Abstract
Treatment of visceral leishmaniasis in Brazil still relies on meglumine antimoniate, with less than ideal efficacy and safety, making new therapeutic tools an urgent need. The oral drug miltefosine was assayed in a phase II clinical trial in Brazil with cure rates lower than previously demonstrated in India. The present study investigated the susceptibility to miltefosine in 73 Brazilian strains of Leishmania infantum from different geographic regions, using intracellular amastigote and promastigote assays. The EC50 for miltefosine of 13 of these strains evaluated in intracellular amastigotes varied between 1.41 and 4.57 μM. The EC50 of the 73 strains determined in promastigotes varied between 5.89 and 23.7 μM. No correlation between in vitro miltefosine susceptibility and the presence of the miltefosine sensitive locus was detected among the tested strains. The relatively low heterogeneity in miltefosine susceptibility observed for the 73 strains tested in this study suggests the absence of decreased susceptibility to miltefosine in Brazilian L. infantum and does not exclude future clinical evaluation of miltefosine for VL treatment in Brazil.
1. Introduction
Visceral leishmaniasis (VL) remains a worldwide public health concern, mainly affecting poor populations across Asia, East Africa, South America, and the Mediterranean region. Over 600 million people live in areas at risk of developing the disease [1]. Overall annual incidence is estimated to be from 50,000 to 90,000 cases, with ten countries concentrating more than 95% of the reported cases: Bangladesh, Brazil, China, Ethiopia, India, Kenya, Nepal, Somalia, South Sudan, and Sudan [1]. In Latin America, VL is a zoonosis caused by Leishmania infantum (syn. L. chagasi) with dog as the main reservoir of infection and Lutzomyia longipalpis the principal vector. It is endemic in 13 countries with an average of 3470 new reported cases every year. In 2019, Brazil reported 2529 new cases, accounting for 97% of cases in Latin America, with around 35% of those in children under 10 years of age [2], and a lethality of 9% [2,3], one of the highest in the world. Another concern is the increasing incidence of HIV/VL coinfection, rising from 0.7% of reported VL cases in Brazil in 2001 to 11.1% in 2019 [2,4].
First line treatment for VL in Brazil is meglumine antimoniate (MA) at a dose of 20 mg intravenous Sb5+/kg/day for 20 days. In 2013, the Brazilian Ministry of Health recommended liposomal amphotericin B (LAMB) as the second line treatment, widening its indications. It is currently recommended for patients aged <1 year and >50 years, during pregnancy, with severe illness based on severity score, renal, hepatic, or cardiac damage, HIV-coinfection or other conditions leading to immunodeficiency, therapeutic failure to MA, or other contraindications to MA use [5].
Between 2011 and 2014, a large multicenter clinical trial sponsored by the Brazilian Ministry of Health was conducted to assess the safety and efficacy of treatments recommended for VL in Brazil (MA, amphotericin B deoxycholate, LAMB) and to test a combination of LAMB (10 mg/kg single dose) plus MA for 10 days. Although no statistically significant differences in cure rates were observed, a better safety profile for 3 mg/kg/day LAMB for 7 days, compared to standard MA treatment, was demonstrated [6]. These results are guiding treatment policy change, recommending LAMB monotherapy as the first line treatment option for VL in Brazil. Nonetheless, while LAMB cure rates around 95% have been reported in clinical trials conducted in other countries [7,8,9,10,11,12,13,14], 87.2% cure rates were observed for LAMB in Brazil, suggesting that there is room to improve drug efficacy. This could be achieved through exploring alternative drug combinations with miltefosine, the only current effective oral agent for VL.
However, a clinical trial to assess the efficacy and safety of miltefosine in patients with VL in Brazil conducted in 2005 in Teresina (Piauí) and Montes Claros (Minas Gerais) [15] showed an unsatisfactory overall efficacy at six months of around 60%. A cure rate of 43% was observed in 14 patients from Montes Claros treated with 2.5 mg/kg/day for 28 days. Even with an extension of treatment to 42 days in Teresina, only a 68% final cure rate was reached in these patients. Although differences were not statistically significant, treatment failure was observed in 52.2% of pediatric patients versus 26.3% in adults [16].
