polychètes. II. Ultrastructure de quelques trophozoïtes. Protistologica 1971, 7, 101–130. [Google Scholar]
- Schrével, J. L’ultrastructure de la région antérieure de la grégarine Selenidium et son intérêt pour l’étude de la nutrition chez les sporozoaires. J. Microsc. 1968, 7, 391–410. [Google Scholar]
- Dyson, J.; Evennett, P.J.; Grahame, J. Histochemical studies of some hydrolytic enzymes in the gregarine Digyalum oweni (Protozoa: Apicomplexa). Arch. Protistenk. 1995, 145, 94–99. [Google Scholar] [CrossRef]
- Roberts, J.B. Studies on Some Gregarines from Polychaete Worms. Ph.D. Thesis, University of Wales, Bangor, UK, 1979. Unpublished. [Google Scholar]
- Desportes, I. Ultrastructure et développement des grégarines du genre Stylocephalus. Ann. Sci. Nat. 1969, 12, 31–96. [Google Scholar]
- Sheffield, H.G.; Garnham, P.C.; Shiroishi, T. The fine structure of the sporozoite of Lankesteria culicis. J. Protozool. 1971, 18, 98–105. [Google Scholar] [CrossRef]
- Hildebrand, H.F. Electron-microscopic investigation on evolution stages of trophozoite of Didymophyes gigantea (Sporozoa, Gregarinida).1. Fine structure of protomerite and epimerite and relationship between host and parasite. Z. Parasitenkd. 1976, 49, 193–215. [Google Scholar] [CrossRef]
- Schrével, J.; Philippe, M. The gregarines. In Parasitic Protozoa, 2nd ed.; Kreier, J.P., Ed.; Academic Press: New York, NY, USA, 1993; Volume 4, pp. 133–245. [Google Scholar]
- Weisser, J. Nemoci Hmyzu; Nakladatelství Československé akademie věd: Prague, Czechoslovakia, 1966; p. 554. [Google Scholar]
- Tronchin, G.; Schrével, J. Chronologie des modifications ultrastructurales au cours de la croissance de Gregarina blaberae. J. Protozool. 1977, 24, 67–82. [Google Scholar] [CrossRef]
- Schrével, J. Observations biologiques et ultrastructurales sur les Selenidiidae et leurs consequences sur la systematique des Gregarinomorphes. J. Protozool. 1971, 18, 448–470. [Google Scholar] [CrossRef]
- Lucarotti, C.J. Cytology of Leidyana canadensis (Apicomplexa: Eugregarinida) in Lambdina fiscellaria fiscellaria larvae (Lepidoptera: Geometridae). J. Invertebr. Pathol. 2000, 75, 117–125. [Google Scholar] [CrossRef]
- Devauchelle, G. Étude ultrastructurale du développement des grégarines du Tenebrio molitor L. Protistologica 1968, 4, 313–332. [Google Scholar]
- Ghazali, M.; Philippe, M.; Deguercy, A.; Gounon, P.; Gallo, J.M.; Schrével, J. Actin and spectrin-like (Mr = 260-240 000) proteins in gregarines. Biol. Cell 1989, 67, 173–184. [Google Scholar]
- Schrével, J.; Vivier, E. Etude au microscope électronique de la région antérieure des Grégarines: Le mucron de Lecudina pellucida (Koll.) Mingazzini et l’épimérite de Sycia inopinata Léger. Protistologica 1966, 2, 17–28. [Google Scholar]
