New Variants of Squash Mosaic Viruses Detected in Human Fecal Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Specimen Collection
2.2. Viral Metagenomics
2.3. Genetic Analysis
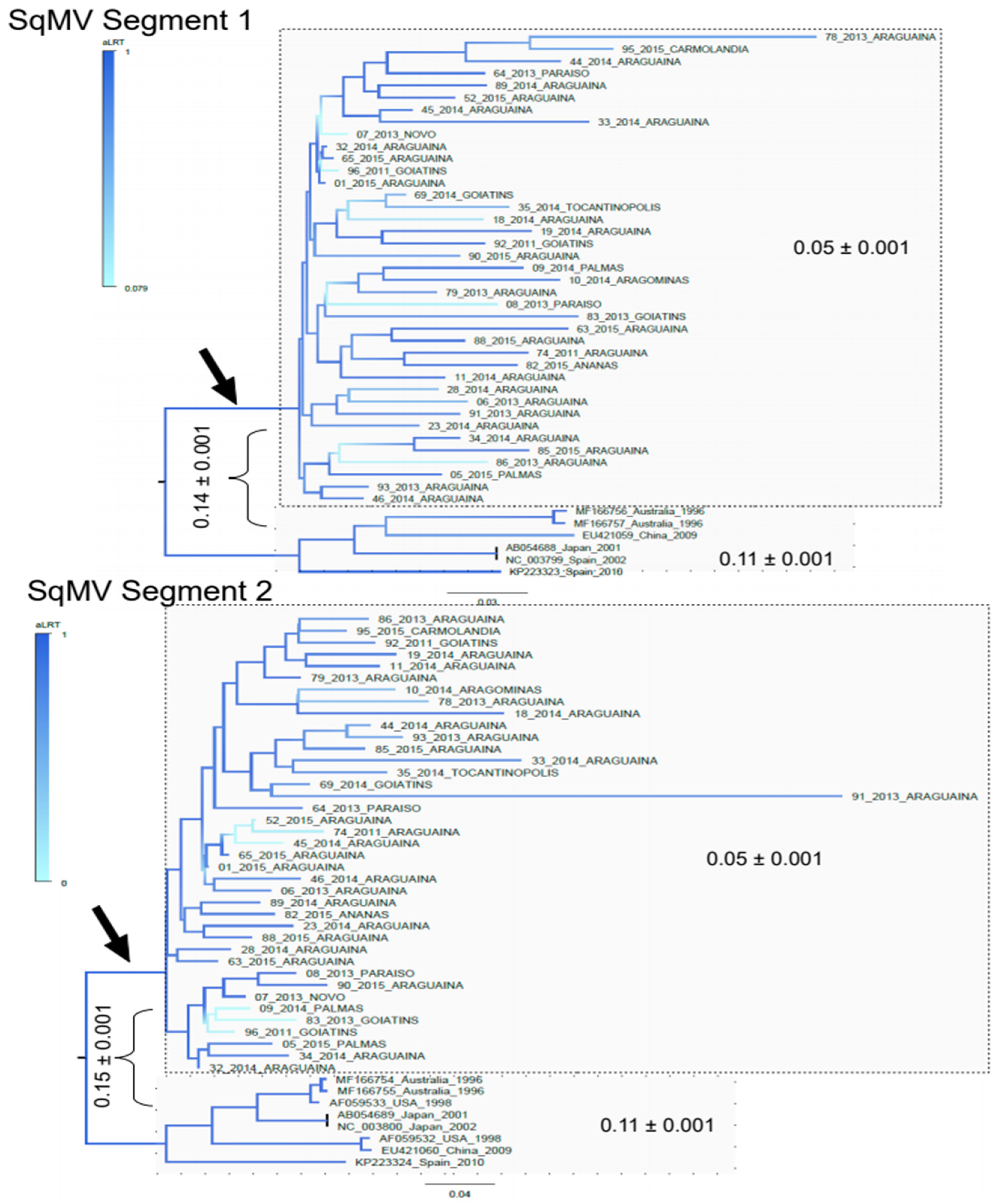
3. Results
3.1. Viral Diversity
3.2. Phylogenetic Analysis
3.3. Genetic Distances and Similarities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- da Silva, F.R.; Lima, J.A.A.; do Nascimento, A.K.Q.; Barbosa, G.S. Biological and Serological of an Isolate of Squash Mosaic Virus and Effects of Mixed Infection with a Virus of the Genus Potyvirus. Rev. Ciência Agronômica 2016, 47, 195. [Google Scholar] [CrossRef]
- Lecoq, H.; Desbiez, C. Viruses of Cucurbit Crops in the Mediterranean Region: An Ever-Changing Picture. Adv. Virus Res. 2012, 84, 67–126. [Google Scholar]
- Thompson, J.; Dasgupta, I.; Fuchs, M.; Iwanami, T.; Karasev, A.V.; Petrzik, K.; Sanfaçon, H.; Tzanetakis, I.; Van Der Vlugt, R.; Wetzel, T.; et al. ICTV Virus Taxonomy Profile: Secoviridae. J. Gen. Virol. 2017, 98, 529–531. [Google Scholar] [CrossRef]
- Carette, J.E.; Verver, J.; Martens, J.; Van Kampen, T.; Wellink, J.; Van Kammen, A. Characterization of plant proteins that interact with cowpea mosaic virus ‘60K’ protein in the yeast two-hybrid system. J. Gen. Virol. 2002, 83, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Mlotshwa, S.; Verver, J.; Sithole-Niang, I.; Gopinath, K.; Carette, J.; Van Kammen, A.; Wellink, J. Subcellular location of the helper component-proteinase of Cowpea aphid-borne mosaic virus. Virus Genes 2002, 25, 207–216. [Google Scholar] [CrossRef]
- Pouwels, J.; Carette, J.E.; Van Lent, J.; Wellink, J. Cowpea mosaic virus: Effects on host cell processes. Mol. Plant. Pathol. 2002, 3, 411–418. [Google Scholar] [CrossRef]
- Carette, J.; Guhl, K.; Wellink, J.; Van Kammen, A. Coalescence of the Sites of Cowpea Mosaic Virus RNA Replication into a Cytopathic Structure. J. Virol. 2002, 76, 6235–6243. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.; Van Lent, J.; Macfarlane, S.A.; Wellink, J.; Van Kammen, A. Cowpea Mosaic Virus 32- and 60-Kilodalton Replication Proteins Target and Change the Morphology of Endoplasmic Reticulum Membranes. J. Virol. 2002, 76, 6293–6301. [Google Scholar] [CrossRef][Green Version]
