Land-Use Type Drives Soil Population Structures of the Entomopathogenic Fungal Genus Metarhizium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites, Sampling Procedure
2.2. Fungal Isolation and DNA Extraction
2.3. Sequence Analysis
2.4. Multilocus Microsatellite Genotyping
2.5. Data Analysis
3. Results
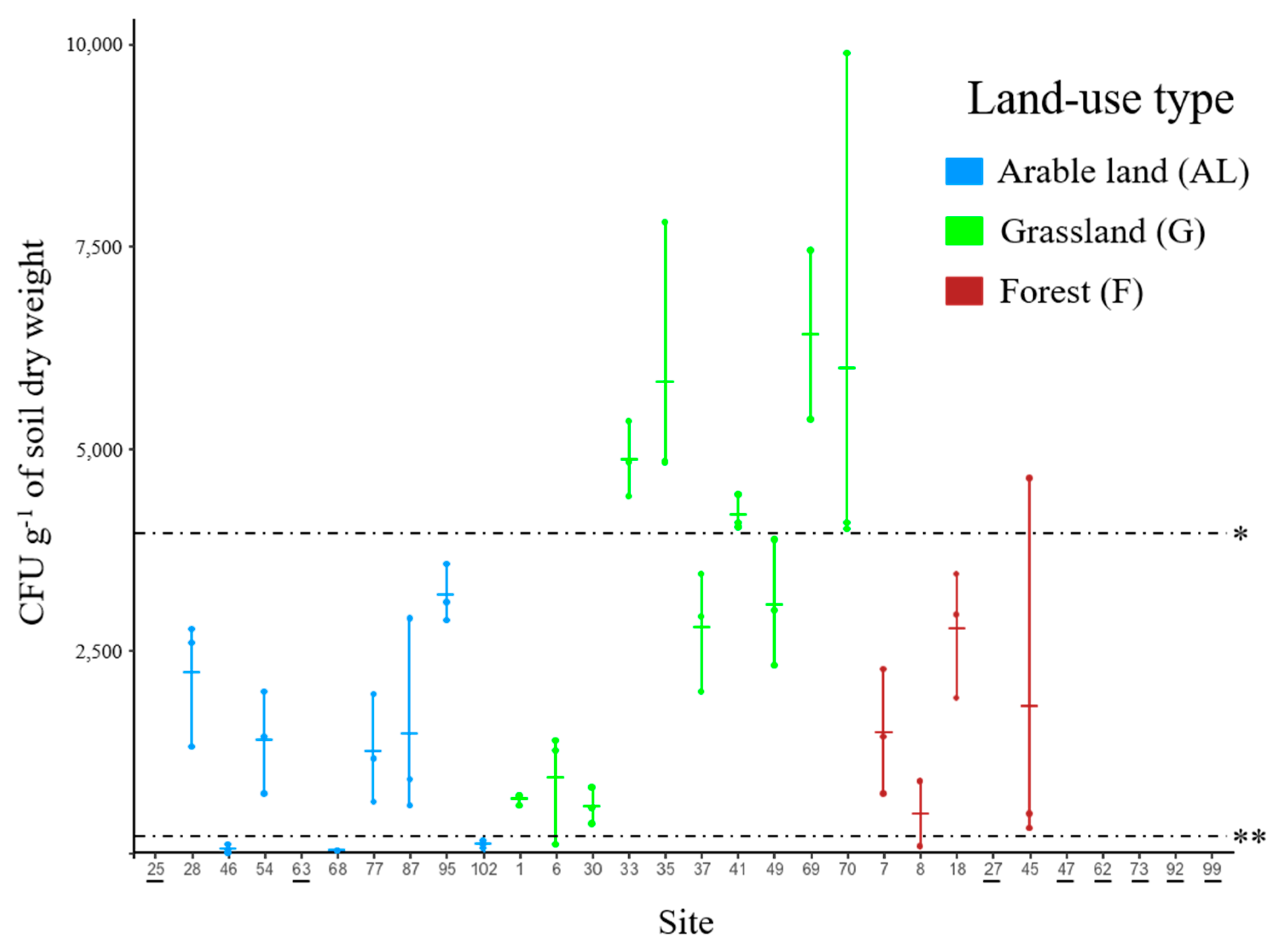
3.1. Abundance of Metarhizium in Soil
3.2. Effect of Environmental Factors on Metarhizium Abundance Groups
3.3. Metarhizium Species Occurrence and Genetic Diversity
3.4. Land-Use Type Effect on Metarhizium Populations
3.5. Effect of Environmental Factors on Metarhizium Populations
4. Discussion
4.1. M. brunneum Populations in Forested vs. Unforested LUTs
4.2. Metarhizium Populations in Arable Land vs. Grassland
4.3. Association Metarhizium Species and Genotypes to LUTs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| GeneBank Accession Number | Isolate | MLG | Species |
|---|---|---|---|
| MZ297396 | 95-III-2 | MLG12 | M. robertsii |
| MZ297397 | 87-III-4 | MLG19 | M. brunneum |
| MZ297398 | 77-III-5 | MLG16 | M. brunneum |
| MZ297399 | 77-I-1 | MLG20 | M. brunneum |
| MZ297400 | 70-III-1 | MLG15 | M. brunneum |
| MZ297401 | 70-II-1 | MLG7 | M. guizhouense |
| MZ297402 | 54-I-4 | MLG1 | M. robertsii |
| MZ297403 | 45-III-6 | MLG4 | M. brunneum |
| MZ297404 | 45-I-1 | MLG6 | M. brunneum |
| MZ297405 | 35-III-3 | MLG8 | M. brunneum |
| MZ297406 | 35-II-5 | MLG9 | M. brunneum |
| MZ297407 | 35-I-6 | MLG17 | M. brunneum |
| MZ297408 | 33-I-6 | MLG2 | M. robertsii |
| MZ297409 | 33-I-5 | MLG3 | M. robertsii |
| MZ297410 | 30-II-6 | MLG21 | M. guizhouense |
| MZ297411 | 30-I-3 | MLG18 | M. brunneum |
| MZ297412 | 28-II-2 | MLG22 | M. guizhouense |
| MZ297413 | 28-I-1 | MLG11 | M. robertsii |
| MZ297414 | 18-III-3 | MLG5 | M. brunneum |
| MZ297415 | 7-III-6 | MLG10 | M. brunneum |
| MZ297416 | 6-I-2 | MLG14 | M. brunneum |
| MZ297417 | 1-III-4 | MLG13 | M. robertsii |
References
- Brunner-Mendoza, C.; Reyes-Montes, M.R.; Moonjely, S.; Bidochka, M.J.; Toriello, C. A review on the genus Metarhizium as an entomopathogenic microbial biocontrol agent with emphasis on its use and utility in Mexico. Biocontrol. Sci. Technol. 2019, 29, 83–102. [Google Scholar] [CrossRef]
- Goettel, M.S.; Eilenberg, J.; Glare, T. Entomopathogenic fungi and their role in regulation of insect populations. In Comprehensive Molecular Insect Science; Gilbert, L.I., Gill, S.S., Eds.; Elsevier: Boston, MA, USA, 2005; pp. 361–406. [Google Scholar]
- Lawo, N.C.; Mahon, R.J.; Milner, R.J.; Sarmah, B.K.; Higgins, T.J.V.; Romeis, J. Effectiveness of bacillus thuringiensis-transgenic chickpeas and the entomopathogenic fungus Metarhizium anisopliae in controlling Helicoverpa armigera (Lepidoptera: Noctuidae). mBio 2008, 74, 4381–4389. [Google Scholar] [CrossRef] [Green Version]
