Mother-to-Neonate Transmission of Antibiotic-Resistant Bacteria: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Bacterial Isolation and Identification
2.4. Antibiotic Susceptibility Testing
2.5. Characterization of blaESBL Genes
2.6. ESBL Typing
2.7. Detection of pvl, mecA and mecC Genes in MRSA Isolates
2.8. Detection of vanA and vanB Genes in VRE Isolates
2.9. Detection of CRE Mechanism
2.10. Detection of Biofilm Formation
2.11. Statistical Analysis
3. Results
3.1. Demographic and Baseline Clinical Data of Participating Pregnant Women, Divided to Carrier and Non-Carriers
3.2. Prevalence of Antibiotic Resistant Bacteria among Mothers and Neonates
3.3. Comparison of Demographic and Clinical Data of All Participating Neonates Divided According to Carriage Status
3.4. Comparison of Demographic and Clinical Data of Neonates Born to a Carrier Mother, Divided According to Carriage Status
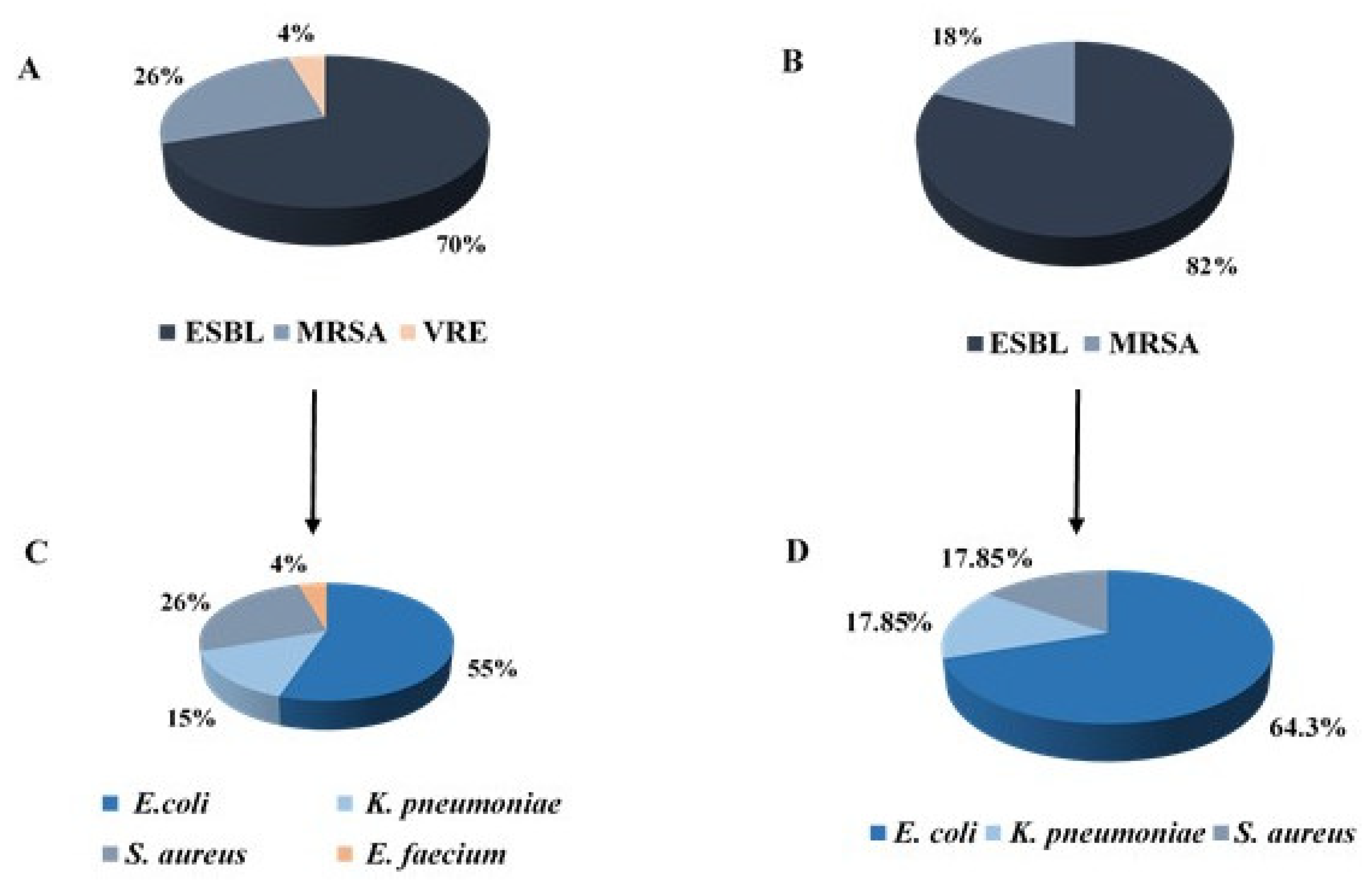
3.5. Resistance Mechanisms and Bacterial Type of Bacterial Isolates
3.6. Bacterial Characteristics of ESBL-Positive Isolates
Biofilm Formation in Transmitted ESBL Isolates Compared to Non-Transmitted Isolates
3.7. Bacterial Characteristics of MRSA Isolates
Biofilm Formation in Transmitted MRSA Isolates Compared to Non-Transmitted Isolates
3.8. Bacterial Characteristics of VRE Isolates
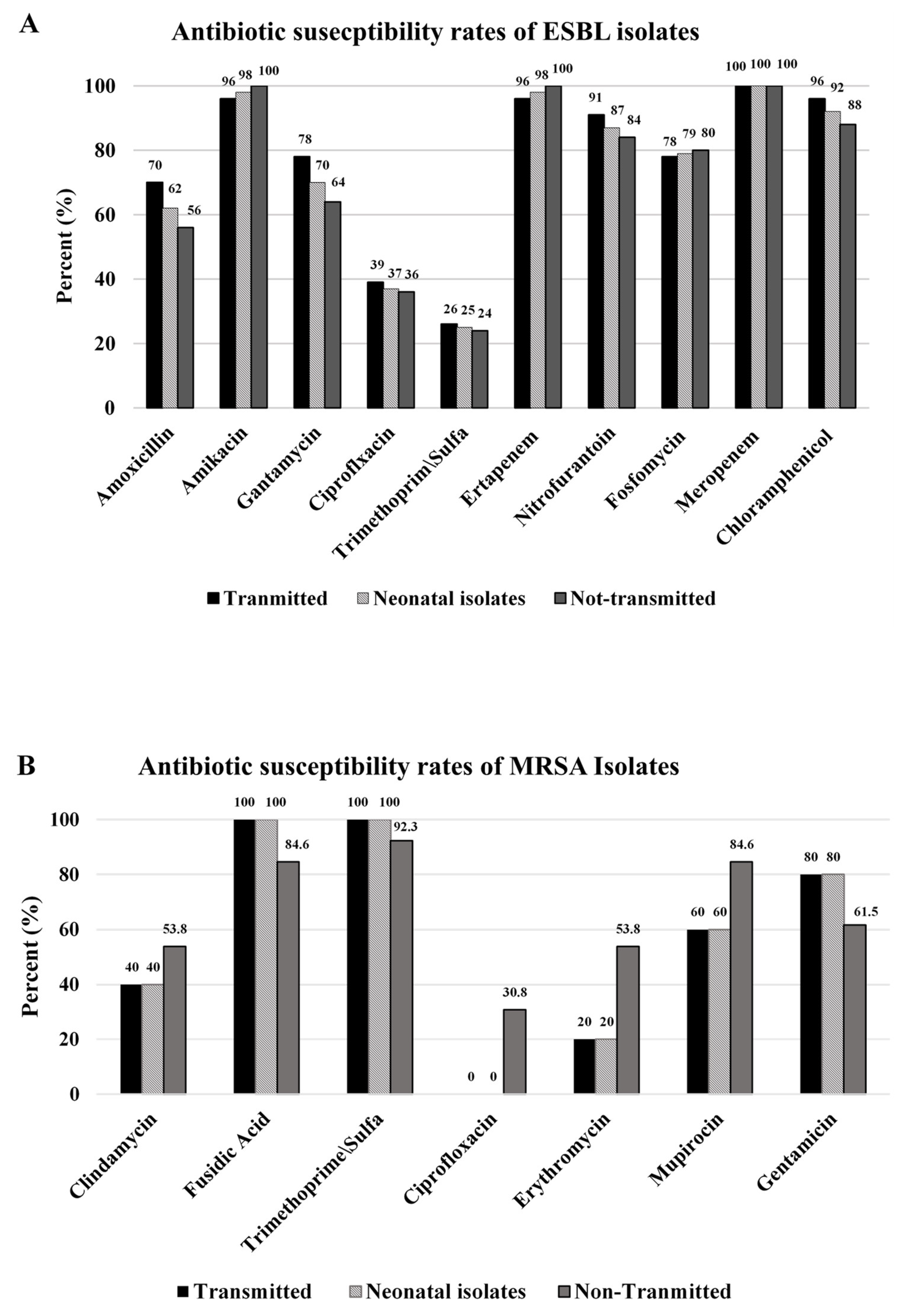
3.9. Antibiotic Susceptibility of the Isolates
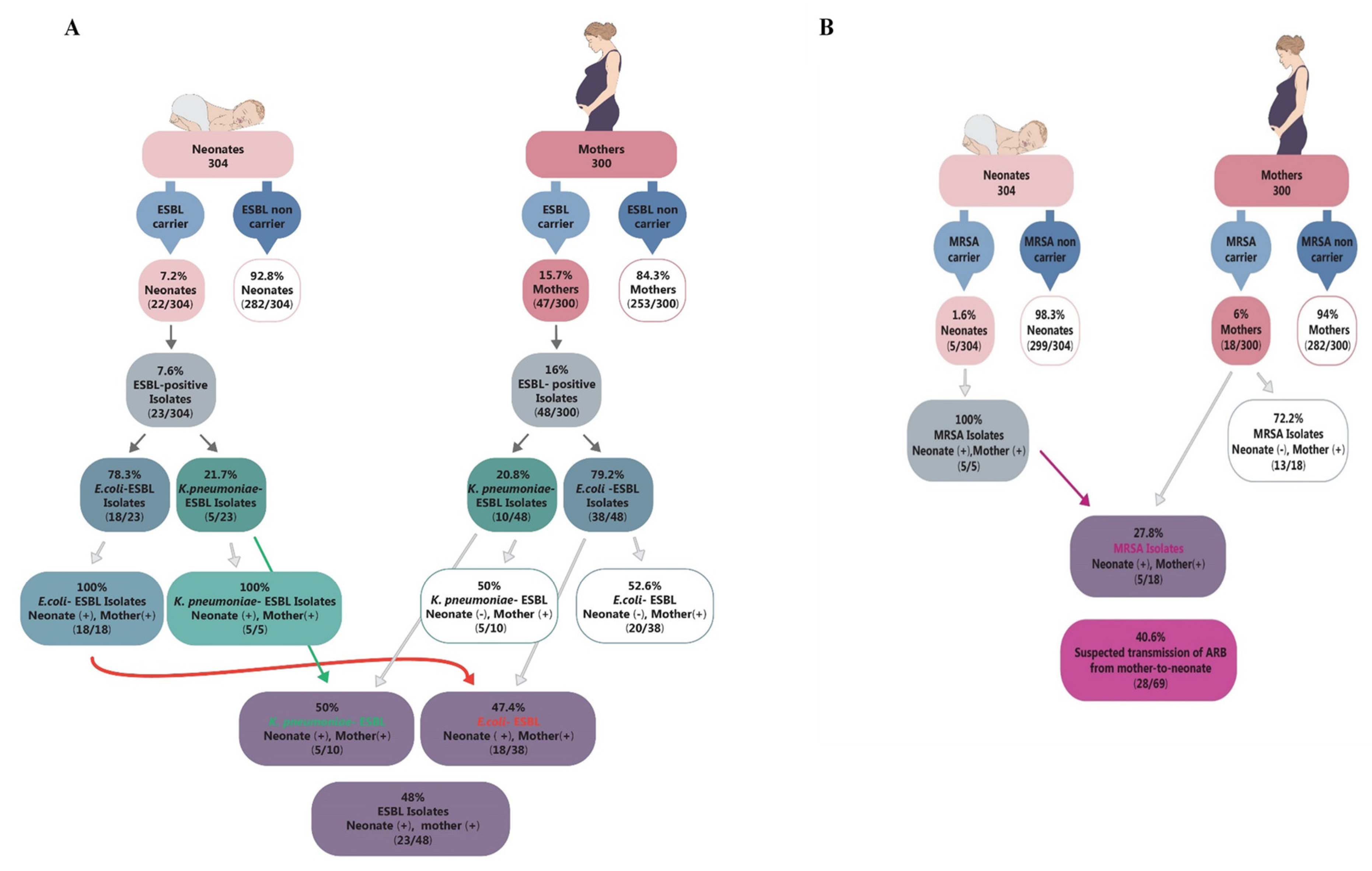
3.10. Maternal-to-Neonate Transmission
4. Discussion
4.1. Risk Factors for Maternal Colonization
4.2. Prevalence of ARB Bacteria among Neonates
4.3. Risk Factors for Neonatal Colonization
4.4. Resistance Mechanisms and Bacterial Type of Bacterial Isolates
4.5. Characteristics of ESBL-Producing Isolates and Comparison between Transmitted Isolates to Non-Transmitted Isolates
4.6. Biofilm Formation in Transmitted ESBL-Positive Isolates Compared to Non-Transmitted Isolates
4.7. Biofilm Formation in Transmitted MRSA Isolates Compared to Non-Transmitted Isolates
4.8. Antibiotic Susceptibility of the Isolates
4.9. Maternal-to-Neonate Transmission
4.10. Clinical Implications
4.11. Research Implications
4.12. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yelin, I.; Snitser, O.; Novich, G.; Katz, R.; Tal, O.; Parizade, M.; Chodick, G.; Koren, G.; Shalev, V.; Kishony, R. Personal clinical history predicts antibiotic resistance of urinary tract infections. Nat. Med. 2019, 25, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Eppes, C.S.; Clark, S.L. Extended-spectrum β-lactamase infections during pregnancy: A growing threat. Am. J. Obstet. Gynecol. 2015, 213, 650–652. [Google Scholar] [CrossRef]