The lower cure rate observed in Brazil compared to India could be due to the relatively low dose given to adults (maximum daily dose of 100 mg, while allometric dosing [17,18,19,20] would have required higher daily doses) and the comparatively lower blood levels of miltefosine in children, in line with previous published pharmacokinetic data from India, Nepal, and Africa. On the other hand, previous investigations on the variability of Brazilian Leishmania braziliensis strains to miltefosine identified up to 15-fold differences between the susceptibility of strains that had not been exposed to the drug and were obtained from patients prior to treatment [21]. Therefore, reduced susceptibility of Brazilian L. infantum strains to the drug could also explain the lower cure rate reported.
A deletion at chromosome 31 detected in several L. infantum strains was recently described and associated with an increased risk of miltefosine treatment failure in VL [16]. This locus was named the miltefosine sensitive locus (MSL) and was studied in a sample set of 26 pre-treatment isolates from Montes Claros/Teresina patients who participated in the clinical trial mentioned above. Infection by deleted isolates (absence of MSL) was associated with relapse after miltefosine treatment [15].
In this context, this collaborative project involving the Natan Portella Institute for Tropical Diseases, Federal University of Piauí, Teresina, PI; the Leishmaniasis Laboratory at the Biomedical Sciences Institute of São Paulo University (ICB-USP), São Paulo, SP; the Laboratory of Research on Leishmaniasis-Oswaldo Cruz Institute (IOC-Fiocruz), Rio de Janeiro, RJ; and Drugs for Neglected Diseases initiative (DNDi) aimed to evaluate a large set of Brazilian L. infantum strains obtained from humans and dogs presenting VL from different endemic areas in Brazil. The strains were characterized for in vitro susceptibility to miltefosine and genotyped for the presence/absence of MSL.
2. Materials and Methods
2.1. Ethics Statement
The project was approved by the institutional ethics committees of all the participating institutions: Oswaldo Cruz Institute, Fiocruz (CAAE 76599317.5.0000.5248); Biomedical Science Institute of São Paulo University (CAAE 76599317.5.3002.5467); and Federal University of Piauí (CAAE 76599317.5.3001.5214). Animals used in this study to obtain bone marrow-derived macrophages were treated in accordance with the regulations of the Brazilian Society of Science in Laboratory Animals (SBCAL) and the National Council for Animal Experiment Control (CONSEA), ICB–USP, having obtained the necessary approvals from the institutional ethical committee for animal use.
2.2. Chemical Compound
Miltefosine (hexadecylphosphocholine) and amphotericin B were purchased from Sigma-Aldrich (M5571, Lots SLBP4444V and SLBW8818).
2.3. Leishmania infantum (syn L. chagasi) Strains
Seventy-three strains isolated between 2002 and 2015 from humans (n = 60) and dogs (n = 13) were included in this study. In addition, one L. infantum (NLC/IOCL3241) and one L. donovani strain were used as internal controls. All L. infantum strains employed in this study are deposited in the Leishmania Collection at the Oswaldo Cruz Foundation (CLIOC), and were shipped to the ICB-USP Leishmaniasis Laboratory. Isolates were chosen to represent different endemic areas of Brazil (Figure 1), and included six isolates from patients who were enrolled in the clinical trial to assess the efficacy and safety of miltefosine in Teresina [15]. Information provided by the institutions that conducted parasite sampling included the absence of previous treatment before sampling. The activities performed with the Leishmania strains were duly registered in SisGen (Sistema Nacional de Gestão do Patrimonio Genético) under the identifier AE5FEC8, as determined by article 20 of Decree No. 8.772, in accordance with the Brazilian Biodiversity Law (Law No. 13.123/2015).
