Impact of Cellulose-Rich Organic Soil Amendments on Growth Dynamics and Pathogenicity of Rhizoctonia solani
Abstract
1. Introduction
2. Materials and Methods
2.1. RsP Experiment: Preparation of Wood Sawdust and Paper Pulp
2.2. RsP Experiment: Assay of Pathogen Performance on Woody Substrates
2.3. RsP Experiment: Measurement of Growth Performance of R. solani
2.4. WT Experiment: Sampling of Soil and Preparation of Materials
2.5. WT Experiment: Bioassay with Wood Sawdust Types and Paper Pulp
2.6. WT Experiment: Determination of Germination Percentages and Plant Disease Incidence
2.7. WT Experiment: Sampling of Soil and Plants
2.8. ToS Experiment: Bioassay with Varying Pre-Incubation Times of Soil Amendments
2.9. ToS Experiment: Sampling of Soil and Plants
2.10. RsP, WT, and ToS Experiments: Fungal Biomass
2.11. ToS Experiment: DNA Extraction, qPCR of R. solani and Fungi, and Sequencing of Fungal and Bacterial Communities
2.12. Bioinformatic and Statistical Analyses
3. Results
3.1. RsP Experiment
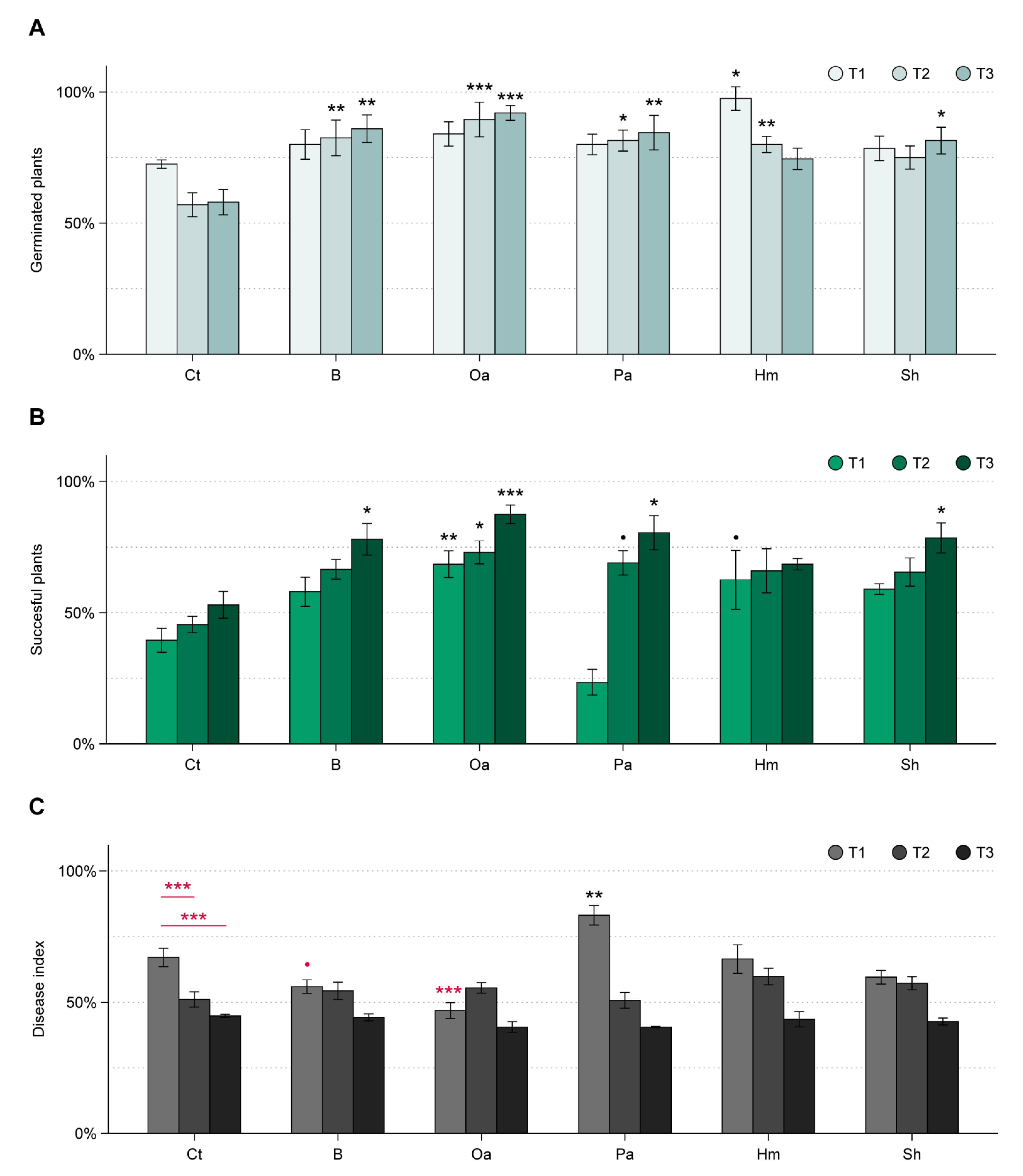
3.2. WT Experiment
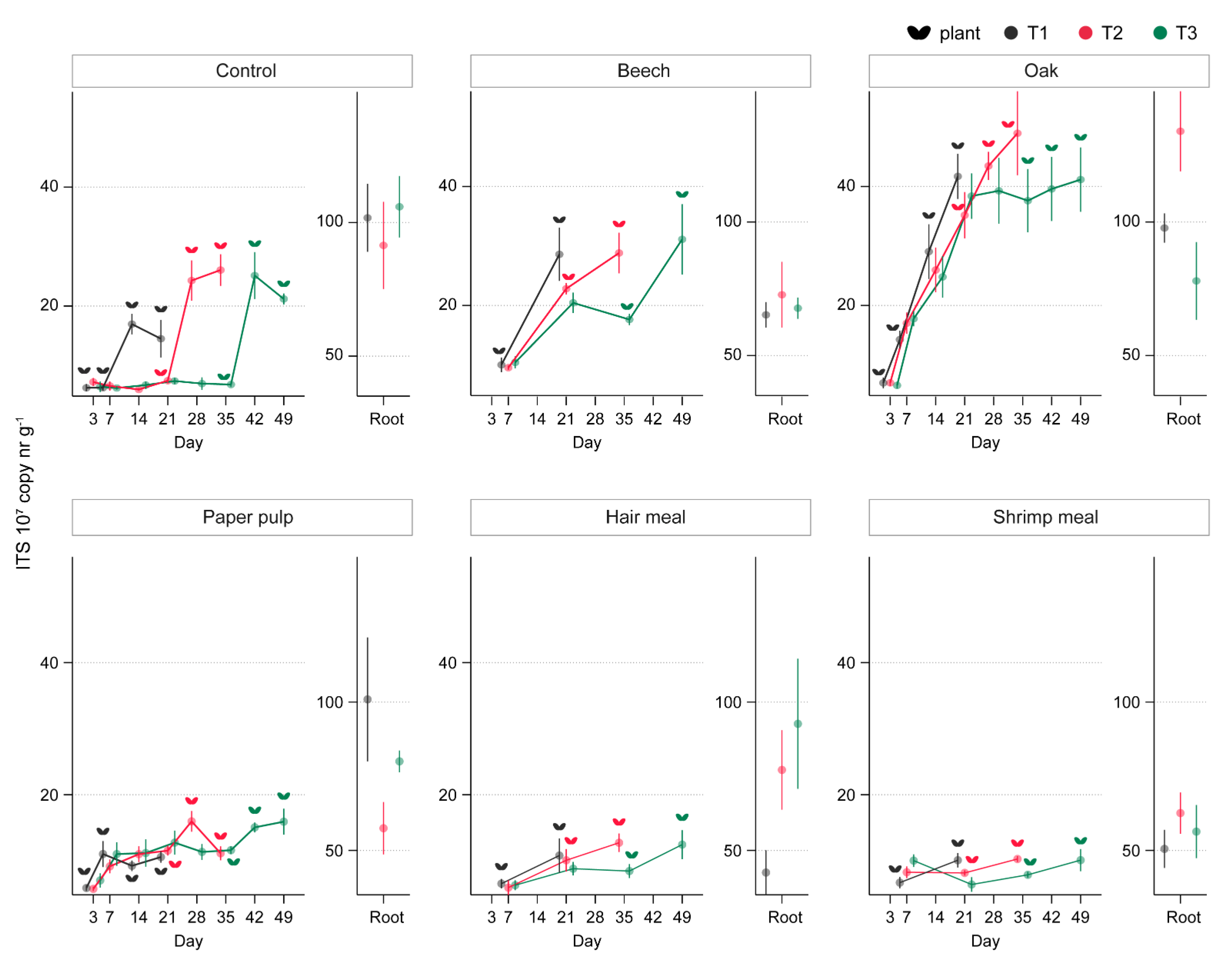
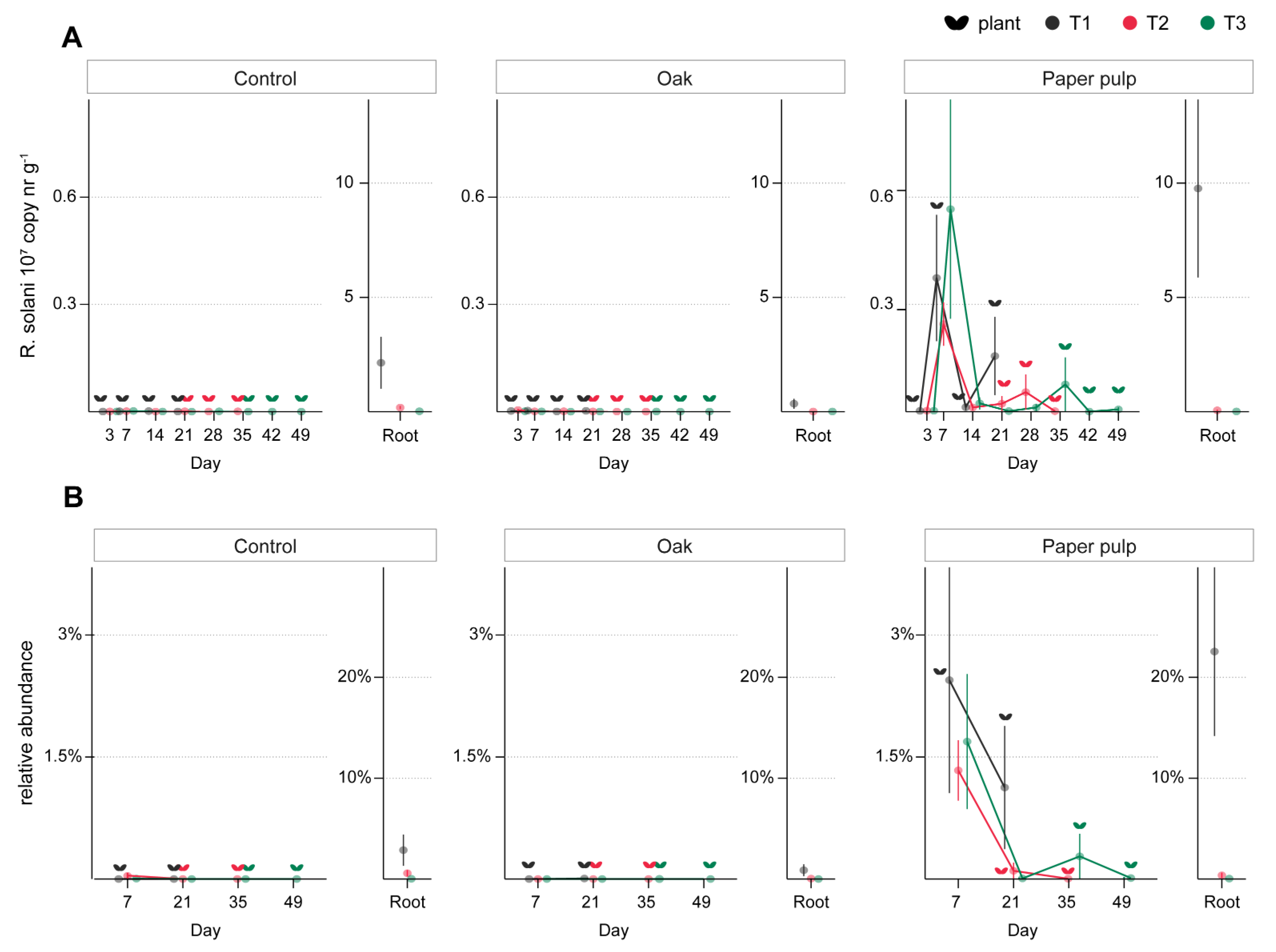
3.3. ToS Experiment
3.3.1. Effect of Organic Amendments on Plants, Total Fungal Biomass, and R. solani
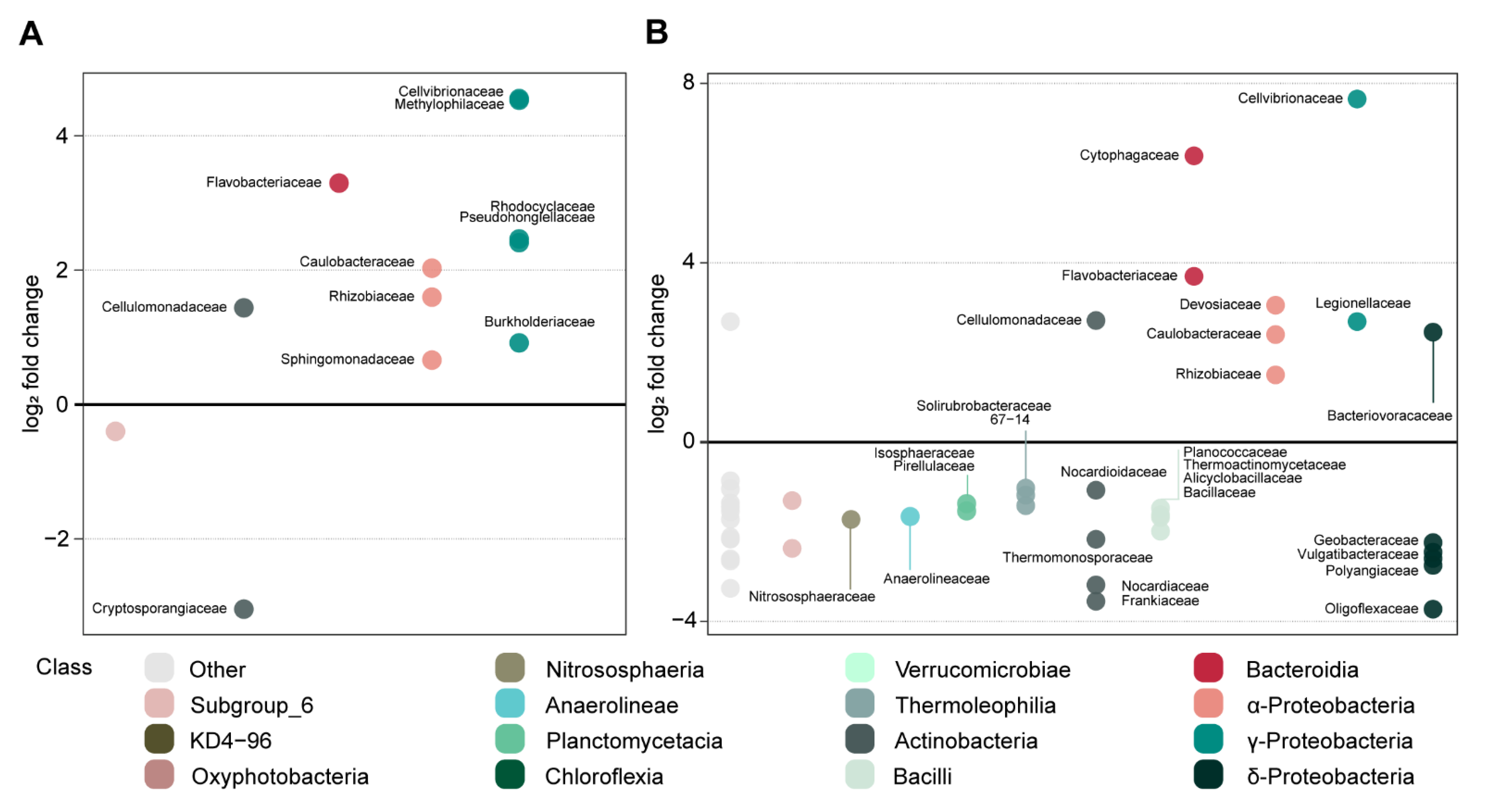
3.3.2. Effect of Oak Sawdust and Paper Pulp on Fungal and Bacterial Communities
4. Discussion
4.1. Growth of R. solani on Woody Substrates and Paper Pulp
4.2. Effect of Wood Sawdusts and Paper Pulp on Fungal Biomass and Beetroot Seedling Performance in R. solani-Infected Soil
4.3. Impact of Timing of Organic Amendments and Sowing on R. solani Population and Disease Dynamics, and on Fungal and Bacterial Communities
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.-H.; Sarthou, J.-P.; Cellier, V.; Messéan, A.; Aubertot, J.-N. Integrated Management of Damping-off Diseases. A Review. Agron. Sustain. Dev. 2017, 37. [Google Scholar] [CrossRef]
- Grimmer, M.K.; van den Bosch, F.; Powers, S.J.; Paveley, N.D. Fungicide Resistance Risk Assessment Based on Traits Associated with the Rate of Pathogen Evolution. Pest Manag. Sci. 2015, 71, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the Soil Microbiome to Increase Soil Health and Plant Fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Arif, I.; Batool, M.; Schenk, P.M. Plant Microbiome Engineering: Expected Benefits for Improved Crop Growth and Resilience. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil Health through Soil Disease Suppression: Which Strategy from Descriptors to Indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The Role of Organic Amendments in Soil Reclamation: A Review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Baker, K.; Cook, R.J. Biological Control of Plant Pathogens; W.H. Freeman and Company: San Francisco, CA, USA, 1974; ISBN 0-7167-0589-3. [Google Scholar]
- Lockwood, J.L. Fungistasis in Soils. Biol. Rev. 1977, 52, 1–43. [Google Scholar] [CrossRef]