- Denkel, L.A.; Schwab, F.; Kola, A.; Leistner, R.; Garten, L.; Von Weizsäcker, K.; Geffers, C.; Gastmeier, P.; Piening, B. The mother as most important risk factor for colonization of very low birth weight (VLBW) infants with extended-spectrum -lactamase-producing Enterobacteriaceae (ESBL-E). J. Antimicrob. Chemother. 2014, 69, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Bulabula, A.N.; Dramowski, A.; Mehtar, S. Transmission of multidrug-resistant Gram-negative bacteria from colonized mothers to their infants: A systematic review and meta-analysis. J. Hosp. Infect. 2020, 104, 57–67. [Google Scholar] [CrossRef]
- Giuffrè, M.; Geraci, D.M.; Bonura, C.; Saporito, L.; Graziano, G.; Insinga, V.; Aleo, A.; Vecchio, D.; Mammina, C. The Increasing Challenge of Multidrug-Resistant Gram-Negative Bacilli: Results of a 5-Year Active Surveillance Program in a Neonatal Intensive Care Unit. Medicine 2016, 95, e3016. [Google Scholar] [CrossRef]
- Danino, D.; Melamed, R.; Sterer, B.; Porat, N.; Hazan, G.; Gushanski, A.; Shany, E.; Greenberg, D.; Borer, A. Mother-to-child transmission of extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J. Hosp. Infect. 2018, 100, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.E.; Blencowe, H.; Mathers, C.; Cousens, S.N.; Oza, S.; You, D.; Lee, A.C.; Waiswa, P.; Lalli, M.; Bhutta, Z.; et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014, 384, 189–205. [Google Scholar] [CrossRef]
- Sehgal, R.; Gaind, R.; Chellani, H.; Agarwal, P. Extended-spectrum β lactamase-producing gram-negative bacteria: Clinical profile and outcome in a neonatal intensive care unit. Ann. Trop. Paediatr. 2007, 27, 45–54. [Google Scholar] [CrossRef]
- Didier, C.; Streicher, M.-P.; Chognot, D.; Campagni, R.; Schnebelen, A.; Messer, J.; Donato, L.; Langer, B.; Meyer, N.; Astruc, D.; et al. Late-onset neonatal infections: Incidences and pathogens in the era of antenatal antibiotics. Eur. J. Nucl. Med. Mol. Imaging 2011, 171, 681–687. [Google Scholar] [CrossRef]
- Oteo, J.; Cercenado, E.; Vindel, A.; Bautista, V.; Fernández-Romero, S.; Saéz, D.; Padilla, B.; Zamora, E.; Campos, J. Outbreak of multidrug-resistant CTX-M-15-producing Enterobacter cloacae in a neonatal intensive care unit. J. Med. Microbiol. 2013, 62, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Sreeramoju, P.; Jaleel, M.; Trevino, S.; Gander, R.; Hynan, L.S.; Hill, J.; Brown, C.; Chung, W.; Siegel, J.D.; et al. Prompt Control of an Outbreak Caused by Extended-Spectrum β-Lactamase–Producing Klebsiella pneumoniae in a Neonatal Intensive Care Unit. J. Pediatr. 2013, 163, 672–679. [Google Scholar] [CrossRef]