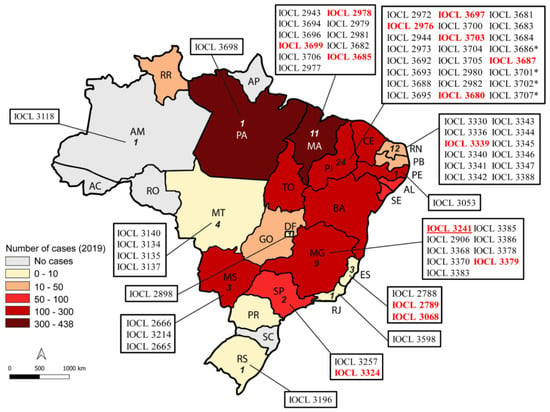
Figure 1.
Geographical distribution of L. infantum clinical strains tested in this study. The map was colored according to the number of cases registered in 2019 as reported in Brasil [22]. Numbers inside each Brazilian state represent the number of strains that originated there. The reference strain NLC is represented in red and underlined. (*) Strains were allocated according to the geographic region in which the parasite was isolated, when the state in which the infection occurred was not confirmed. Strains used in the initial amastigote assays are highlighted in red.
2.4. Parasite Culture
L. infantum promastigotes were grown in sterile M199 medium (Sigma-Aldrich, St. Louis, MO, USA), prepared by diluting the powder in distilled water. Medium was supplemented with 10% heat-inactivated fetal calf serum, 0.25% hemin, 100 μg/mL penicillin/streptomycin, and 2% sterile human urine. Cultures were maintained in an incubator at 25 °C and weekly passages were performed.
2.5. Intracellular Amastigote Assay
To determine drug activity in intracellular L. infantum amastigotes, bone marrow-derived macrophages (BMDM) from BALB/c mice were obtained using the method previously described [23]. Briefly, 3 × 105 BMDM were left to adhere to round glass coverslips in 24-well culture plates. After incubation in a 5% CO2 atmosphere for 24 h at 37 °C, wells were washed with PBS to remove non-adhered cells. Macrophages were then exposed to stationary-phase promastigotes of L. infantum at a multiplicity of infection (MOI) of 15 parasites per macrophage for 4 h at 37 °C in a 5% CO2 atmosphere.
Non-internalized parasites were removed by successive washings with PBS, and infected macrophages were incubated for 72 h at 37 °C at 5% CO2 in RPMI medium with increasing miltefosine concentrations (0.5 to 40 μM). Coverslips were washed with PBS, stained with the Instant Prov kit (Newprov, Pinhais, PR, Brazil) and examined by optical microscopy (Nikon Eclipse E200; Nikon Corporation, Tokyo, Japan). The percentage of infected macrophages, number of amastigotes per infected macrophage, and infectivity index (percentage of infected macrophages multiplied by the average number of amastigotes per macrophage) were determined by counting 100 cells in each triplicate slide. The EC50 was determined using the values of the infectivity index through the nonlinear, dose response, sigmoidal model using the GraphPad Prism 6 program. At least two independent experiments were performed in triplicate for each miltefosine concentration and L. infantum NCL/IOCL3241 and L. donovani Ld15 strains were used as controls in some experiments.
2.6. Promastigote Susceptibility Assay
Drug activity was determined by incubating promastigotes of the L. infantum strains in the presence of increasing miltefosine (2.5 to 200 µM) and amphotericin B (6.25 to 400 nM) concentrations. After 24 h incubation, viability was assessed by MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide) assay as previously described [23]. EC50 values were obtained by plotting optical density values measured by absorbance at 595 and 690 nm in nonlinear, dose response, sigmoidal models using the GraphPad Prism 6 program. At least three independent experiments were performed in triplicate and L. infantum NCL/IOCL3241 and L. donovani Ld15 strains were used as controls.