- Ling, K.-S.; Wechter, W.P.; Walcott, R.R.; Keinath, A.P. Development of a Real-time RT-PCR Assay for Squash Mosaic Virus Useful for Broad Spectrum Detection of Various Serotypes and its Incorporation into a Multiplex Seed Health Assay. J. Phytopathol. 2011, 159, 649–656. [Google Scholar] [CrossRef]
- Moura, M.C.C.L.; Lima, J.A.A.; Oliveira, V.B.; Gonçalves, M.F.B. Serological Identification of Virus Species Infecting Cucurbits in Producing Areas of the State of Maranhão, Brazil. Fitopatol. Bras. 2001, 26, 90–92. [Google Scholar] [CrossRef]
- Alencar, N.E.; Figueira, A.R.; Almeida, J.E.M.; Lucas, M.A.; Santos, L.B.; Nascimento, I.R. Molecular Biological Identification of Detected Viruses in Cucurbit Species from the State of Tocantins. J. Biotechnol. Biodivers. 2012, 3, 32–37. [Google Scholar]
- Balique, F.; Lecoq, H.; Raoult, D.; Colson, P. Can Plant Viruses Cross the Kingdom Border and Be Pathogenic to Humans? Viruses 2015, 7, 2074–2098. [Google Scholar] [CrossRef]
- Dolja, V.V.; Koonin, E.V. Common origins and host-dependent diversity of plant and animal viromes. Curr. Opin. Virol. 2011, 1, 322–331. [Google Scholar] [CrossRef]
- Bengone-Abogourin, J.G.; Chelkha, N.; Verdin, E.; Colson, P. Sequence Similarities between Viroids and Human Micrornas. Intervirology 2019, 62, 227–234. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, S.; Park, Y.; Kim, S.; Ryu, C. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]
- Rebolledo-Mendez, J.D.; Vaishnav, R.A.; Cooper, N.G.; Friedland, R.P. Cross-kingdom sequence similarities between human micro-RNAs and plant viruses. Commun. Integr. Biol. 2013, 6, e24951. [Google Scholar] [CrossRef]
- Da Costa, A.C.; Thézé, J.; Komninakis, S.; Duro, R.L.S.; Felinto, M.R.L.; Moura, L.C.C.; Barroso, I.M.D.O.; Santos, L.E.C.; Nunes, M.A.D.L.; Moura, A.A.; et al. Spread of Chikungunya Virus East/Central/South African Genotype in Northeast Brazil. Emerg. Infect. Dis. 2017, 23, 1742–1744. [Google Scholar] [CrossRef]
- Deng, X.; Naccache, S.N.; Ng, T.; Federman, S.; Li, L.; Chiu, C.; Delwart, E.L. An ensemble strategy that significantly improves de novo assembly of microbial genomes from metagenomic next-generation sequencing data. Nucleic Acids Res. 2015, 43, e46. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; The UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. Mafft: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Cilli, A.; Luchs, A.; Leal, E.; Gill, D.; Milagres, F.A.D.P.; Komninakis, S.V.; Brustulin, R.; Teles, M.D.A.R.; Lobato, M.C.A.B.S.; Das Chagas, R.T.; et al. Human sapovirus GI.2 and GI.3 from children with acute gastroenteritis in northern Brazil. Memórias do Instituto Oswaldo Cruz 2019, 114, e180574. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.C.; Leal, E.; Gill, D.; Milagres, F.A.D.P.; Komninakis, S.V.; Brustulin, R.; Teles, M.D.A.R.; Lobato, M.C.A.B.S.; Das Chagas, R.T.; Abrão, M.D.F.N.D.S.; et al. Discovery of Cucumis melo endornavirus by deep sequencing of human stool samples in Brazil. Virus Genes 2019, 55, 332–338. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.C.; Luchs, A.; Milagres, F.A.P.; Komninakis, S.V.; Gill, D.E.; Lobato, M.; Brustulin, R.; Chagas, R.T.d.; Abrao, M.; Soares, C.; et al. Recombination Located over 2a-2b Junction Ribosome Frameshifting Region of Saffold Cardiovirus. Viruses 2018, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.C.; Luchs, A.; Milagres, F.A.P.; Komninakis, S.V.; Gill, D.E.; Lobato, M.; Brustulin, R.; Chagas, R.T.d.; Abrao, M.; Soares, C.; et al. Near Full Length Genome of a Recombinant (E/D) Cosavirus Strain from a Rural Area in the Central Region of Brazil. Sci. Rep. 2018, 8, 12304. [Google Scholar] [CrossRef]
- Ribeiro, G.D.O.; Luchs, A.; Milagres, F.A.D.P.; Komninakis, S.V.; Gill, D.E.; Lobato, M.C.A.B.S.; Brustulin, R.; Das Chagas, R.T.; Abrão, M.D.F.N.D.S.; Soares, C.V.D.D.A.; et al. Detection and Characterization of Enterovirus B73 from a Child in Brazil. Viruses 2018, 11, 16. [Google Scholar] [CrossRef]
- Tahmasebi, R.; Costa, A.C.D.; Tardy, K.; Tinker, R.J.; Milagres, F.A.P.; Brustulin, R.; Teles, M.; Chagas, R.T.D.; Soares, C.; Watanabe, A.S.A.; et al. Genomic Analyses of Potential Novel Recombinant Human Adenovirus C in Brazil. Viruses 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, P.C.; Ochman, H. Resurrection of a Global, Metagenomically Defined Gokushovirus. eLife 2020, 9, e51599. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Rakibuzzaman, A.; Ramamoorthy, S. Torque teno viruses in health and disease. Virus Res. 2020, 285, 198013. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística (IBGE); Pesquisa Nacional De Saneamento Básico. Abastecimento De Água E Esgotamento Sanitário, Coordenação De População E Indicadores Sociais; Governo Federal do Brasil: Rio de Janeiro, Brazil, 2020. [Google Scholar]
| Sequence Name | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) 96_2011_GOIATINS | 2.20% | 1.10% | 1.50% | 5.29% | 5.29% | 5.40% | 5.40% | 6.55% | 6.55% | |