- Stapleton, P.J.M.; Murphy, M.; McCallion, N.; Brennan, M.; Cunney, R.; Drew, R.J. Outbreaks of extended spectrum beta-lactamase-producing Enterobacteriaceae in neonatal intensive care units: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, 72–78. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Changes in Pathogens Causing Early-Onset Sepsis in Very-Low-Birth-Weight Infants. N. Engl. J. Med. 2002, 347, 240–247. [Google Scholar] [CrossRef]
- Giuffrè, M.; Bonura, C.; Cipolla, D.; Mammina, C. MRSA infection in the neonatal intensive care unit. Expert Rev. Anti-Infect. Ther. 2013, 11, 499–509. [Google Scholar] [CrossRef]
- Longardt, A.C.; Piening, B.; Von Weizsäcker, K.; Dame, C.; Bührer, C.; Garten, L. Screening for Third-Generation Cephalosporin-Resistant Bacteria Reduces the Incidence on Late-Onset Sepsis and Antibiotic use in Neonates. Klinische Pädiatrie 2020, 232, 203–209. [Google Scholar] [CrossRef]
- Rettedal, S.; Lohr, I.H.; Bernhoff, E.; Natas, O.; Sundsfjord, A.; Oymar, K. Extended-spectrum β-lactamase-producing Enterobacteriaceae among pregnant women in Norway: Prevalence and maternal–neonatal transmission. J. Perinatol. 2015, 35, 907–912. [Google Scholar] [CrossRef]
- Koizumi, A.; Maruyama, K.; Ohki, Y.; Nakayama, A.; Yamada, Y.; Kurosawa, H.; Tsukagoshi, H.; Fujiu, T.; Takahashi, M.; Kimura, T.; et al. Prevalence and Risk Factor for Antibiotic-resistant Escherichia coli Colonization at Birth in Premature Infants: A Prospective Cohort Study. Pediatr. Infect. Dis. J. 2020, 39, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Rámila, C.J.; López-Cerero, L.; Martín, M.V.A.; Martín, C.V.; Serrano, L.; Pascual, Á.; Rodríguez-Baño, J. Vagino-rectal colonization and maternal–neonatal transmission of Enterobacteriaceae producing extended-spectrum β-lactamases or carbapenemases: A cross-sectional study. J. Hosp. Infect. 2019, 101, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Foessleitner, P.; Gasser, J.; Kiss, H.; Flunt, A.; Presterl, E.; Petricevic, L.; Farr, A. Vaginal colonization of extended-spectrum beta-lactamase-producing bacteria during pregnancy: An observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Heigl, K.; Zamfir, M.; Adler, A.C.; Dammeyer, A.; Schomacher, L.; Karlin, B.; Franitza, M.; Hörmansdorfer, S.; Tuschak, C.; Valenza, G.; et al. Prevalence of methicillin-sensitive, methicillin-resistant Staphylococcus aureus, and extended-spectrum beta-lactamase-producing Escherichia coli in newborns: A cross-sectional study. J. Matern. Neonatal Med. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Peretz, A.; Skuratovsky, A.; Khabra, E.; Adler, A.; Pastukh, N.; Barak, S.; Perlitz, Y.; Ben-Ami, M.; Kushnir, A. Peripartum maternal transmission of extended-spectrum β-lactamase organism to newborn infants. Diagn. Microbiol. Infect. Dis. 2017, 87, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz. J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef]