2.7. PCR Detection of the Presence/Absence of Miltefosine Sensitive Locus (MSL)
Leishmania strains were characterized as MSL- or MSL+ either by whole genome deep sequence analyses or by qPCR. Both methodologies and results are available at Schwabl, et al. [24]. Briefly, to obtain DNA, Leishmania strains were grown in biphasic NNN + Schneider’s medium until the end of the log phase, and genomic DNA was extracted using a commercial kit following the manufacturer’s instructions (Qiagen, Valencia, CA, USA). qPCR amplification of the MSL on chromosome 31 or whole genome sequencing was accomplished according to the strategies published [24].
2.8. Statistical Analysis
All statistical analyses were performed using Graph Pad Prism 6.0. Correlation between infectivity and EC50 of intracellular amastigotes was tested using Spearman’s correlation test. For the analysis of correlation between the presence/absence of MSL locus and EC50 to miltefosine, Mann Whitney test was used. Analysis of possible correlations between susceptibility and year of isolation were done using two-tailed unpaired t-test with Welch’s. Statistical significance was considered in all these cases for p-value under 0.05 (p < 0.05).
3. Results
The susceptibility to miltefosine was determined in 13 strains isolated before treatment from 10 humans and 3 dogs, from 5 different Brazilian states and in a control strain (NLC), that was included in these experiments as a reference. The EC50 determined in intracellular amastigotes varied from 1.41 to 4.57 μM between the strains (Figure 2A and Table 1). The EC50 obtained for the L. infantum reference strain NLC to miltefosine was 1.79 ± 0.42 μM (Table 1). Infectivity was also analyzed (Table 1), and correlation tests between EC50 of intracellular amastigotes and the percentage of infection revealed that these two variables were not correlated (Spearman’s correlation test: R = −0.2992 and p = 0.2965).
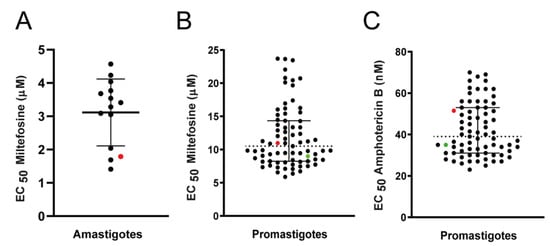
Figure 2.
Susceptibility of L. infantum strains to miltefosine. Black dots represent the EC50 determined for each clinical strain. The red and green dots represent the EC50 for L. infantum NLC/IOCL3241 (reference strain) and L. donovani LD-15, respectively, used as internal controls. Graphics show median EC50 (dotted line) and 25% and 75% percentiles (solid lines). (A) Susceptibility of L. infantum intracellular amastigotes to miltefosine, calculated from at least two independent experiments, each performed in triplicate. (B) Susceptibility of L. infantum promastigotes to miltefosine. The results shown are the mean of at least three independent experiments, each performed in triplicate. (C) Susceptibility of L. infantum promastigotes to amphotericin B; mean of two experiments performed in triplicate.

Table 1.
Descriptive analysis of Brazilian isolates and reference strains included in this study.
In previous studies that evaluated the susceptibility of Leishmania strains to miltefosine, we and others observed that, for this drug, there was a direct correlation between susceptibility in amastigotes and promastigotes [21,25]. As promastigote assays are more amenable for larger samples, sensitivity to miltefosine was determined for promastigotes in a total of 73 strains of L. infantum, including the 13 strains tested in intracellular amastigotes.
Sensitivity to miltefosine was determined for promastigotes in a total of 73 strains of L. infantum, sampled before treatment from 60 humans and 13 dogs. A reference strain was again used as an internal control for all experiments (strain NLC). The L. donovani strain LD-15 was also employed as an internal control (Figure 2B, Table 1). The EC50 for miltefosine in promastigotes varied from 5.89 to 23.7 μM (Figure 2B, Table 1). The EC50 for miltefosine in the L. infantum reference strain NLC and the L. donovani strain LD-15 were 10.98 ± 1.95 and 9.00 ± 0.80 µM respectively. Calculation of the activity indexes (ratio between the EC50 of the clinical strains and EC50 of the reference strain) revealed that the highest EC50 observed among the clinical strains was 2.16-fold higher than the EC50 of the NLC reference strain.