- Pilz, C.; Wegensteiner, R.; Keller, S. Selection of entomopathogenic fungi for the control of the western corn rootworm Diabrotica virgifera virgifera. J. Appl. Entomol. 2007, 131, 426–431. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol. Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Maniania, N.K.; Ekesi, S. The use of entomopathogenic fungi in the control of tsetse flies. J. Invertebr. Pathol. 2013, 112, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.A.; Schumann, M.; Przyklenk, M.; Patel, A.; Vidal, S. Wireworm damage reduction in potatoes with an attract-and-kill strategy using Metarhizium brunneum. J. Pest. Sci. 2016, 90, 479–493. [Google Scholar] [CrossRef]
- Faria, M.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzon, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; St. Leger, J. Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl. Environ. Microbiol. 2002, 68, 6383–6387. [Google Scholar] [CrossRef] [Green Version]
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Ubiquity of insect-derived nitrogen transfer to plants by endophytic insect-pathogenic fungi: An additional branch of the soil nitrogen cycle. Appl. Environ. Microbiol. 2012, 80, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. Fungi with multifunctional lifestyles: Endophytic insect pathogenic fungi. Plant. Mol. Biol. 2016, 90, 657–664. [Google Scholar] [CrossRef]
- Raya-Diaz, S.; Sanchez-Rodriguez, A.R.; Segura-Fernandez, J.M.; del Campillo, M.C.; Quesada-Moraga, E. Entomopathogenic fungi-based mechanisms for improved Fe nutrition in sorghum plants grown on calcareous substrates. PLoS ONE 2017, 12, e0185903. [Google Scholar] [CrossRef] [Green Version]
- Jaber, L.R.; Enkerli, J. Fungal entomopathogens as endophytes: Can they promote plant growth? Biocontrol. Sci. Technol. 2017, 27, 28–41. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Alder-Rangel, A.; Dadachova, E.; Finlay, R.D.; Dijksterhuis, M.K.J.; Braga, G.U.L.; Corrochano, L.M.; Hallsworth, J.E. Fungal stress biology: A preface to the fungal stress responses special edition. Curr. Genet. 2015, 61, 231–238. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2013, 35, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control. 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Foley, J.A.; Defries, R.S.; Asner, G.P. Global consequences of land use. Science. 2005, 309. [Google Scholar] [CrossRef] [Green Version]
- Vänninen, I. Distribution and occurrence of four entomopathogenic fungi in Finland: Effect of geographical location, habitat type and soil type. Mycol. Res. 1996, 100, 93–101. [Google Scholar] [CrossRef]
- Jabbour, R.; Barbercheck, M.E. Soil management effects on entomopathogenic fungi during the transition to organic agriculture in a feed grain rotation. Biol. Control. 2009, 51, 435–443. [Google Scholar] [CrossRef]
- Kepler, R.M.; Ugine, T.A.; Maul, J.E.; Cavigelli, M.A.; Rehner, S.A. Community composition and population genetics of insect pathogenic fungi in the genus Metarhizium from soils of a long-term agricultural research system. Environ. Microbiol. 2015, 8, 2791–2804. [Google Scholar] [CrossRef]