- Gangashettappa, N.; Raksha, L.; Shantala, G.B.; Nandan, B.R.; Sinha, D. Study of biofilm formation in bacterial isolates from contact lens wearers. Indian J. Ophthalmol. 2020, 68, 23–28. [Google Scholar] [CrossRef]
- Rawstron, S.A.; Jackman, J.M.; Serebro, E.; Johnson, G.; Cabbad, M.; Bromberg, K.; Kondamudi, V.; Sepkowitz, D.; Landman, D. Perirectal Screening for Carbapenem-Resistant Enterobacteriaceae Obtained from 100 Consecutive Healthy Pregnant Women in Labor at a Brooklyn Hospital: Results and Risk Factors. Infect. Control. Hosp. Epidemiol. 2018, 39, 369–371. [Google Scholar] [CrossRef]
- Mairi, A.; Touati, A.; Bessai, S.A.; Boutabtoub, Y.; Khelifi, F.; Sotto, A.; Lavigne, J.-P.; Pantel, A. Carbapenemase-producing Enterobacteriaceae among pregnant women and newborns in Algeria: Prevalence, molecular characterization, maternal-neonatal transmission, and risk factors for carriage. Am. J. Infect. Control. 2019, 47, 105–108. [Google Scholar] [CrossRef]
- Schaumburg, F.; Alabi, A.S.; Grobusch, M.P.; Kremsner, P.G.; Köck, R.; Peters, G.; Ramharter, M.; Becker, K.; Mombo-Ngoma, G.; Kaba, H.; et al. Transmission of Staphylococcus aureus between mothers and infants in an African setting. Clin. Microbiol. Infect. 2014, 20, O390–O396. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yao, Z. Maternal-Infant Correlation of Multidrug-Resistant Staphylococcus aureus Carriage: A Prospective Cohort Study. Front. Pediatr. 2018, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.I.; Kaufman, H.K. Nasopharyngeal carriage of methicillin-resistant Staphylococcus aureus: Incidence and outcomes in pregnant women. J. Am. Osteopat. Assoc. 2011, 111, 389–395. [Google Scholar]
- Lazenby, G.B. Opportunistic Infections in Women with HIV AIDS. Clin. Obstet. Gynecol. 2012, 55, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois-Nicolaos, N.; Lucet, J.-C.; Daubié, C.; Benchaba, F.; Rajguru, M.; Ruimy, R.; Andremont, A.; Armand--Lefèvre, L. Maternal vaginal colonisation by Staphylococcus aureus and newborn acquisition at delivery. Paediatr. Peérinat. Epidemiol. 2010, 24, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Top, K.A.; Buet, A.; Whittier, S.; Ratner, A.J.; Saiman, L. Predictors of Staphylococcus aureus Rectovaginal Colonization in Pregnant Women and Risk for Maternal and Neonatal Infections. J. Pediatr. Infect. Dis. Soc. 2012, 1, 7–15. [Google Scholar] [CrossRef]
- Miller, M.B.; Allen, S.L.; Mangum, M.E.; Doutova, A.; Gilligan, P.H. Prevalence of Vancomycin-Resistant Enterococcus in Prenatal Screening Cultures. J. Clin. Microbiol. 2004, 42, 855–857. [Google Scholar] [CrossRef]