Sensitivity to amphotericin B was also determined in these strains, and a 2.8-fold variation between the lowest (25 nM) and the highest (70 nM) EC50 values was observed (Figure 2C, Table 1). The EC50 of the L. infantum reference strain NLC and of the L. donovani LD-15 strain to amphotericin were 51.00 ± 5.00 and 35.00 ± 0.50 nM, respectively.
Forty-nine strains (out of 73; 67%) were genotyped for the presence or absence of MSL, recently described and associated with the treatment outcome in Brazilian patients and L. infantum in vitro susceptibility to miltefosine [15,16]. Twenty strains (2 from dogs and 18 from humans) were positive (presented) for MSL and 29 (7 from dogs and 22 from humans) were deleted for the locus. No correlations were observed between the EC50 for miltefosine and the absence (MSL-) or presence (MSL+) of the locus in amastigotes (p = 0.2468; Median for MSL- parasites = 3.86 μM, and for MSL+ = 3.09 μM) (Figure 3A) or promastigotes (p = 0.2885; median for MSL- parasites = 10.51 μM, and for MSL+ = 11.25 μM) (Figure 3B).
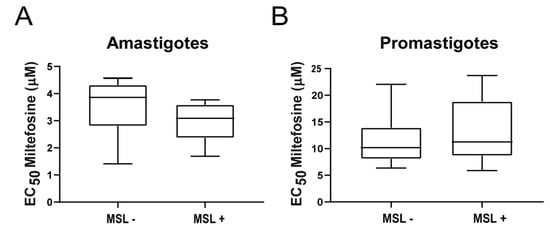
Figure 3.
Susceptibility to miltefosine in L. infantum strains presenting (MSL+) or not (MSL-) the MSL locus. EC50 was determined in amastigotes (A) or promastigotes (B) and tested for correlation with the genotypic analysis of MSL locus. The central line in the box-plot represents the median values of each analyzed group.
Statistical analysis was also conducted using only the human L. infantum strains from Piauí, Maranhão and Minas Gerais (amastigotes: 2MSL-; 5MSL+; promastigotes: 9MSL-, 18MSL+), aiming to mimic the parasite diversity analyzed previously [16], but even in this restricted population, no statistical differences were observed in the median EC50 of MSL- and MSL+ parasites (p = 0.3810 and p = 0.4948, for amastigotes and promastigotes, respectively) (Figure S1).
Analysis of possible correlations between susceptibility and year of isolation detected no significant trends. The same was true for samples isolated from humans and dogs (Figure 4). The analysis of correlation between EC50 and geographical origin was not reliable due to low representation for some states (Table 1).
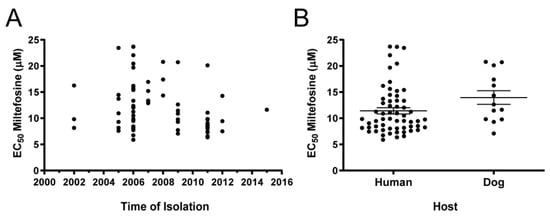
Figure 4.
Dispersion analysis of the EC50 for miltefosine determined in 73 Brazilian L. infantum strains according to (A) year of isolation and (B) provenance of host. Each black dot represents one clinical strain. In (B), the central lines represent mean EC50 and SEM of strains. No significant differences between samples originating from humans or dogs were observed (p = 0.0950 two-tailed unpaired t-test with Welch’s correction).