- Clifton, E.H.; Jaronski, S.T.; Hodgson, E.W.; Gassmann, A.J. Abundance of soil-borne entomopathogenic fungi in organic and conventional fields in the mid-western USA with an emphasis on the effect of herbicides and fungicides on fungal persistence. Biol. Control. 2015, 51, 435–443. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Kasperski, J.E.; Wild, G.A.M. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Canad. J. Bot. 1998, 76, 1198–1204. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Jurado, I.; Fernández-Bravo, M.; Campos, C.; Quesada-Moraga, E. Diversity of entomopathogenic Hypocreales in soil and phylloplane of five Mediterranean cropping systems. J. Invertebr. Pathol. 2015, 130, 97–106. [Google Scholar] [CrossRef]
- Nielsen, C.; Skovgard, H.; Steenberg, T. Effect of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) on survival and reproduction of the filth fly parasitoid, Spalangia cameroni (Hymenoptera: Pteromalidae). Environ. Entomol. 2004, 34, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Kessler, P.; Schweizer, C. Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metharhizium anisopliae. BioControl. 2003, 48, 307–319. [Google Scholar] [CrossRef]
- Schneider, S.; Widmer, F.; Jacot, K.; Kolliker, R.; Enkerli, J. Spatial distribution of Metarhizium clade 1 in agricultural landscapes with arable land and different semi-natural habitats. Appl. Soil. Ecol. 2012, 52, 20–28. [Google Scholar] [CrossRef]
- Klingen, I.; Eilenberg, J.; Meadow, R. Effects of farming system, field margins and bait insect on the occurrence of insect pathogenic fungi in soils. Agric. Ecosyst. Environ. 2002, 91, 191–198. [Google Scholar] [CrossRef]
- Miętkiewski, R.T.; Pell, K.J.; Clark, S.J. Influence of pesticide use on the natural occurrence of entomopathogenic fungi in arable soil in the UK: Field and laboratory comparisons. Biocontrol. Sci. Technol. 1997, 7, 565–575. [Google Scholar] [CrossRef]
- Cabrera-Mora, J.A.; Guzmán-Franco, A.W.; Santillán-Galicia, M.T.; Tamayo-Mejía, F. Niche separation of species of entomopathogenic fungi within the genera Metarhizium and Beauveria in different cropping systems in Mexico. Fungal Ecol. 2019, 39, 349–355. [Google Scholar] [CrossRef]
- Wyrebek, M.; Huber, C.; Sasan, R.K.; Bidochka, M.J. Three sympatrically occurring species of Metarhizium show plant rhizosphere specificity. Microbiology 2011, 157, 2904–2911. [Google Scholar] [CrossRef] [Green Version]
- Bidochka, M.J.; Kamp, A.M.; Lavender, T.M.; Dekoning, J.; De Croos, J.N.A. Habitat association in two genetic groups of the insect-pathogenic fungus Metarhizium anisopliae: Uncovering cryptic species? Appl. Environ. Microbiol. 2001, 67, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidochka, M.J.; Small, C.L. Phylogeography of Metarhizium, an insect pathogenic fungus. In Insect-Fungal Associations: Ecology and Evolution; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 75–118. [Google Scholar]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia. 2009, 101, 512–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Thorup-Kristensen, K.; Meyling, N.V. Molecular diversity of the entomopathogenic fungal Metarhizium community within an agroecosystem. J. Invertebr. Pathol. 