- Ghasemi, E.; Mansouri, S.; Shahabinejad, N. Vaginal Colonization and Susceptibility to Antibiotics of Enterococci During Late Pregnancy in Kerman City, Iran. Arch. Clin. Infect. Dis. 2016, 11, e35428. [Google Scholar] [CrossRef]
- Andrews, W.W.; Schelonka, R.; Waites, K.; Stamm, A.; Cliver, S.P.; Moser, S. Genital Tract Methicillin-Resistant Staphylococcus aureus: Risk of vertical transmission in pregnant women. Obstet. Gynecol. 2008, 111, 113–118. [Google Scholar] [CrossRef]
- Wang, X.; Towers, S.; Panchanathan, S.; Chowell, G. A Population Based Study of Seasonality of Skin and Soft Tissue Infections: Implications for the Spread of CA-MRSA. PLoS ONE 2013, 8, e60872. [Google Scholar] [CrossRef]
- Ramharter, M.; Chai, S.K.; Kremsner, P.G.; Adegnika, A.A.; Klöpfer, A.; Längin, M.; Agnandji, S.T.; Oyakhirome, S.; Schwarz, N.G.; Grobusch, M.P.; et al. Shared breastfeeding in central Africa. AIDS 2004, 18, 1847–1849. [Google Scholar] [CrossRef] [PubMed]
- Baier, C.; Pirr, S.; Ziesing, S.; Ebadi, E.; Hansen, G.; Bohnhorst, B.; Bange, F.-C. Prospective surveillance of bacterial colonization and primary sepsis: Findings of a tertiary neonatal intensive and intermediate care unit. J. Hosp. Infect. 2019, 102, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Yuce, A.; Karaman, M.; Gulay, Z.; Yulug, N. Vancomycin-resistant Enterococci in Neonates. Scand. J. Infect. Dis. 2001, 33, 803–805. [Google Scholar] [CrossRef]
- Bromiker, R.; Ernest, N.; Bar Meir, M.; Kaplan, M.; Hammerman, C.; Schimmel, M.S.; Schlesinger, Y. Correlation of Bacterial Type and Antibiotic Sensitivity with Maternal Antibiotic Exposure in Early-Onset Neonatal Sepsis. Neonatology 2013, 103, 48–53. [Google Scholar] [CrossRef]
- Nübel, U.; Nachtnebel, M.; Falkenhorst, G.; Benzler, J.; Hecht, J.; Kube, M.; Bröcker, F.; Moelling, K.; Bührer, C.; Gastmeier, P.; et al. MRSA Transmission on a Neonatal Intensive Care Unit: Epidemiological and Genome-Based Phylogenetic Analyses. PLoS ONE 2013, 8, e54898. [Google Scholar] [CrossRef]
- Rettedal, S.; Löhr, I.H.; Natås, O.; Sundsfjord, A.; Øymar, K. Risk factors for acquisition of CTX-M-15 extended-spectrum beta-lactamase-producing Klebsiella pneumoniae during an outbreak in a neonatal intensive care unit in Norway. Scand. J. Infect. Dis. 2012, 45, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Erosini, R.; Margarit, I. Biofilm formation by Streptococcus agalactiae: Influence of environmental conditions and implicated virulence factors. Front. Cell. Infect. Microbiol. 2015, 5, 6. [Google Scholar] [CrossRef]