- Baudoin, J. Sur l’ultrastructure de la région antérieure de la grégarine Ancyrophora puytoraci. Protistologica 1969, 5, 431–439. [Google Scholar]
- Ormières, R. Pyxinia firmus (Leger, 1892), Eugregarine parasite of the Coleoptera Dermestes frischi Kugel. An ultrastructural study. Z. Parasitenkd. 1977, 53, 13–22. [Google Scholar] [CrossRef]
- Desai, R.N. Cytochemical demonstration of some hydrolytic enzymes in some gregarines (Protozoa: Sporozoa). I. The nonspecific acid and alkaline phosphatases. Acta Protozool. 1985, 24, 319–326. [Google Scholar]
- Desai, R.N. Cytochemical studies of some hydrolytic enzymes in gregarines. II: β-glucuronidase activity in different stages of the gregarine Stylocephalus conoides Devdhar, 1962 (Protozoa: Sporozoa). Acta Protozool. 1986, 25, 71–74. [Google Scholar]
- MacMillan, W.G. Some Aspects of the Biology of Nematocystis magna Schmidt. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 1970. Unpublished. [Google Scholar]
- Desai, R.N.; Nadkarni, V.B. Studies on steroidogenic potential of the gregarine Stylocephalus conoides Devdhar, 1962 (Protozoa: Sporozoa). A cytochemical study. Arch. Protistenkd. 1987, 133, 301–308. [Google Scholar] [CrossRef]
- Ghazali, M.; Schrével, J. Myosin-like protein (M(r)175,000) in Gregarina blaberae. J. Eukaryot. Microbiol. 1993, 40, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Harry, O.G. Gregarines: Their effect on the growth of the desert locust (Schistocerca gregaria). Nature 1970, 225, 964–966. [Google Scholar] [CrossRef]
- Heintzelman, M.B. Actin and myosin in Gregarina polymorpha. Cell Motil. Cytoskeleton 2004, 58, 83–95. [Google Scholar] [CrossRef]
- Valigurová, A.; Vaškovicová, N.; Musilová, N.; Schrével, J. The enigma of eugregarine epicytic folds: Where gliding motility originates? Front. Zool. 2013, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Vávra, J. Lankesteria barretti n. sp. (Eugregarinida, Diplocystidae), a parasite of the mosquito Aedes triseriatus (Say) and a review of the genus Lankesteria Mingazzini. J. Protozool. 1969, 16, 546–570. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.D., Jr.; Callaway, C.S. The fine structure of the gregarine Lankesteria culicis parasitic in the yellow fever mosquito Aedes aegypti. J. Protozool. 1969, 16, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Tronchin, G.; Philippe, M.; Mocquard, J.P.; Schrével, J. Cycle biologique de Gregarina blaberae: Description, chronologie, étude de la croissance, influence du cycle de l’hôte, Blaberus craniifer. Protistologica 1986, 22, 127–142. [Google Scholar]
- Martin, R.E. The transportome of the malaria parasite. Biol. Rev. 2020, 95, 305–332. [Google Scholar] [CrossRef]
- Spielmann, T.; Gras, S.; Sabitzki, R.; Meissner, M. Endocytosis in Plasmodium and Toxoplasma parasites. Trends Parasitol. 2020, 36, 520–532. [Google Scholar] [CrossRef]