2014, 123, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.; Mayerhofer, J.; Enkerli, J.; Eilenberg, J.; Meyling, N.V.; Moral, R.D.; Demetrio, C.G.B.; Delalibera, I. Persistence of Brazilian isolates of the entomopathogenic fungi Metarhizium anisopliae and M. robertsii in strawberry crop soil after soil drench application. Agric. Ecosyst. Environ. 2016, 233, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Inglis, G.D.; Duke, G.M.; Goettel, M.S.; Kabaluk, J.T.; Ortega-Polo, R. Biogeography and genotypic diversity of Metarhizium brunneum and Metarhizium robertsii in northwestern North American soils. Can. J. Microbiol. 2019, 65, 261–281. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Ampong-Nyarko, K. Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci. Technol. 1999, 9, 177–185. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Anderson, A.J.; Roberts, D.W. Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic regions. J. Invertebr. Pathol. 2005, 88, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, M.; Flores-León, A.; Calero-López, S.; Gutiérrez-Sánchez, F.; Valverde-García, P.; Quesada-Moraga, E. UV-B radiation-related effects on conidial inactivation and virulence against Ceratitis capitata (Wiedemann) (Diptera; Tephritidae) of phylloplane and soil Metarhizium sp. Strains. J. Invertebr. Pathol. 2017, 148, 142–151. [Google Scholar] [CrossRef]
- McGuire, A.V.; Northfield, T.D. Tropical Occurrence and Agricultural Importance of Beauveria bassiana and Metarhizium anisopliae. Front. Sustain. Food. Syst. 2020, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Uzman, D.; Pliestera, J.; Leyerb, I.; Entlingc, M.H.; Reinekea, A. Drivers of entomopathogenic fungi presence in organic and conventional vineyard soils. Appl. Soil Ecol. 2019, 133, 89–97. [Google Scholar] [CrossRef]
- Medo, J.; Cagán, M. Factors affecting the occurrence of entomopathogenic fungi in soils of Slovakia as revealed using two methods. Biol. Control. 2011, 59, 200–208. [Google Scholar] [CrossRef]
- Gubler, A.; Schwab, P.; Wächter, D.; Meuli, R.G.; Keller, A. Ergebnisse der Nationalen Bodenbeobachtung (NABO) 1985–2009. Zustand und Veränderungen der anorganischen Schadstoffe und Bodenbegleitparameter; Bundesamt für Umwelt—Umwelt-Zustand: Bern, Germany, 2015; pp. 1–81. [Google Scholar]
- Gschwend, F.; Hartmann, M.; Hug, A.; Enkerli, J.; Gubler, A.; Frey, B.; Meuli, R.G.; Widmer, F. Long-term stability of soil bacterial and fungal community structures revealed in their abundant and rare fractions. Mol. Ecol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gubler, A.; Wächter, D.; Schwab, P.; Müller, M.; Keller, A. Twenty-five years of observations of soil organic carbon in Swiss croplands showing stability overall but with some divergent trends. Environ. Monit. Assess. 2019, 191, 277. [Google Scholar] [CrossRef] [Green Version]