- Parker, R.E.; Laut, C.; Gaddy, J.A.; Zadoks, R.N.; Dele Davies, H.; Manning, S.D. Association between genotypic diversity and biofilm production in group B Streptococcus. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Ho, Y.-R.; Li, C.-M.; Yu, C.-H.; Lin, Y.-J.; Wu, C.-M.; Harn, I.-C.; Tang, M.-J.; Chen, Y.-T.; Shen, F.-C.; Lu, C.-Y.; et al. The enhancement of biofilm formation in Group B streptococcal isolates at vaginal pH. Med. Microbiol. Immunol. 2012, 202, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Schilcher, K.; Burcham, L.R.; Kwiecinski, J.M.; Johnson, P.M.; Head, S.R.; Heinrichs, D.E.; Horswill, A.R.; Doran, K.S. Identification of Key Determinants ofStaphylococcus aureusVaginal Colonization. mBio 2019, 10, e02321-19. [Google Scholar] [CrossRef]
| Characteristic | Non-Carrier (N = 236) | Carrier | Total | p Value |
|---|---|---|---|---|
| (N = 64) | (N = 300) | |||
| Mean age | ||||
| (min–max) | 29.5 (18–47) | 29.9 (22–42) | 29.6 (18–47) | 0.602 |
| Nationality (n, %) | ||||
| Jews | 68 (28.8) | 25 (39.1) | 93 (31) | |
| Arabs | 137 (58) | 30 (46.9) | 167 (55.7) | 0.238 |
| Other | 31 (13.2) | 9 (14) | 40 (13.3) | |
| Settlement type (n, %) | ||||
| City | 96 (40.7) | 32 (50) | 128 (42.7) | |
| Village | 2 (0.8) | 2 (3.2) | 4 (1.3) | 0.123 |
| Other | 138 (58.5) | 30 (46.8) | 168 (56) | |
| Gravidity (Average) | 2.17 | 2.04 | 2.14 | 0.517 |
| Antibiotic use during pregnancy | ||||
| No | 200 (84.7) | 60 (93.75) | 260 (86.6) | |
| Yes | 36 (15.3) | 4 (6.25) | 40 (13.3) | 0.124 |
| Delivery type | ||||
| Vacuum | 24 (10.2) | 12 (18.75) | 36 (12) | |
| Spontaneous | 212 (89.8) | 52 (81.25) | 264 (88) | 0.061 |
| Gestational week (n, %) | ||||
| ≤37 | 19 (8) | 4 (6.3) | 23 (7.7) | |
| 38–39 | 117 (49.6) | 33 (51.5) | 150 (50) | 0.881 |
| ≥40 | 100 (42.4) | 27 (42.2) | 127 (42.3) | |
| Amniotic fluid port (n, %) | ||||
| Spontaneous rupture of membrane (Srm) | 126 (53.4) | 22 (34.4) | 148 (49.3) | |
| Artificial ruptureof membrane (Arm) | 110 (46.6) | 42 (65.6) | 152 (50.7) | 0.007 |
| Antibiotic use during delivery | ||||
| No | 189 (80.1) | 47 (73.5) | 236 (78.7) | |
| Yes | 47 (19.9) | 17 (26.5) | 64 (21.3) | 0.25 |
| Characteristic | Non-Carrier | Carrier | Total | p Value |
|---|---|---|---|---|
| (N = 278) | (N = 26) | (N = 304) | ||
| Mean birth weight | ||||
| (gr, min–max) | 3345.5 (1740–4450) | 3282.3 (2180–4380) | 3351.4 (1740–4450) | 0.436 |
| Gender (n, %) | 0.749 | |||
| Female | 125 (45) | 11 (42.3) | 136 (44.7) | |
| Male | 153 (55) | 15 (57.7) | 168 (55.3) | |
| Gravidity (Average) | 2.16 | 2.11 | 0.885 | |
| Antibiotic use during pregnancy (n, %) | ||||
| No | 240 (86.3) | 22 (84.6) | 262 (86.2) | 0.808 |
| Yes | 38 (13.7) | 4 (15.4) | 42 (13.8) | |
| Delivery type (n, %) | 0.684 | |||