- Prensier, G.; Dubremetz, J.F.; Schrével, J. The unique adaptation of the life cycle of the coelomic gregarine Diplauxis hatti to its host Perinereis cultrifera (Annelida, Polychaeta): An experimental and ultrastructural study. J. Eukaryot. Microbiol. 2008, 55, 541–553. [Google Scholar] [CrossRef]
- Vivier, E.; Devauchelle, G.; Petitprez, A.; Porchet-Hennere, E.; Prensier, G.; Schrével, J.; Vinckier, D. Observations on comparative cytology in sporozoans. Part 1. The sperficial structures in vegetative forms. Protistologica 1970, 6, 127–150. [Google Scholar]
- Schrével, J. Polysaccharides of cell-surface of gregarines (Protozoa Parasites). 1. Ultrastructure and cytochemistry. J. Microsc. 1972, 15, 21–40. [Google Scholar]
- Warner, F.D. The fine structure of Rhynchocystis pilosa (Sporozoa, Eugregarinida). J. Protozool. 1968, 15, 59–73. [Google Scholar] [CrossRef]
- Kováčiková, M.; Vaškovicová, N.; Nebesářová, J.; Valigurová, A. Effect of jasplakinolide and cytochalasin D on cortical elements involved in the gliding motility of the eugregarine Gregarina garnhami (Apicomplexa). Eur. J. Protistol. 2018, 66, 97–114. [Google Scholar] [CrossRef]
- Schrével, J.; Caigneaux, E.; Gros, D.; Philippe, M. The 3 cortical membranes of the gregarines. 1. Ultrastructural organization of Gregarina blaberae. J. Cell Sci. 1983, 61, 151–174. [Google Scholar] [CrossRef]
- Walker, M.H.; Lane, N.J.; Lee, W.M. Freeze-fracture studies on the pellicle of the eugregarine, Gregarina garnhami (Eugregarinida, Protozoa). J. Ultrastruct. Res. 1984, 88, 66–76. [Google Scholar] [CrossRef]
- Kováčiková, M.; Paskerova, G.G.; Diakin, A.; Simdyanov, T.G.; Vaškovicová, N.; Valigurová, A. Motility and cytoskeletal organisation in the archigregarine Selenidium pygospionis (Apicomplexa): Observations on native and experimentally affected parasites. Parasitol. Res. 2019, 118, 2651–2667. [Google Scholar] [CrossRef] [PubMed]
- Leander, B.S. Molecular phylogeny and ultrastructure of Selenidium serpulae (Apicomplexa, Archigregarinia) from the calcareous tubeworm Serpula vermicularis (Annelida, Polychaeta, Sabellida). Zool. Scr. 2007, 36, 213–227. [Google Scholar] [CrossRef]
- Valigurová, A.; Jirků, M.; Koudela, B.; Gelnar, M.; Modrý, D.; Šlapeta, J. Cryptosporidia: Epicellular parasites embraced by the host cell membrane. Int. J. Parasitol. 2008, 38, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Melicherová, J.; Hofmannová, L.; Valigurová, A. Response of cell lines to actual and simulated inoculation with Cryptosporidium proliferans. Eur. J. Protistol. 2018, 62, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Butaeva, F.; Paskerova, G.; Entzeroth, R. Ditrypanocystis sp. (Apicomplexa, Gregarinia, Selenidiidae): The mode of survival in the gut of Enchytraeus albidus (Annelida, Oligochaeta, Enchytraeidae) is close to that of the coccidian genus Cryptosporidium. Tsitologiia 2006, 48, 695–704. [Google Scholar] [PubMed]