- Garbeva, P.; Hol, W.H.G.; Termorshuizen, A.J.; Kowalchuk, G.A.; de Boer, W. Fungistasis and General Soil Biostasis—A New Synthesis. Soil Biol. Biochem. 2011, 43, 469–477. [Google Scholar] [CrossRef]
- Watson, A.G.; Ford, E.J. Soil Fungistasis—A Reappraisal. Annu. Rev. Phytopathol. 1972, 10, 327–346. [Google Scholar] [CrossRef]
- Kepler, R.M.; Maul, J.E.; Rehner, S.A. Managing the Plant Microbiome for Biocontrol Fungi: Examples from Hypocreales. Curr. Opin. Microbiol. 2017, 37, 48–53. [Google Scholar] [CrossRef]
- De Boer, W.; Wagenaar, A.-M.; Klein Gunnewiek, P.J.A.; van Veen, J.A. In Vitro Suppression of Fungi Caused by Combinations of Apparently Non-Antagonistic Soil Bacteria. FEMS Microbiol. Ecol. 2007, 59, 177–185. [Google Scholar] [CrossRef]
- Cornforth, D.; Foster, K. Competition Sensing: The Social Side of Bacterial Stress Responses. Nat. Rev. Microbiol. 2013, 11. [Google Scholar] [CrossRef]
- De Boer, W.; Hundscheid, M.P.J.; Klein Gunnewiek, P.J.A.; de Ridder-Duine, A.S.; Thion, C.; van Veen, J.A.; van der Wal, A. Antifungal Rhizosphere Bacteria Can Increase as Response to the Presence of Saprotrophic Fungi. PLoS ONE 2015, 10, e0137988. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial–Fungal Interactions: Ecology, Mechanisms and Challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef]
- De Vries, F.T.; Bardgett, R.D. Plant–Microbial Linkages and Ecosystem Nitrogen Retention: Lessons for Sustainable Agriculture. Front. Ecol. Environ. 2012, 10, 425–432. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W.; et al. Soil Networks Become More Connected and Take up More Carbon as Nature Restoration Progresses. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Clocchiatti, A.; Hannula, S.E.; van den Berg, M.; Korthals, G.; de Boer, W. The Hidden Potential of Saprotrophic Fungi in Arable Soil: Patterns of Short-Term Stimulation by Organic Amendments. Appl. Soil Ecol. 2020, 147, 103434. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the Characteristics of Organic Soil Amendments That Suppress Soilborne Plant Diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Termorshuizen, A.J.; van Rijn, E.; van der Gaag, D.J.; Alabouvette, C.; Chen, Y.; Lagerlöf, J.; Malandrakis, A.A.; Paplomatas, E.J.; Rämert, B.; Ryckeboer, J.; et al. Suppressiveness of 18 Composts against 7 Pathosystems: Variability in Pathogen Response. Soil Biol. Biochem. 2006, 38, 2461–2477. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Di Silverio, N.; Carrino, L.; Cesarano, G.; Assaeed, A.M.; Abd-ElGawad, A.M. Decomposition and Organic Amendments Chemistry Explain Contrasting Effects on Plant Growth Promotion and Suppression of Rhizoctonia solani Damping Off. PLoS ONE 2020, 15, e0230925. [Google Scholar] [CrossRef] [PubMed]
- Hoitink, H.; Boehm, M. Biocontrol within the Context of Soil Microbial Communities: A Substrate-Dependent Phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef]
- Termorshuizen, A.J.; Jeger, M.J. Strategies of Soilborne Plant Pathogenic Fungi in Relation to Disease Suppression. Fungal Ecol. 2008, 1, 108–114. [Google Scholar] [CrossRef]