4. Discussion
Inadequate efficacy and serious side effects are major and widespread problems of VL treatment. The range of drugs for VL is narrow and fraught with limitations [26]. Miltefosine, though displaying some less than ideal characteristics such as potential teratogenicity, gastrointestinal side effects, and a long plasma half-life of 150–200 h [27], is and will remain for the next few years the only oral drug available for leishmaniasis treatment. Miltefosine also presents a series of advantages compared to the other available treatment options, and care must be taken to avoid the loss of this oral drug. Employed as monotherapy in the Indian subcontinent for several years and in combination therapy with paromomycin as the second line treatment in the framework of the Kala-azar Elimination program [28], miltefosine is not currently in use in Brazil for human visceral leishmaniasis [29,30].
Only one clinical trial to test miltefosine monotherapy for treatment of VL has been conducted in Brazil, with patients enrolled at Montes Claros and Teresina. An overall cure rate of 59% was documented in a sample of 42 treated patients. This study was carried out in two different locations and using two different treatment schedules. In all cases, adults were treated with a 100 mg miltefosine/day total dose, which did not necessarily correspond to the desired dose of 2.5 mg/kg/day [15]. The efficacy in this trial was lower than the 94% cure rate observed in India when miltefosine was implemented [31] and the 90% cure rate observed after a decade of use [29].
Differences in efficacy of the same drug or treatment schedule found in different geographical areas have been demonstrated, but there is no clear understanding of the reasons for this, even given the differences between L. donovani and L. infantum parasite species [28]. This information, and the results obtained in the Brazilian miltefosine trial, raised concerns about the possibility of Brazilian L. infantum being intrinsically tolerant to miltefosine. In this context, we gathered efforts to investigate the susceptibility to miltefosine of a panel of clinical strains of L. infantum from different geographic regions of Brazil.
The susceptibility to miltefosine of 13 clinical strains was determined for intracellular amastigotes. The EC50 varied from 1.41 to 4.57 μM, or 3.2-fold, between the least and the most susceptible isolates. The EC50 values in these isolates were strikingly homogenous compared to, for example, Brazilian L. (V.) braziliensis clinical isolates, which presented 6- and 15-fold differences in the EC50 between the most and the least susceptible parasites, in promastigotes and amastigotes, respectively [21]. This low variability was also reported by Faral-Tello, et al. [32] that evaluated the infectivity and susceptibility of 5 L. infantum clinical strains to leishmanicidal drugs including miltefosine and found EC50 values that varied from 3.4 to 5.3 μM.
The characterization of susceptibility to miltefosine in a total of 73 strains was done in cultured promastigotes. The promastigote model is less laborious and eliminates the need of animals for macrophage isolation. The susceptibility to amphotericin B was also determined to widen the scope of the study and also to serve as an independent variable in case a heterogeneous behavior toward miltefosine was discovered. The analysis in promastigotes confirmed a low degree of variation (5.89 to 23.7 μM), when compared to the differences found among L. braziliensis clinical strains (22.9 to 144.2 μM) [21].
The evaluation of a small percentage of strains in the amastigote stage is a limitation of this study. A correlation between miltefosine susceptibility in promastigotes and amastigotes of L. braziliensis [21] and L. donovani [25] had already been demonstrated. This correlation was not present among the 13 strains in this study and we hypothesize this could be due to the low variability in the EC50 values determined. However, it would be interesting to widen the spectrum of strains tested as amastigotes in the future. Another aspect that remains to be addressed in future studies is to improve the distribution of strains between the different geographical areas and continuing the evaluation with samples collected more recently. In our sample, some states and samples isolated after 2013 were underrepresented.
The analysis in promastigotes, confirmed a low degree of variation (5.89 to 23.7 μM), when compared to the differences found among L. braziliensis clinical strains (22.9 to 144.2 μM) [21].