- Ryan, U.; Paparini, A.; Monis, P.; Hijjawi, N. It’s official-Cryptosporidium is a gregarine: What are the implications for the water industry? Water Res. 2016, 105, 305–313. [Google Scholar] [CrossRef]
- Clode, P.L.; Koh, W.H.; Thompson, R.C.A. Life without a host cell: What is Cryptosporidium? Trends Parasitol. 2015, 31, 614–624. [Google Scholar] [CrossRef]
- Borowski, H.; Thompson, R.C.; Armstrong, T.; Clode, P.L. Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitology 2010, 137, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T. Gregarine site-heterogeneous 18S rDNA trees, revision of gregarine higher classification, and the evolutionary diversification of Sporozoa. Eur. J. Protistol. 2014, 50, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Melicherová, J.; Ilgová, J.; Kváč, M.; Sak, B.; Koudela, B.; Valigurová, A. Life cycle of Cryptosporidium muris in two rodents with different responses to parasitization. Parasitology 2014, 141, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Jirků, M.; Valigurová, A.; Koudela, B.; Křížek, J.; Modrý, D.; Šlapeta, J. New species of Cryptosporidium Tyzzer, 1907 (Apicomplexa) from amphibian host: Morphology, biology and phylogeny. Folia Parasitol. 2008, 55, 81–94. [Google Scholar] [CrossRef]
- Lumb, R.; Smith, K.; Odonoghue, P.J.; Lanser, J.A. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue-culture cells. Parasitol. Res. 1988, 74, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.B.; O’Hara, S.P.; Small, A.J.; Tietz, P.S.; Choudhury, A.K.; Pagano, R.E.; Chen, X.M.; LaRusso, N.F. Cryptosporidium parvum infects human cholangiocytes via sphingolipid-enriched membrane microdomains. Cell. Microbiol. 2006, 8, 1932–1945. [Google Scholar] [CrossRef][Green Version]
- Vetterling, J.M.; Takeuchi, A.; Madden, P.A. Ultrastructure of Cryptosporidium wrairi from the guinea pig. J. Protozool. 1971, 18, 248–260. [Google Scholar] [CrossRef]
- Forney, J.R.; DeWald, D.B.; Yang, S.G.; Speer, C.A.; Healey, M.C. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect. Immun. 1999, 67, 844–852. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; Small, A.J.; Chen, X.M.; LaRusso, N.F. Host cell actin remodeling in response to Cryptosporidium. Subcell. Biochem. 2008, 47, 92–100. [Google Scholar]
- Beyer, T.V.; Svezhova, N.V.; Sidorenko, N.V.; Khokhlov, S.E. Cryptosporidium parvum (Coccidia, Apicomplexa): Some new ultrastructural observations on its endogenous development. Eur. J. Protistol. 2000, 36, 151–159. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Paperna, I. Ultrastructural study of the coccidian Cryptosporidium sp. from stomachs of juvenile cichlid fish. Dis. Aquat. Organ. 1986, 2, 13–20. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Koh, W.H.; Clode, P.L. Cryptosporidium—What is it? Food Waterborne Parasitol. 2016, 4, 54–61. [Google Scholar] [CrossRef]