- Croteau, G.A.; Zibilske, L.M. Influence of Papermill Processing Residuals on Saprophytic Growth and Disease Caused by Rhizoctonia solani. Appl. Soil Ecol. 1998, 10, 103–115. [Google Scholar] [CrossRef]
- Chapelle, E.; Mendes, R.; Bakker, P.A.H.; Raaijmakers, J.M. Fungal Invasion of the Rhizosphere Microbiome. ISME J. 2016, 10, 265–268. [Google Scholar] [CrossRef]
- Gonzalez Garcia, V.; Portal Onco, M.A.; Rubio Susan, V. Review. Biology and Systematics of the Form Genus Rhizoctonia. Span. J. Agric. Res. 2006, 4, 55. [Google Scholar] [CrossRef]
- Henis, Y.; Sneh, B.; Katan, J. Effect of Organic Amendments on Rhizoctonia and Accompanying Microflora in Soil Accompanyng Microflora in Soil. Can. J. Microbiol. 1967, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, D.K.; Roberts, D.P.; Garrett, W.M.; Natarajan, S.S.; Darwish, O.; Alkharouf, N.; Pain, A.; Khan, F.; Jambhulkar, P.P.; Mitra, A. Proteomic Investigation of Rhizoctonia solani AG 4 Identifies Secretome and Mycelial Proteins with Roles in Plant Cell Wall Degradation and Virulence. J. Agric. Food Chem. 2016, 64, 3101–3110. [Google Scholar] [CrossRef]
- Wibberg, D.; Andersson, L.; Tzelepis, G.; Rupp, O.; Blom, J.; Jelonek, L.; Pühler, A.; Fogelqvist, J.; Varrelmann, M.; Schlüter, A.; et al. Genome Analysis of the Sugar Beet Pathogen Rhizoctonia solani AG2-2IIIB Revealed High Numbers in Secreted Proteins and Cell Wall Degrading Enzymes. BMC Genom. 2016, 17, 245. [Google Scholar] [CrossRef]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and How to Kill a Plant Cell: Infection Strategies of Plant Pathogenic Fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef]
- Scheuerell, S.J.; Sullivan, D.M.; Mahaffee, W.F. Suppression of Seedling Damping-off Caused by Pythium ultimum, P. irregulare, and Rhizoctonia solani in Container Media Amended with a Diverse Range of Pacific Northwest Compost Sources. Phytopathology 2005, 95, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.; Schilder, M.T. Enhancement of Soil Suppressiveness against Rhizoctonia solani in Sugar Beet by Organic Amendments. Appl. Soil Ecol. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Parmeter, J.R. Rhizoctonia Solani, Biology and Pathology; University of California Press: Berkeley, CA, USA, 1970; ISBN 978-0-520-01497-8. [Google Scholar]
- Engelkes, C.A.; Windels, C.E. Susceptibility of Sugar Beet and Beans to Rhizoctonia solani AG-2-2 IIIB and AG-2-2 IV. Am. Phytopathol. Soc. 1996, 80, 1413–1417. [Google Scholar]
- Chiang, K.S.; Liu, H.I.; Bock, C.H. A Discussion on Disease Severity Index Values. Part I: Warning on Inherent Errors and Suggestions to Maximise Accuracy. Ann. Appl. Biol. 2017, 171, 139–154. [Google Scholar] [CrossRef]