| Vacuum | 35 (12.6) | 4 (15.4) | 39 (12.8) | |
| Spontaneous | 243 (87.4) | 22 (84.6) | 265 (87.2) | |
| Gestational week (n, %) | 0.376 | |||
| ≤37 | 24 (8.7) | 1 (3.8) | 25 (8.3) | |
| 38–39 | 140 (50.5) | 11 (42.3) | 151 (49.8) | |
| ≥40 | 113 (40.8) | 14 (53.8) | 127 (41.9) | |
| Amniotic fluid port (n, %) | ||||
| Spontaneous rupture of membrane (Srm) | 143 (51.4%) | 6 (23.1) | 149 (49%) | 0.006 |
| Artificial rupture of membrane (Arm) | 135 (48.6%) | 20 (76.9) | 155 (51%) | |
| Antibiotic use during delivery (n, %) | ||||
| No | 223 (80.5) | 15 (57.7) | 238 (78.5) | 0.007 |
| Yes | 54 (19.5) | 11 (42.3) | 65 (21.5) |
| Characteristic | Non-Carrier | Carrier | Total | p Value |
|---|---|---|---|---|
| (N = 39) | (N = 26) | (N = 65) | ||
| Mean birth weight | 3280.8 (1740–4156) | 3282.3 (2180–4380) | 3281.4 (1740–4380) | 0.99 |
| (gr, min–max) | ||||
| Gender (n, %) | 0.760 | |||
| Female | 18 (46.2) | 11 (42.3) | 29 (44.6) | |
| Male | 21 (53.8) | 15 (57.7) | 36 (55.4) | |
| Gravidity (Average) | 2.05 | 2.11 | 2.07 | 0.846 |
| Antibiotic use during pregnancy (n, %) | ||||
| No | 37 (94.9) | 22 (84.6) | 59 (90.8) | 0.162 |
| Yes | 2 (5.1) | 4 (15.4) | 6 (8.2) | |
| Delivery type (n, %) | 0.602 | |||
| Vacuum | 8 (20.5) | 4 (15.4) | 12 (18.5) | |
| Spontaneous | 31 (79.5) | 22 (84.6) | 53 (81.5) | |
| Gestational week (n, %) | 0.220 | |||
| ≤37 | 4 (10.3) | 1 (3.8) | 5 (7.7) | |
| 38–39 | 22 (56.4) | 11 (42.3) | 33 (50.7) | |
| ≥40 | 13 (33.3) | 14 (53.8) | 27 (41.5) | |
| Amniotic fluid port (n, %) | ||||
| Spontaneous rupture of membrane (Srm) | 16 (41.1) | 6 (23.1) | 22 (33.8) | 0.134 |
| Artificial rupture of membrane (Arm) | 23 (58.9) | 20 (76.9) | 43 (66.2) | |
| Antibiotic use during delivery (n, %) | ||||
| No | 32 (82) | 15 (57.7) | 47 (72.3) | 0.032 |
| Yes | 7 (18) | 11 (42.3) | 18 (21.7) |
| Characteristic | Non-Transmitted | Transmitted | Total | p Value |
|---|---|---|---|---|
| (N = 41) | (N = 28) | (N = 69) | ||
| Resistance mechanism | 0.114 | |||
| ESBL | 25 (61) | 23 (82.1) | 48 (69.6) | |
| MRSA | 13 (31.7) | 5 (17.9) | 18 (26.1) | |
| VRE | 3 (7.3) | 0 (0) | 3 (4.3) | |
| Bacterial type | 0.225 | |||
| E. coli | 20 (48.8) | 18 (64.3) | 38 (55.1) | |
| E. faecium | 3 (7.3) | 0 (0) | 3 (4.3) | |
| K. pneumonia | 5 (12.2) | 5 (17.85) | 10 (14.5) | |
| S. aureus | 13 (31.7) | 5 (17.85) | 18 (26.1) |
| Characteristic | Maternal Isolates without Transmission to Newborn (NTN) | Maternal Isolates with Transmission to Newborn (TN) | Total | p Value | Neonatal Isolates |
|---|---|---|---|---|---|
| (n, %), N = 25 | (n, %), N = 23 | N = 48 | (n, %), N = 23 | ||
| Bacterial type | 0.882 | ||||
| E. coli | 20 (80) | 18 (78.3) | 38 (79.2) | 18 (78.3) | |