- Tzipori, S.; Griffiths, J.K. Natural history and biology of Cryptosporidium parvum. Adv. Parasitol. 1998, 40, 5–36. [Google Scholar] [CrossRef]
- Perkins, M.E.; Riojas, Y.A.; Wu, T.W.; Le Blancq, S.M. CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host-parasite boundary in intracellular stages. Proc. Natl. Acad. Sci. USA 1999, 96, 5734–5739. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Iseki, M. Freeze-fracture study of the site of attachment of Cryptosporidium muris in gastric glands. J. Protozool. 1992, 39, 539–544. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; Chen, X.-M. The cell biology of Cryptosporidium infection. Microbes Infect. 2011, 13, 721–730. [Google Scholar] [CrossRef]
- Koh, W.; Thompson, A.; Edwards, H.; Monis, P.; Clode, P.L. Extracellular excystation and development of Cryptosporidium: Tracing the fate of oocysts within Pseudomonas aquatic biofilm systems. BMC Microbiol. 2014, 14, 281. [Google Scholar] [CrossRef]
- Dyková, I.; Lom, J. Fish coccidia: Critical notes on life cycles, classification and pathogenicity. J. Fish Dis. 1981, 4, 487–505. [Google Scholar] [CrossRef]
- Lukeš, J. Life cycle of Goussia pannonica (Molnar, 1989) (Apicomplexa, Eimeriorina), an extracytoplasmic coccidium from the white bream Blicca bjoerkna. J. Protozool. 1992, 39, 484–494. [Google Scholar] [CrossRef]
- Molnar, K.; Baska, F. Light and electron microscopic studies on Epieimeria anguillae (Léger & Hollande, 1922), a coccidium parasitizing the European eel, Anguilla anguilla L. J. Fish Dis. 1986, 9, 99–110. [Google Scholar]
- Beyer, T.V.; Sidorenko, N.V.; Svezhova, N.V. Cell interaction with intracellular parasitism of cryptosporidia. I. The influence of Cryptosporidium parvum (Coccidia, Sporozoa) on phosphatase activity in the small intestine of experimentally infected new-born rats. Cytology 1995, 37, 829–837. [Google Scholar]
- Beyer, T.V.; Svezhova, N.V.; Radchenko, A.I.; Sidorenko, N.V. Parasitophorous vacuole: Morphofunctional diversity in different coccidian genera (a short insight into the problem). Cell Biol. Int. 2002, 26, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Benajiba, M.H.; Marques, A.; Lom, J.; Bouix, G. Ultrastructure and sporogony of Eimeria (syn. Epieimeria) anguillae (Apicomplexa) in the eel (Anguilla anguilla). J. Eukaryot. Microbiol. 1994, 41, 215–222. [Google Scholar] [CrossRef]
- Bonnin, A.; Lapillonne, A.; Petrella, T.; Lopez, J.; Chaponnier, C.; Gabbiani, G.; Robine, S.; Dubremetz, J.F. Immunodetection of the microvillous cytoskeleton molecules villin and ezrin in the parasitophorous vacuole wall of Cryptosporidium parvum (Protozoa: Apicomplexa). Eur. J. Cell Biol. 1999, 78, 794–801. [Google Scholar] [CrossRef]