- Dubey, S.C.; Tripathi, A.; Upadhyay, B.K.; Kumar, A. Development of Conventional and Real Time PCR Assay for Detection and Quantification of Rhizoctonia solani Infecting Pulse Crops. Biologia 2016, 71, 133–138. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New Primers to Amplify the Fungal ITS2 Region—Evaluation by 454-Sequencing of Artificial and Natural Communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Rivers, A.; Weber, K.; Gardner, T.; Liu, S.; Armstrong, S. ITSxpress: Software to Rapidly Trim Internally Transcribed Spacer Sequences with Quality Scores for Marker Gene Analysis. F1000Research 2018, 7, 1418. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE Database for Molecular Identification of Fungi—Recent Updates and Future Perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and Microbial Differential Abundance Strategies Depend upon Data Characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef]
- Eriksson, K.-E.L. Biotechnology in the Pulp and Paper Industry. Wood Sci. Technol. 1990, 24, 79–101. [Google Scholar] [CrossRef]
- Bora, P.; Hardy, G.E.S.J.; O’Brien, P.A. Laccase Activity and Maceration of Lupin Tissue by Rhizoctonia solani Is Inhibited by Arginine. Australas. Plant Pathol. 2005, 34, 591. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen Additions and Litter Decomposition: A Meta-Analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen Additions and Microbial Biomass: A Meta-Analysis of Ecosystem Studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K.; Cornelissen, J.H.C.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.M.; Scarff, F.; Weedon, J.T.; Wirth, C.; Zanne, A.E. Plant Traits and Wood Fates across the Globe: Rotted, Burned, or Consumed? Glob. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Cesarino, I.; Araújo, P.; Domingues Júnior, A.P.; Mazzafera, P. An Overview of Lignin Metabolism and Its Effect on Biomass Recalcitrance. Braz. J. Bot. 2012, 35, 303–311. [Google Scholar] [CrossRef]
- Hart, J.H. The Role of Wood Exudates and Extractives in Protecting Wood from Decay. In Natural Products of Woody Plants: Chemicals Extraneous to the Lignocellulosic Cell Wall; Rowe, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 861–880. ISBN 978-3-642-74075-6. [Google Scholar]
- Grayer, R.J.; Harborne, J.B. A Survey of Antifungal Compounds from Higher Plants, 1982–1993. Phytochemistry 1994, 37, 19–42. [Google Scholar] [CrossRef]
- Valette, N.; Perrot, T.; Sormani, R.; Gelhaye, E.; Morel-Rouhier, M. Antifungal Activities of Wood Extractives. Fungal Biol. Rev. 2017, 31, 113–123. [Google Scholar] [CrossRef]
- Hosseini Hashemi, S.K.; Latibari, A. Evaluation and Identification of Walnut Heartwood Extractives for Protection of Poplar Wood. BioResources 2011, 6, 59–69. [Google Scholar] [CrossRef]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.C.; Zanne, A.E.; Wirth, C.; Coomes, D.A. Global Meta-Analysis of Wood Decomposition Rates: A Role for Trait Variation among Tree Species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef]
- Rowe, J.W.; Conner, A.H. Extractives in Eastern Hardwoods—A Review; Gen. Tech. Rep. Fpl-18; U.S. Department of Agriculture: Madison, WI, USA, 1979; p. 72.