Recently Carnielli, et al. [16] reported that a deletion of four genes on chromosome 31 found by whole genome sequencing of 26 L. infantum strains isolated from cured and relapsed patients from two Brazilian endemic regions was correlated to the treatment outcome. An association between the presence of this locus and a positive response to miltefosine treatment was found, and the genomic region was named miltefosine sensitive locus (MSL) [16]. The susceptibility profile of intracellular amastigotes from strains isolated from patients that relapsed or were cured in the Montes Claros/Teresina clinical trial [15] identified a mean EC50 in strains from cured patients of approximately 5 μM whereas for relapsed patients this value was approximately 13 μM. The threshold for predicting treatment outcome was calculated as 8 μM.
In our sample, all the strains presented miltefosine EC50 against amastigotes below 5 μM. Due to the high prevalence of MSL deletion in L. infantum strains in the Americas [24] more than 60% of the strains studied were genotyped for the presence or absence of the MSL following the previously published protocol [24]. The prevalence of MSL deletion detected in the present study (59%) was in agreement with that found previously (54%) [16]. However, no associations were found between L. infantum in vitro susceptibility to miltefosine and the presence/absence of the MSL after testing 49 strains from 11 localities. This lack of association was still observed when the L. infantum population analyzed was reduced to geographically mimic the sample previously studied (only from Minas Gerais, Piauí and Maranhão).
Even with the relative homogeneity in the determined miltefosine EC50 found in our sample, an important limitation of our study is that the evaluation of in vitro susceptibility does not immediately correlate with the clinical efficacy. Nevertheless, infections of BALB/c mice with the reference strain NLC were successfully treated with 20 mg/kg/day miltefosine for 5 days. When followed up for 4 weeks, these mice showed complete resolution of clinical disease and absence of parasites (M. Galuppo and S. Uliana, personal communication) which supports interpreting the results of the present study as indicating the overall susceptibility to miltefosine of the 73 isolates.
The lack of concordance between data from this and the previous study might reflect differences in methodology, the number of in vitro passages of parasites used, or differences in the populations studied, meaning that there is a need to extend the analysis to a wider sample of strains. However, it is worth considering whether the high failure rates observed in the previous Brazilian clinical trial might have been due to factors other than parasite susceptibility. In the case of L. donovani VL, it has been shown that the exposure to miltefosine is lower in children than in adults; indeed, by using an allometric dose in children, efficacy increases from 59% to 90% [19]. Further studies performed to understand the pharmacokinetics and pharmacodynamics of miltefosine in different populations found that the probability of VL relapse was strongly correlated with the time during which the plasmatic concentrations of miltefosine were higher than the EC90 values or above 10 × EC50 [20]. In African patients, the 2.5 mg/kg/day for 28 days schedule resulted in concentrations above the desired threshold for 51.4 days (CI 30.5–77.1). To analyze the likelihood of failure, the authors employed miltefosine EC50 and EC90 for L. donovani intracellular amastigotes of 4.95 and 25.9 µM, respectively [17]. These values are comparable to the median EC50 values for L. infantum clinical isolates found in the present study. If pharmacokinetic and pharmacodynamics characteristics in Brazilian patients are comparable to those previously characterized in Nepal and Africa, it is likely that the necessary plasmatic concentrations will be achieved upon use of allometric dosing.
In conclusion, this study indicates that isolates of L. infantum obtained over a wide geographical area in Brazil do not appear to be significantly heterogeneous in their susceptibility to miltefosine. Further clinical evaluation of miltefosine for treatment of VL in Brazil is advisable, using an allometric dose in children and associated with pharmacokinetic studies, possibly in combination with other leishmanicidal drugs, in order to evaluate the possibility of incorporating this drug into the therapeutic arsenal for VL treatment in Brazil.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9061228/s1. Figure S1: Susceptibility to miltefosine in L. infantum strains presenting (MSL+) or not (MSL-) the MSL locus.