- Walker, D.M.; Oghumu, S.; Gupta, G.; McGwire, B.S.; Drew, M.E.; Satoskar, A.R. Mechanisms of cellular invasion by intracellular parasites. Cell. Mol. Life Sci. 2014, 71, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, S.A.; Schepis, A.; Reynolds, L.; Kappe, S.H.I. Secretory organelle function in the Plasmodium sporozoite. Trends Parasitol. 2021, 37, 651–663. [Google Scholar] [CrossRef]
- Bannister, L.H. The interactions of intracellular Protista and their host cells, with special reference to heterotrophic organisms. Proc. R. Soc. Lond. B. Biol. Sci. 1979, 204, 141–163. [Google Scholar]
- Goldberg, D.E.; Zimmerberg, J. Hardly vacuous: The parasitophorous vacuolar membrane of malaria parasites. Trends Parasitol. 2020, 36, 138–146. [Google Scholar] [CrossRef]
- Rudzinska, M.A.; Trager, W.; Lewengrub, S.J.; Gubert, E. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 1976, 169, 323–334. [Google Scholar] [CrossRef]
- Beck, J.R.; Ho, C.-M. Transport mechanisms at the malaria parasite-host cell interface. PLoS Pathog. 2021, 17, e1009394. [Google Scholar] [CrossRef]
- Goldberg, D.E. Hemoglobin degradation. In Malaria: Drugs, Disease and Post-Genomic Biology; Compans, R.W., Cooper, M.D., Honjo, T., Koprowski, H., Melchers, F., Oldstone, M.B.A., Olsnes, S., Potter, M., Vogt, P.K., Wagner, H., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 275–291. [Google Scholar]
- Wunderlich, J.; Rohrbach, P.; Dalton, J.P. The malaria digestive vacuole. Front. Biosci. 2012, 4, 1424–1448. [Google Scholar]
- Xie, S.C.; Ralph, S.A.; Tilley, L. K13, the cytostome, and artemisinin resistance. Trends Parasitol. 2020, 36, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Žižka, Z. Fine structure of the neogregarine Farinocystis tribolii Weiser, 1953. Developmental stages in sporogony and parasite-host relations. Z. Parasitenkd. 1977, 54, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Schrével, J.; Desportes, I. Gregarines. In Encyclopedia of Parasitology; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1142–1188. [Google Scholar]
- Vávra, J.; McLaughlin, R.E. Fine Structure of some developmental stages of Mattesia grandis Mclaughlin (Sporozoa, Neogregarinida), a parasite of boll weevil Anthonomus grandis Boheman. J. Protozool. 1970, 17, 483–496. [Google Scholar] [CrossRef]
- Larsson, J.I.R. On the cytology and fine structure of the neogregarine Syncystis aeshnae Tuzet and Manier, 1953 (Apicomplexa, Syncystidae). J. Protozool. 1991, 38, 383–392. [Google Scholar] [CrossRef]
- Žižka, Z. Formation of a parasitophorous vacuole in a nonadequate experimental host: Electron microscopical and X-ray microanalytical study. Folia Microbiol. 2005, 50, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Naville, A. Recherches cytologiques sur les schizogrégarines. Z. Zellforsch. Mikrosk. Anat. 1930, 11, 375–396. [Google Scholar] [CrossRef]