- Bonanomi, G.; Cesarano, G.; Lombardi, N.; Motti, R.; Scala, F.; Mazzoleni, S.; Incerti, G. Litter Chemistry Explains Contrasting Feeding Preferences of Bacteria, Fungi, and Higher Plants. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.E. Preformed Antimicrobial Compounds and Plant Defense against Fungal Attack. Plant Cell 1996, 8, 1821–1831. [Google Scholar] [CrossRef]
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Scalbert, A. Tannins in Woods and Their Contribution to Microbial Decay Prevention. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 935–952. ISBN 978-1-4615-3476-1. [Google Scholar]
- Tomak, E.D.; Gonultas, O. The Wood Preservative Potentials of Valonia, Chestnut, Tara and Sulphited Oak Tannins. J. Wood Chem. Technol. 2018, 38, 183–197. [Google Scholar] [CrossRef]
- Broda, M. Natural Compounds for Wood Protection against Fungi-A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef]
- Pospelov, S.V.; Pospelova, A.D.; Onipko, V.V.; Semenko, M.V. Fungistatic Properties of Lectin-Containing Extracts of Medicinal Plants. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 91–105. ISBN 978-0-12-819304-4. [Google Scholar]
- De Menezes, A.B.; Richardson, A.E.; Thrall, P.H. Linking Fungal–Bacterial Co-Occurrences to Soil Ecosystem Function. Curr. Opin. Microbiol. 2017, 37, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.K.; Singh, B.; Sharma, O.P. Microbial Degradation of Tannins—A Current Perspective. Biodegradation 1998, 9, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.P.; Alva, A.; Boydston, R.A.; Cochran, R.L.; Hamm, P.B.; McGuire, A.; Riga, E. Soil Microbial, Fungal, and Nematode Responses to Soil Fumigation and Cover Crops under Potato Production. Biol. Fertil. Soils 2006, 42, 247–257. [Google Scholar] [CrossRef]
- Mutabaruka, R.; Hairiah, K.; Cadisch, G. Microbial Degradation of Hydrolysable and Condensed Tannin Polyphenol–Protein Complexes in Soils from Different Land-Use Histories. Soil Biol. Biochem. 2007, 39, 1479–1492. [Google Scholar] [CrossRef]
- Van Beneden, S.; Roobroeck, D.; França, S.C.; De Neve, S.; Boeckx, P.; Höfte, M. Microbial Populations Involved in the Suppression of Rhizoctonia solani AG1-1B by Lignin Incorporation in Soil. Soil Biol. Biochem. 2010, 42, 1268–1274. [Google Scholar] [CrossRef]