| (2) 65_2015_ARAGUAINA | 1.14% | 1.10% | 0.90% | 4.36% | 4.36% | 4.46% | 4.46% | 5.71% | 5.61% | |
| (3) 01_2015_ARAGUAINA | 0.98% | 1.08% | 0.60% | 4.46% | 4.46% | 4.56% | 4.56% | 5.71% | 5.71% | |
| (4) 32_2014_ARAGUAINA | 0.81% | 0.76% | 0.92% | 3.94% | 3.94% | 4.05% | 4.05% | 5.19% | 5.19% | |
| (5) MF166754_Australia_1996 | 4.98% | 6.12% | 5.89% | 5.72% | 0.00% | 1.40% | 1.40% | 3.74% | 2.81% | |
| (6) MF166755_Australia_1996 | 5.09% | 6.23% | 6.00% | 5.83% | 0.11% | 1.40% | 1.40% | 3.74% | 2.81% | |
| (7) AB054689_Japan_2001 | 4.47% | 5.54% | 5.32% | 5.15% | 2.40% | 2.51% | 0.00% | 3.43% | 2.81% | |
| (8) NC_003800_Japan_2002 | 4.47% | 5.54% | 5.32% | 5.15% | 2.40% | 2.51% | 0.00% | 3.43% | 2.81% | |
| (9) EU421060_China_2009 | 5.32% | 6.46% | 6.23% | 6.06% | 3.51% | 3.62% | 3.35% | 3.35% | 4.67% | |
| (10) KP223324_Spain_2010 | 4.81% | 5.94% | 5.72% | 5.54% | 2.40% | 2.51% | 1.91% | 1.91% | 3.68% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanova, F.; Marcatti, R.; Bertanhe, M.; Morais, V.d.S.; Milagres, F.A.d.P.; Brustulin, R.; Araújo, E.L.L.; Tahmasebi, R.; Witkin, S.S.; Deng, X.; et al. New Variants of Squash Mosaic Viruses Detected in Human Fecal Samples. Microorganisms 2021, 9, 1349. https://doi.org/10.3390/microorganisms9071349
Villanova F, Marcatti R, Bertanhe M, Morais VdS, Milagres FAdP, Brustulin R, Araújo ELL, Tahmasebi R, Witkin SS, Deng X, et al. New Variants of Squash Mosaic Viruses Detected in Human Fecal Samples. Microorganisms. 2021; 9(7):1349. https://doi.org/10.3390/microorganisms9071349
Chicago/Turabian StyleVillanova, Fabiola, Roberta Marcatti, Mayara Bertanhe, Vanessa dos Santos Morais, Flavio Augusto de Padua Milagres, Rafael Brustulin, Emerson Luiz Lima Araújo, Roozbeh Tahmasebi, Steven S. Witkin, Xutao Deng, and et al. 2021. "New Variants of Squash Mosaic Viruses Detected in Human Fecal Samples" Microorganisms 9, no. 7: 1349. https://doi.org/10.3390/microorganisms9071349
APA StyleVillanova, F., Marcatti, R., Bertanhe, M., Morais, V. d. S., Milagres, F. A. d. P., Brustulin, R., Araújo, E. L. L., Tahmasebi, R., Witkin, S. S., Deng, X., Delwart, E., Sabino, E. C., Abreu-Junior, C. H., Leal, É., & Costa, A. C. d. (2021). New Variants of Squash Mosaic Viruses Detected in Human Fecal Samples. Microorganisms, 9(7), 1349. https://doi.org/10.3390/microorganisms9071349