- Valigurová, A.; Koudela, B. Ultrastructural study of developmental stages of Mattesia dispora (Neogregarinorida: Lipotrophidae), a parasite of the flour moth Ephestia kuehniella (Lepidoptera). Eur. J. Protistol. 2006, 42, 313–323. [Google Scholar] [CrossRef]
- Weiser, J. Schizogregariny z hmyzu škodícího zásobám mouky II. Mattesia dispora Naville 1930 a Coelogregarina ephestiae Gheleovitch 1947. Věstník Československé Zoologické Společnosti 1954, 17, 73–90. [Google Scholar]
- Žižka, Z. An electron microscope study of autoinfection in neogregarines (Sporozoa, Neogregarinida). J. Protozool. 1972, 19, 275–280. [Google Scholar] [CrossRef]
- Tanada, Y.; Kaya, H.K. Insect Pathology; Academic Press: San Diego, CA, USA, 1993; p. 666. [Google Scholar]
- Ormières, R.; Massot, M.; Massot, N. Ophryocystis schneideri Lég. Hagenm., 1900 néogrégarine parasite de Blaps ďAlgérie. Cycle et étude ultrastructurale. Protistologica 1978, 14, 483–492. [Google Scholar]
- Žižka, Z. Fine structure of the neogragarine Farinocystis tribolii Weiser, 1953. Syzygy and gametes formation. Protistologica 1978, 14, 209–215. [Google Scholar]
- ŽIžka, Z. Fine structure of the neogregarine Farinocystis tribolii Weiser, 1953: Free gametocytes. Acta Protozool. 1978, 17, 255–259. [Google Scholar]
- Žižka, Z. Fine structure of the neogregarine Farinocystis tribolii Weiser, 1953. Developmental stages in merogony. J. Protozool. 1978, 25, 50–56. [Google Scholar] [CrossRef]
- Žižka, Z. The fine structure of some developmental stages of Farinocystis tribolii Weiser (Sporozoa, Neogregarinida) from the fat body of the beetle Tribolium castaneum Herbst. J. Protozool. 1971, 17, 46. [Google Scholar]
- Leander, B.S.; Ramey, P.A. Cellular identity of a novel small subunit rDNA sequence clade of apicomplexans: Description of the marine parasite Rhytidocystis polygordiae n. Sp (Host: Polygordius sp., polychaeta). J. Eukaryot. Microbiol. 2006, 53, 280–291. [Google Scholar] [CrossRef]
- Rueckert, S.; Leander, B.S. Phylogenetic position and description of Rhytidocystis cyamus sp. n. (Apicomplexa, Rhytidocystidae): A novel intestinal parasite of the north-eastern Pacific ‘stink worm’ (Polychaeta, Opheliidae, Travisia pupa). Mar. Biodivers. 2009, 39, 227–234. [Google Scholar] [CrossRef]
- Miroliubova, T.S.; Simdyanov, T.G.; Mikhailov, K.V.; Aleoshin, V.V.; Janouškovec, J.; Belova, P.A.; Paskerova, G.G. Polyphyletic origin, intracellular invasion, and meiotic genes in the putatively asexual agamococcidians (Apicomplexa incertae sedis). Sci. Rep. 2020, 10, 15847. [Google Scholar] [CrossRef] [PubMed]
- Guérardel, Y.; Leleu, D.; Coppin, A.; Liénard, L.; Slomianny, C.; Strecker, G.; Ball, S.; Tomavo, S. Amylopectin biogenesis and characterization in the protozoan parasite Toxoplasma gondii, the intracellular development of which is restricted in the HepG2 cell line. Microbes Infect. 2005, 7, 41–48. [Google Scholar] [CrossRef]