- Van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; de Boer, W. A Thready Affair: Linking Fungal Diversity and Community Dynamics to Terrestrial Decomposition Processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, S.A.; Kuske, C.R. Identification of Cellulose-Responsive Bacterial and Fungal Communities in Geographically and Edaphically Different Soils by Using Stable Isotope Probing. Appl. Environ. Microbiol. 2012, 78, 2316. [Google Scholar] [CrossRef]
- Koechli, C.; Campbell, A.N.; Pepe-Ranney, C.; Buckley, D.H. Assessing Fungal Contributions to Cellulose Degradation in Soil by Using High-Throughput Stable Isotope Probing. Soil Biol. Biochem. 2019, 130, 150–158. [Google Scholar] [CrossRef]
- McBride, M.J.; Liu, W.; Lu, X.; Zhu, Y.; Zhang, W. The Family Cytophagaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 577–593. ISBN 978-3-642-38953-5. [Google Scholar]
- DeBoy, R.T.; Mongodin, E.F.; Fouts, D.E.; Tailford, L.E.; Khouri, H.; Emerson, J.B.; Mohamoud, Y.; Watkins, K.; Henrissat, B.; Gilbert, H.J.; et al. Insights into Plant Cell Wall Degradation from the Genome Sequence of the Soil Bacterium Cellvibrio japonicus. J. Bacteriol. 2008, 190, 5455. [Google Scholar] [CrossRef] [PubMed]
- Schellenberger, S.; Kolb, S.; Drake, H.L. Metabolic Responses of Novel Cellulolytic and Saccharolytic Agricultural Soil Bacteria to Oxygen: Metabolic Response of Soil Cellulose Degraders. Environ. Microbiol. 2009, 12, 845–861. [Google Scholar] [CrossRef]
- Hoppe, B.; Krüger, D.; Kahl, T.; Arnstadt, T.; Buscot, F.; Bauhus, J.; Wubet, T. A Pyrosequencing Insight into Sprawling Bacterial Diversity and Community Dynamics in Decaying Deadwood Logs of Fagus sylvatica and Picea abies. Sci. Rep. 2015, 5, 9456. [Google Scholar] [CrossRef]
- Johnston, S.R.; Boddy, L.; Weightman, A.J. Bacteria in Decomposing Wood and Their Interactions with Wood-Decay Fungi. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Yin, C.; Vargas, J.; Schlatter, D.; Hagerty, C.; Hulbert, S.; Paulitz, T. Rhizosphere Community Selection Reveals Bacteria Associated with Reduced Root Disease. Microbiome 2020. [Google Scholar] [CrossRef]