| K. pneumoniae | 5 (20) | 5 (21.7) | 10 (20.8) | 5 (21.7) | |
| CTX-M presence | 0.281 | ||||
| Yes | 25 (100) | 21 (91.3) | 46 (95.8) | 21 (91.3) | |
| No | 0 (0) | 2 (8.7) | 2 (4.2) | 2 (9.7) | |
| SHV presence | 0.132 | ||||
| Yes | 0 (0) | 2 (8.7) | 2 (4.2) | 2 (9.7) | |
| No | 25 (100) | 21 (91.3) | 46 (95.8) | 20 (91.3) | |
| Number of colonies | <0.001 | ||||
| <5 | 10 (40) | 1 (4.3) | 11 (22.9) | 2 (8.7) | |
| 5–10 | 9 (36) | 5 (21.7) | 14 (29.2) | 5 (21.7) | |
| >10 | 6 (24) | 17 (74) | 23 (47.9) | 16 (69.6) | |
| Biofilm formation | <0.001 | ||||
| Negative | 23 (92) | 5 (21.7) | 28 (58.3) | 6 (26.1) | |
| Moderate | 2 (8) | 5 (21.7) | 7 (14.6) | 5 (21.7) | |
| Strong | 0 | 13 (56.6) | 13 (27.1) | 12 (52.2) |
| Characteristic | Maternal Isolates without Transmission to Newborn (NTN) | Maternal Isolates with Transmission to Newborn (TN) | Total | Neonatal Isolates |
|---|---|---|---|---|
| (n, %), N = 13 | (n, %), N = 5 | N = 18 | (n, %), N = 5 | |
| MecA presence | ||||
| yes | 13 (100) | 5 (100) | 18 (100) | 5 (100) |
| no | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pvl presence | ||||
| Yes | 4 (30.8) | 0 (0) | 4 (22.2) | 0 (0) |
| No | 9 (69.2) | 5 (100) | 14 (77.8) | 5 (100) |
| Number of colonies | ||||
| <5 | 2 (15.4) | 0 (4.3) | 2 (11.1) | 1 (20) |
| 5–10 | 5 (38.5) | 2 (40) | 7 (38.9) | 3 (60) |
| >10 | 6 (46.1) | 3 (60) | 9 (50) | 1 (20) |
| Biofilm formation | ||||
| Negative | 12 (92.3) | 0 (0) | 12 (66.7) | 0 (0) |
| Moderate | 1 (7.7) | 2 (40) | 3 (16.6) | 2 (40) |
| Strong | 0 (0) | 3 (60) | 3 (16.6) | 3 (60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matok, L.A.; Azrad, M.; Leshem, T.; Abuzahya, A.; Khamaisi, T.; Smolkin, T.; Peretz, A. Mother-to-Neonate Transmission of Antibiotic-Resistant Bacteria: A Cross-Sectional Study. Microorganisms 2021, 9, 1245. https://doi.org/10.3390/microorganisms9061245
Matok LA, Azrad M, Leshem T, Abuzahya A, Khamaisi T, Smolkin T, Peretz A. Mother-to-Neonate Transmission of Antibiotic-Resistant Bacteria: A Cross-Sectional Study. Microorganisms. 2021; 9(6):1245. https://doi.org/10.3390/microorganisms9061245
Chicago/Turabian StyleMatok, Lital Ashtamkar, Maya Azrad, Tamar Leshem, Anan Abuzahya, Thanaa Khamaisi, Tatiana Smolkin, and Avi Peretz. 2021. "Mother-to-Neonate Transmission of Antibiotic-Resistant Bacteria: A Cross-Sectional Study" Microorganisms 9, no. 6: 1245. https://doi.org/10.3390/microorganisms9061245
APA StyleMatok, L. A., Azrad, M., Leshem, T., Abuzahya, A., Khamaisi, T., Smolkin, T., & Peretz, A. (2021). Mother-to-Neonate Transmission of Antibiotic-Resistant Bacteria: A Cross-Sectional Study. Microorganisms, 9(6), 1245. https://doi.org/10.3390/microorganisms9061245