- Strasser, H.; Forer, A.; Schinner, F. Development of media for the selective isolation and maintenance of virulence of Beauveria brongniartii. In Microbial Control of Soil Dwelling Pests; Jackson, T., Glare, T., Eds.; Christchurch, AgResearch Publisher: Lincoln, New Zealand, 1996; pp. 125–130. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; APS Press: St. Paul, MN, USA, 2006. [Google Scholar]
- Rehner, S.A.; Buckley, E.P. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Rauch, H.; Hartmann, M.; Widmer, F.; Gschwend, F.; Strasser, H.; Leuchtmann, A.; Enkerli, J. Response of soil microbial communities to the application of a formulated Metarhizium brunneum biocontrol strain. Biocontrol Sci. Technol. 2019, 29, 547–564. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Lutz, A.; Widmer, F.; Rehner, S.A.; Leuchtmann, A.; Enkerli, J. Multiplexed microsatellite markers for seven Metarhizium species. J. Invertebr. Pathol. 2015, 132, 132–134. [Google Scholar] [CrossRef] [Green Version]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Pielou, E. Ecological Diversity; John Wiley and Sons: Hoboken, NJ, USA, 1975. [Google Scholar]
- Ludwig, J.; Reynolds, J. Statistical Ecology. A Primer on Methods and Computing; John Wiley and Sons: Hoboken, NY, USA, 1988. [Google Scholar]
- Grünwald, N.J.; Goodwin, S.B.; Milgroom, M.G.; Fry, W.E. Analysis of genotypic diversity data for populations of microorganisms. Phytopathology 2003, 93, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-69-3; RStudoi: Boston, MA, USA, 2018. [Google Scholar]
- Oksanen, J.; Blanchet, G.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.4-6; RStudoi: Boston, MA, USA, 2018; Available online: http://CRAN.R-project.org/package=vegan (accessed on 27 May 2021).
- R-Core-Team. R: A Language and Environment For Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Gower, J.C. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 1966, 53, 325–328. [Google Scholar] [CrossRef]
- Shah, F.A.; Wang, C.S.; Butt, T.M. Nutrition influence growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 2005, 251, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Sun, M.H.; Liu, X.Z.; Che, Y.S. Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycol. Res. 2007, 111, 87–92. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Garrido-Jurado, I.; Borrego, A.; Quesada-Moraga, E. Effects of cultural conditions on fungal biomass, blastospore yields and toxicity of fungal secreted proteins in batch cultures of Metarhizium anisopliae (Ascomycota: Hypocreales). Pest. Manag. Sci. 2010, 66, 725–735. [Google Scholar] [CrossRef]
- Sehler, R.; Li, J.; Reager, J.T.; Ye, H. Investigating relationship between soil moisture and precipitation globally using remote sensing observations. J. Contemp. Water Res. Educ. 2019, 168, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Jurado, I.; Valverde-Garcia, P.; Quesada-Moraga, E. Use of a multiple logistic regression model to determine the effects of soil moisture and temperature on the virulence of entomopathogenic fungi against pre-imaginal Mediterranean fruit fly Ceratitis capitata. Biol. Control. 2011, 59, 366–372. [Google Scholar] [CrossRef]