- Gómez Expósito, R.; de Bruijn, I.; Postma, J.; Raaijmakers, J.M. Current Insights into the Role of Rhizosphere Bacteria in Disease Suppressive Soils. Front. Microbiol. 2017, 8, 2529. [Google Scholar] [CrossRef] [PubMed]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-Induced Activation of Disease-Suppressive Functions in the Endophytic Root Microbiome. Science 2019, 366, 606. [Google Scholar] [CrossRef]
- Sarrocco, S.; Guidi, L.; Fambrini, S.; Degl’Innocenti, E.; Vannacci, G. Competition for Cellulose Exploitation between Rhizoctonia solani and Two Trichoderma Isolates in the Decomposition of Wheat Straw. J. Plant Pathol. 2009, 9. [Google Scholar] [CrossRef]
- Anees, M.; Edel-Hermann, V.; Steinberg, C. Build up of Patches Caused by Rhizoctonia solani. Soil Biol. Biochem. 2010, 42, 1661–1672. [Google Scholar] [CrossRef]
- Debode, J.; De Tender, C.; Soltaninejad, S.; Van Malderghem, C.; Haegeman, A.; Van der Linden, I.; Cottyn, B.; Heyndrickx, M.; Maes, M. Chitin Mixed in Potting Soil Alters Lettuce Growth, the Survival of Zoonotic Bacteria on the Leaves and Associated Rhizosphere Microbiology. Front. Microbiol. 2016, 7, 565. [Google Scholar] [CrossRef]
- Andreo-Jimenez, B.; Schilder, M.T.; Nijhuis, E.H.; te Beest, D.E.; Bloem, J.; Visser, J.H.M.; van Os, G.; Brolsma, K.; de Boer, W.; Postma, J. Chitin- and Keratin-Rich Soil Amendments Suppress Rhizoctonia solani Disease via Changes to the Soil Microbial Community. Appl. Environ. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]

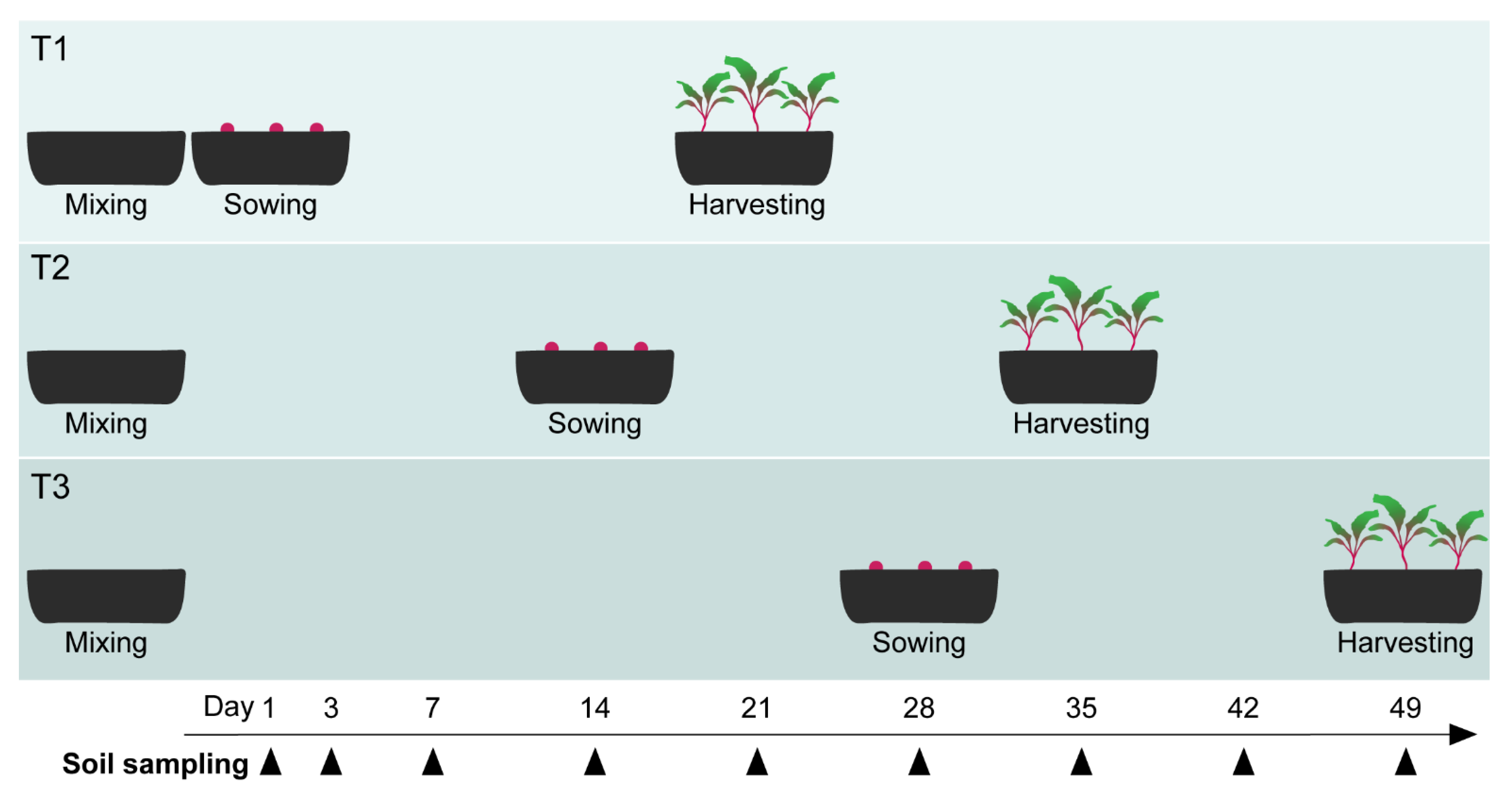
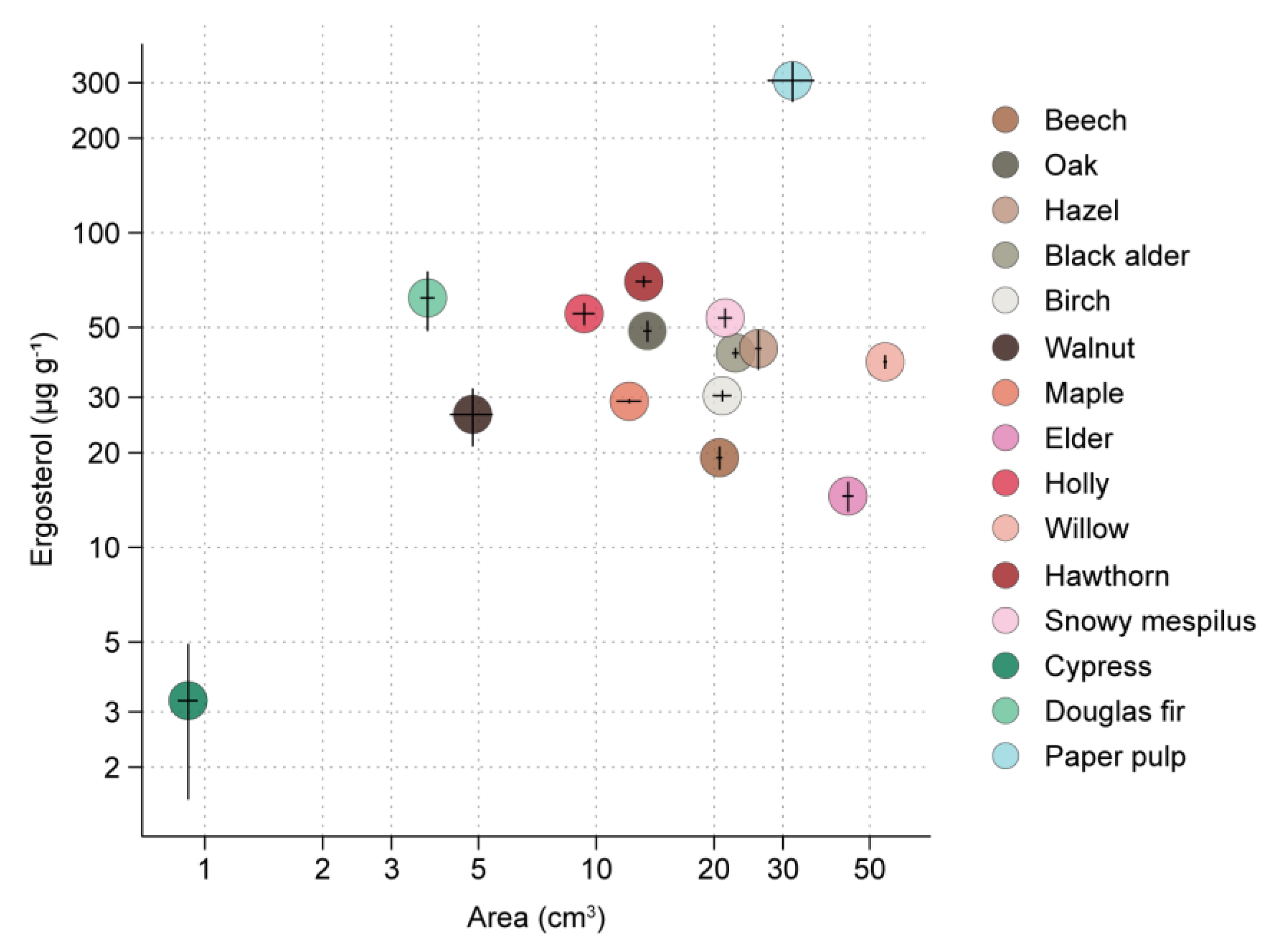
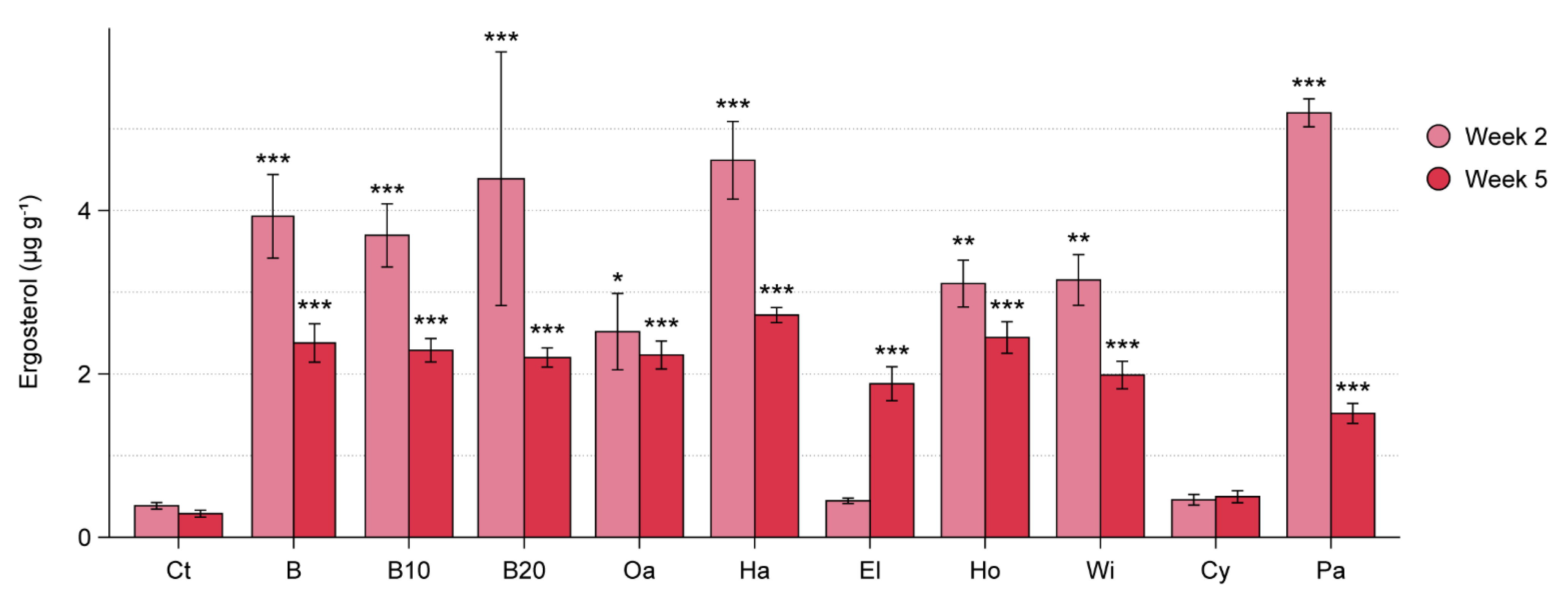
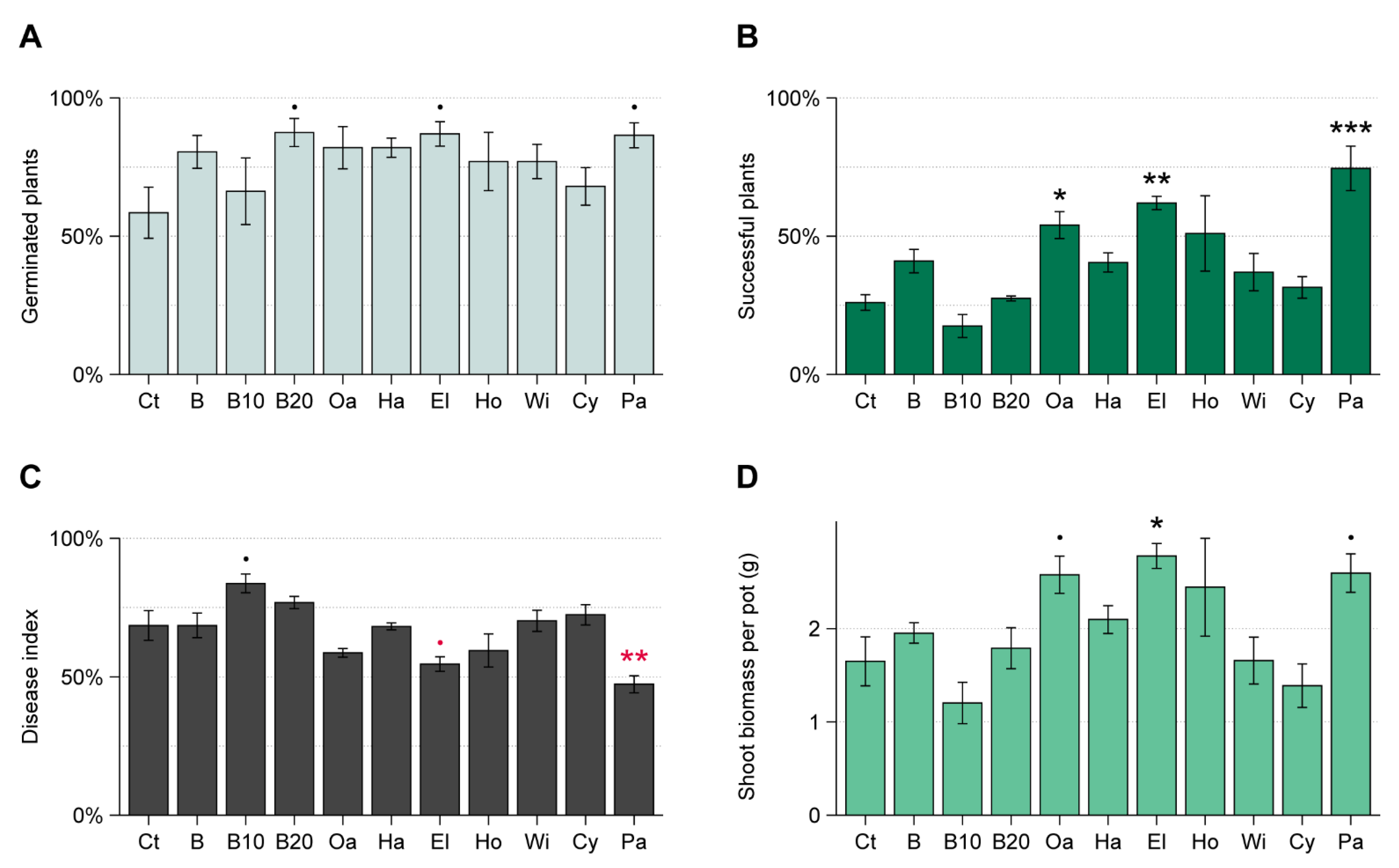
| Material | Species | Exp. RsP | Exp. WT | Exp. ToS |
|---|---|---|---|---|
| Beech | Fagus sylvatica | • | • | • |
| 10% decomposed beech | Fagus sylvatica | • | ||
| 20% decomposed beech | Fagus sylvatica | • | ||
| Oak | Quercus robur | • | • | • |
| Hazel | Corylus avellana | • | • | |
| Black alder | Alnus glutinosa | • | ||
| Birch | Betula sp. | • | ||
| Walnut | Juglans sp. | • | ||
| Maple | Acer sp. | • | ||
| Elder | Sambucus sp. | • | • | |
| Holly | Ilex sp. | • | • | |
| Willow | Salix alba | • | • | |
| Hawthorn | Crataegus sp. | • | ||
| Snowy mespilus | Amelanchier sp. | • | ||
| Cypress | Cupressus sempervirens | • | • | |
| Douglas fir | Pseudotsuga menziesii | • | ||
| Paper pulp | - | • | • | • |