- Harris, J.R.; Adrian, M.; Petry, F. Amylopectin: A major component of the residual body in Cryptosporidium parvum oocysts. Parasitology 2004, 128, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Weppelman, R.M.; Lopez-Ramos, B. Isolation of amylopectin granules and identification of amylopectin phosphorylase in the oocysts of Eimeria tenella. J. Protozool. 1975, 22, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Schrével, J.; Stark, J.R. The storage polysaccharide (paraglycogen) of the gregarine, Gregarina blaberae: Cytology and biochemistry. Comp. Biochem. Physiol. B Comp. Biochem. 1973, 44, 1001–1010. [Google Scholar] [CrossRef]
- Ryley, J.F.; Bentley, M.; Manners, D.J.; Stark, J.R. Amylopectin, the storage polysaccharide of the Coccidia Eimeria brunetti and E. tenella. J. Parasitol. 1969, 55, 839–845. [Google Scholar] [CrossRef]
- Nakai, Y.; Ogimoto, K. Utilization of carbohydrate by Eimeria tenella sporozoites. Nihon Juigaku Zasshi 1983, 45, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Michalski, W.P.; Edgar, J.A.; Prowse, S.J. Mannitol metabolism in Eimeria tenella. Int. J. Parasitol. 1992, 22, 1157–1163. [Google Scholar] [CrossRef]
- Coppin, A.; Varré, J.S.; Lienard, L.; Dauvillée, D.; Guérardel, Y.; Soyer-Gobillard, M.O.; Buléon, A.; Ball, S.; Tomavo, S. Evolution of plant-like crystalline storage polysaccharide in the protozoan parasite Toxoplasma gondii argues for a red alga ancestry. J. Mol. Evol. 2005, 60, 257–267. [Google Scholar] [CrossRef]


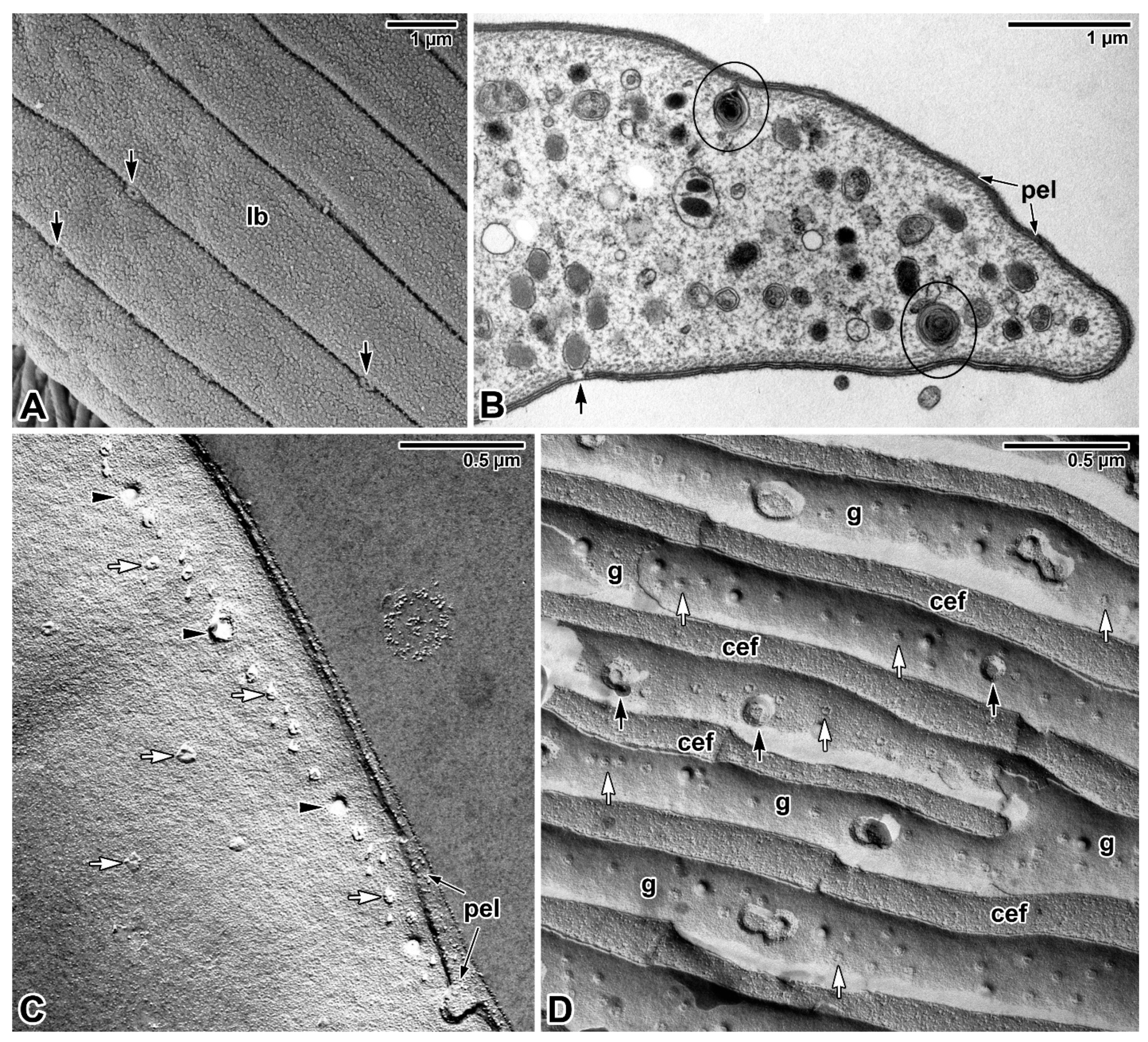

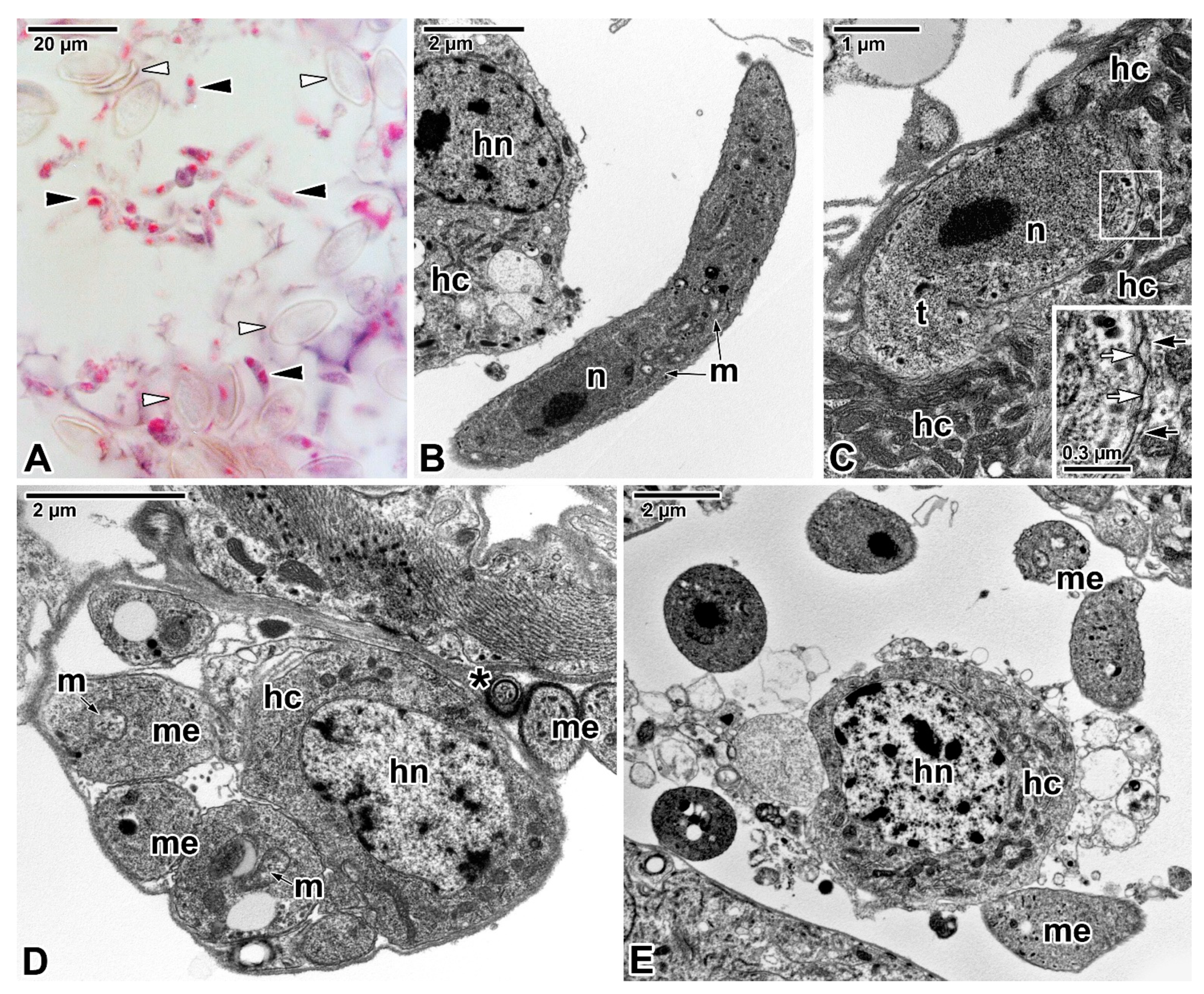
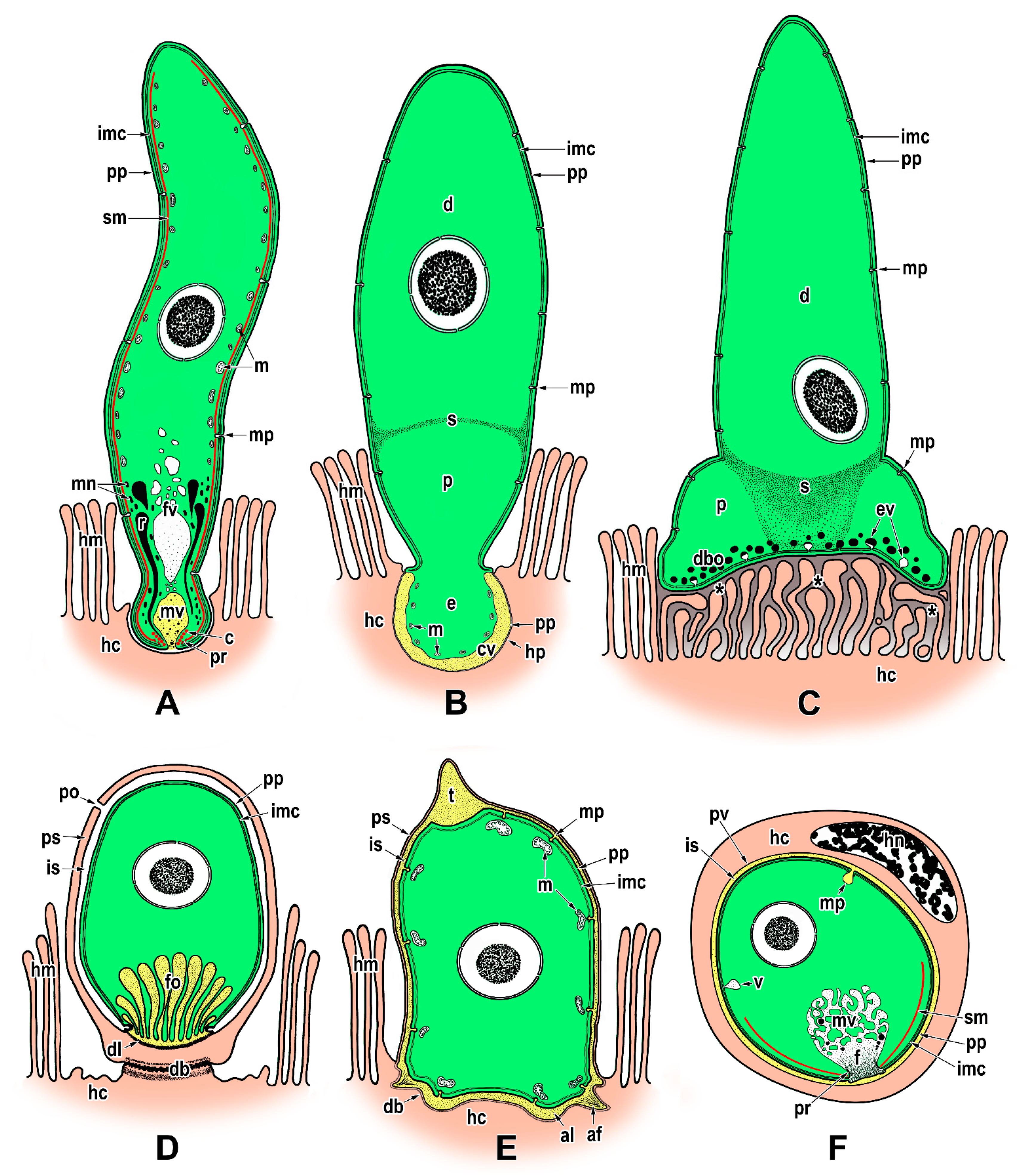
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valigurová, A.; Florent, I. Nutrient Acquisition and Attachment Strategies in Basal Lineages: A Tough Nut to Crack in the Evolutionary Puzzle of Apicomplexa. Microorganisms 2021, 9, 1430. https://doi.org/10.3390/microorganisms9071430
Valigurová A, Florent I. Nutrient Acquisition and Attachment Strategies in Basal Lineages: A Tough Nut to Crack in the Evolutionary Puzzle of Apicomplexa. Microorganisms. 2021; 9(7):1430. https://doi.org/10.3390/microorganisms9071430
Chicago/Turabian StyleValigurová, Andrea, and Isabelle Florent. 2021. "Nutrient Acquisition and Attachment Strategies in Basal Lineages: A Tough Nut to Crack in the Evolutionary Puzzle of Apicomplexa" Microorganisms 9, no. 7: 1430. https://doi.org/10.3390/microorganisms9071430
APA StyleValigurová, A., & Florent, I. (2021). Nutrient Acquisition and Attachment Strategies in Basal Lineages: A Tough Nut to Crack in the Evolutionary Puzzle of Apicomplexa. Microorganisms, 9(7), 1430. https://doi.org/10.3390/microorganisms9071430