- Sosa-Gómez, D.R.; Delpin, K.E.; Moscardi, F.; Farias, J.R.B. Natural occurrence of the entomopathogenic fungi Metarhizium, Beauveria and Paecilomyces in soybean under till and no-till cultivation systems. Neotrop. Entomol. 2001, 30, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Hummel, R.L.; Walgenbach, J.F.; Barbercheck, M.E.; Kennedy, G.G.; Hoyt, G.D.; Arellano, C. Effects of production practices on soil-borne entomopathogens in western North Carolina vegetable systems. Environ. Entomol. 2002, 31, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Inglis, G.D.; Duke, G.M.; Goettel, M.S.; Kabaluk, J.T. Genetic diversity of Metarhizium anisopliae var. anisopliae in southwestern British Columbia. J. Invertebr. Pathol. 2007, 98, 101–113. [Google Scholar] [CrossRef]
- Rehner, S.A. Genetic structure of Metarhizium species in western USA: Finite populations composed of divergent clonal lineages with limited evidence for recent recombination. J. Invertebr. Pathol. 2020, 177, 107491. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Rehner, S.A.; Bruck, D.J. Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. J. Invertebr. Pathol. 2011, 106, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Arribas, P.; Andújar, C.; Salces-Castellano, A.; Emerson, B.C.; Vogler, A.P. The limited spatial scale of dispersal in soil arthropods revealed with whole-community haplotype-level metabarcoding. Mol. Ecol. 2021, 30, 48–61. [Google Scholar] [CrossRef]
- Dromph, K.M. Collembolans as vectors of entomopathogenic fungi. Pedobiologia 2003, 47, 245–256. [Google Scholar] [CrossRef]



| LUT | Factor (1) | High | Medium | Low | ANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | F-Value | p-Value | Pattern (2) | ||
| All LUT | C:N ratio | 9.6 | 0.9 | 8.9 | 10.0 | 11.1 | 3.1 | 8.0 | 17.7 | 14.2 | 5.5 | 8.6 | 27.0 | 8.72 | 0.0004 | H = M ≠ L |
| MAP [mm] | 1496.2 | 321.0 | 1090.0 | 1910.0 | 1342.4 | 363.2 | 962.0 | 2140.0 | 1116.7 | 368.5 | 528.0 | 1838.0 | 6.77 | 0.0019 | H = M ≠ L | |
| Arable land | Altitude [masl] | - (3) | - | - | - | 549.4 | 167.9 | 336.0 | 830.0 | 449.6 | 55.6 | 379.0 | 545.0 | 4.78 | 0.0374 | M ≠ L |
| Clay [% sdw] | - | - | - | - | 17.6 | 4.6 | 11.5 | 23.8 | 32.5 | 19.1 | 5.8 | 59.0 | 8.69 | 0.0064 | M ≠ L | |
| Sand [% sdw] | - | - | - | - | 42.2 | 9.8 | 31.0 | 54.0 | 22.4 | 11.4 | 11.0 | 36.1 | 26.15 | <0.0001 | M ≠ L | |
| Soil skeleton [% sv] | - | - | - | - | 4.0 | 0.8 | 3.1 | 4.9 | 0.7 | 0.8 | 0.0 | 2.0 | 135.85 | <0.0001 | M ≠ L | |
| Total Carbon [% sdw] | - | - | - | - | 2.0 | 0.7 | 1.1 | 3.2 | 2.8 | 0.9 | 1.8 | 4.3 | 8.55 | 0.0068 | M ≠ L | |
| DNA [µg/mg sdw] | - | - | - | - | 25.5 | 9.1 | 14.0 | 45.0 | 17.9 | 3.8 | 13.0 | 26.0 | 8.85 | 0.006 | M ≠ L | |
| Grassland | Silt [% sdw] | 42.3 | 8.4 | 34.3 | 55.0 | 35.6 | 7.9 | 27.0 | 49.9 | - | - | - | 5.10 | 0.0319 | H ≠ M | |
| Forest | Altitude [masl] | - | - | - | - | 745.3 | 269.9 | 525.00 | 1180.0 | 1115.8 | 408.7 | 505.0 | 1655.0 | 7.60 | 0.0101 | M ≠ L |