| Hair meal | Sus scrofa | • | ||
| Shrimp meal | Crangon crangon | • |
| A | |||||||||||||||
| Comparison | Day 7 | Day 21 | Day 35 | Day 49 | Root | ||||||||||
| Distance | R2 | Distance | R2 | Distance | R2 | Distance | R2 | Distance | R2 | ||||||
| Oak–control | 0.55 | 0.68 | ** | 0.68 | 0.73 | ** | 0.70 | 0.79 | ** | 0.69 | 0.72 | * | 0.32 | 0.21 | ** |
| Paper pulp–control | 0.50 | 0.56 | ** | 0.40 | 0.42 | ** | 0.34 | 0.34 | ** | 0.36 | 0.30 | * | 0.57 | 0.40 | ** |
| B | |||||||||||||||
| Comparison | Day 7 | Day 21 | Day 35 | Day 49 | Root | ||||||||||
| Distance | R2 | Distance | R2 | Distance | R2 | Distance | R2 | Distance | R2 | ||||||
| Oak–control | 0.27 | 0.05 | * | 0.25 | 0.04 | • | 0.30 | 0.06 | 0.37 | 0.10 | 0.26 | 0.04 | |||
| Paper pulp–control | 0.43 | 0.12 | * | 0.30 | 0.06 | * | 0.33 | 0.07 | * | 0.47 | 0.16 | • | 0.33 | 0.06 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clocchiatti, A.; Hannula, S.E.; Rizaludin, M.S.; Hundscheid, M.P.J.; klein Gunnewiek, P.J.A.; Schilder, M.T.; Postma, J.; de Boer, W. Impact of Cellulose-Rich Organic Soil Amendments on Growth Dynamics and Pathogenicity of Rhizoctonia solani. Microorganisms 2021, 9, 1285. https://doi.org/10.3390/microorganisms9061285
Clocchiatti A, Hannula SE, Rizaludin MS, Hundscheid MPJ, klein Gunnewiek PJA, Schilder MT, Postma J, de Boer W. Impact of Cellulose-Rich Organic Soil Amendments on Growth Dynamics and Pathogenicity of Rhizoctonia solani. Microorganisms. 2021; 9(6):1285. https://doi.org/10.3390/microorganisms9061285
Chicago/Turabian StyleClocchiatti, Anna, Silja Emilia Hannula, Muhammad Syamsu Rizaludin, Maria P. J. Hundscheid, Paulien J. A. klein Gunnewiek, Mirjam T. Schilder, Joeke Postma, and Wietse de Boer. 2021. "Impact of Cellulose-Rich Organic Soil Amendments on Growth Dynamics and Pathogenicity of Rhizoctonia solani" Microorganisms 9, no. 6: 1285. https://doi.org/10.3390/microorganisms9061285
APA StyleClocchiatti, A., Hannula, S. E., Rizaludin, M. S., Hundscheid, M. P. J., klein Gunnewiek, P. J. A., Schilder, M. T., Postma, J., & de Boer, W. (2021). Impact of Cellulose-Rich Organic Soil Amendments on Growth Dynamics and Pathogenicity of Rhizoctonia solani. Microorganisms, 9(6), 1285. https://doi.org/10.3390/microorganisms9061285