| Clay [% sdw] | - | - | - | - | 31.1 | 9.1 | 18.8 | 42.0 | 17.8 | 8.3 | 7.0 | 30.5 | 17.30 | 0.0003 | M ≠ L | |
| Sand [% sdw] | - | - | - | - | 31.1 | 8.7 | 18.0 | 39.0 | 47.2 | 21.0 | 17.5 | 71.0 | 6.35 | 0.0177 | M ≠ L | |
| C:N ratio | - | - | - | - | 15.5 | 2.2 | 12.0 | 17.7 | 18.2 | 4.3 | 13.7 | 27.0 | 4.25 | 0.0488 | M ≠ L | |
| Metarhizium spp. | M. brunneum | M. robertsii | M. guizhouense | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Land-Use Type | N | MLG | N | MLG | H | E.5 | N | MLG | H | E.5 | N | MLG | H | E.5 |
| Arable land | 115 | 10 | 66 | 4 | 0.99 | 0.75 | 46 | 5 | 1.09 | 0.60 | 3 | 1 | - | - |
| Grassland | 171 | 12 | 130 | 7 | 1.33 | 0.75 | 34 | 3 | 0.70 | 0.74 | 7 | 2 | - | - |
| Forest | 63 | 4 | 63 | 4 | 0.96 | 0.80 | - | - | - | - | - | - | ||
| Total | 349 | 22 | 259 | 13 | 1.84 | 0.72 | 80 | 6 | 1.17 | 0.74 | 10 | 3 | - | - |
| M. brunneum | M. robertsii | |||||||
|---|---|---|---|---|---|---|---|---|
| Among 3 LUTs | Grassland– Arable Land | Arable Land | Grassland | Forest | Between 2 LUTs | Arable Land | Grassland | |
| Variable | p-Value (R2) | p-Value (R2) | p-Value (R2) | p-Value (R2) | p-Value (R2) | p-Value (R2) | p-Value (R2) | p-Value (R2) |
| Land-use type | 0.0001 (37%) | 0.0513 | - | - | - | 0.4665 | - | - |
| C:N ratio | 0.0001 (25%) | 0.1149 | 0.9821 | 0.0074 (26%) | 0.2083 | 0.5366 | 0.4599 | 0.8333 |
| Basal respiration | 0.0071 (13%) | 0.0928 | 0.3016 | 0.7186 | 0.1667 | 0.1327 | 0.4595 | 0.6668 |
| Organic Carbon | 0.0039 (12%) | 0.0861 | 0.5563 | 0.6421 | 0.2083 | 0.2562 | 0.6476 | 0.8333 |
| Total Carbon | 0.0055 (11%) | 0.1657 | 0.5891 | 0.6488 | 0.2083 | 0.1403 | 0.3230 | 0.8333 |
| Bulk density | 0.0055 (13%) | 0.2375 | 0.5190 | 0.0893 | 0.2500 | 0.1333 | 0.6103 | 0.1667 |
| Soil skeleton | 0.5821 | 0.4503 | 0.8032 | 0.5633 | 0.8333 | 0.0098 (33%) | 0.3008 | 0.3333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Bravo, M.; Gschwend, F.; Mayerhofer, J.; Hug, A.; Widmer, F.; Enkerli, J. Land-Use Type Drives Soil Population Structures of the Entomopathogenic Fungal Genus Metarhizium. Microorganisms 2021, 9, 1380. https://doi.org/10.3390/microorganisms9071380
Fernández-Bravo M, Gschwend F, Mayerhofer J, Hug A, Widmer F, Enkerli J. Land-Use Type Drives Soil Population Structures of the Entomopathogenic Fungal Genus Metarhizium. Microorganisms. 2021; 9(7):1380. https://doi.org/10.3390/microorganisms9071380
Chicago/Turabian StyleFernández-Bravo, María, Florian Gschwend, Johanna Mayerhofer, Anna Hug, Franco Widmer, and Jürg Enkerli. 2021. "Land-Use Type Drives Soil Population Structures of the Entomopathogenic Fungal Genus Metarhizium" Microorganisms 9, no. 7: 1380. https://doi.org/10.3390/microorganisms9071380
APA StyleFernández-Bravo, M., Gschwend, F., Mayerhofer, J., Hug, A., Widmer, F., & Enkerli, J. (2021). Land-Use Type Drives Soil Population Structures of the Entomopathogenic Fungal Genus Metarhizium. Microorganisms, 9(7), 1380. https://doi.org/10.3390/microorganisms9071380