Author Contributions
Conceptualization, E.C., C.H.N.C., S.R.B.U., J.R., J.A.; methodology, C.R.E., E.V.d.C.L., M.C.B.,E.C., C.H.N.C., S.R.B.U.; validation, C.R.E., E.V.d.C.L., M.C.B.; formal analysis, C.R.E., S.R.B.U.; investigation, C.R.E., E.V.d.C.L., M.C.B.; resources, E.C., C.H.N.C., S.R.B.U.; data curation, C.R.E., S.R.B.U.; Writing—Original Draft Preparation, C.R.E., E.C., S.R.B.U., J.R.; Writing—review & editing, C.R.E., E.V.d.C.L., M.C.B., D.L., J.A., E.C., C.H.N.C., J.R., S.R.B.U.; visualization, C.R.E., S.R.B.U.; supervision, E.C., C.H.N.C., S.R.B.U., J.R., J.A.; project administration, E.C., C.H.N.C., S.R.B.U., J.R.; funding acquisition, E.C., C.H.N.C., S.R.B.U. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Drugs for Neglected Diseases initiative—http://www.dndi.org. DNDi would like to thank the following donors for their support to this work: Associação Bem-Te-Vi Diversidade, Brazil; UK aid, UK; and Dutch Ministry of Foreign Affairs (DGIS), the Netherlands. DNDi also thanks Médecins sans Frontières and the Swiss Agency for Development and Cooperation (SDC), Switzerland for supporting its overall mission. This research was funded by grants from CNPq -National Council for Scientific and Technological Development (CNPq fellows—302622/2017-9 and 306971/2018-6), FAPERJ—Carlos Chagas Filho Research Foundation of Rio de Janeiro State (E26/202.569/2019), PAEF-IOC-FIOCRUZ, Oswaldo Cruz Foundation (Support for Biological Collections), FAPESP—Fundação de Apoio à Pesquisa do Estado de São Paulo (2015/09080-2 and 2016/23405-4) and Capes—Brazilian Federal Agency for Support and Evaluation of Graduate Education (Finance code 001)—Programa Institucional de Internacionalização (PrInt). S.R.B.U. is the recipient of a senior researcher scholarship from CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Boards of Instituto Oswaldo Cruz (CAAE: 76599317.5.0000.5248, approved 11 October 2017), Biomedical Science Institute São Paulo University (CAAE 76599317.5.3002.5467) and Federal University of Piauí (CAAE 76599317.5.3001.5214). Animals used in this study to obtain bone marrow-derived macrophages were treated in accordance with the regulations of the Brazilian Society of Science in Laboratory Animals (SBCAL) and the National Council for Animal Experiment Control (CONSEA), having obtained the necessary approval from the institutional ethical committee for animal use (ICB–USP 178-12-CEUA, June 19, 2015).
Informed Consent Statement
Patient consent was waived by the Institutional Review Boards because parasites used in this work were available at the IOC Culture Collection. Part of the sample had been obtained from patients participating in research projects approved by an Ethical Committee and part was from parasites isolated as part of routine diagnostic procedures in humans or dogs. These latter samples were not attached to patient information beyond clinical form of the disease and geographical origin.
Data Availability Statement
The data presented in this study are available in the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- WHO. Leishmaniasis. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 22 February 2021).
- Pan American Health Organization. Leishmaniasis: Epidemiological Report in the Americas; PAHO: Washington, DC, USA, December 2020; Available online: https://iris.paho.org/handle/10665.2/51742 (accessed on 25 March 2021).
- Brasil, Ministério da Saúde. Letalidade de Leishmaniose Visceral: Brasil, Grandes Regiões e Unidades Federadas 2000 a 2019. Available online: https://antigo.saude.gov.br/images/pdf/2020/August/25/LV-Letalidade.pdf (accessed on 25 March 2021).
- Lindoso, J.A.; Cota, G.F.; da Cruz, A.M.; Goto, H.; Maia-Elkhoury, A.N.; Romero, G.A.; de Sousa-Gomes, M.L.; Santos-Oliveira, J.R.; Rabello, A. Visceral leishmaniasis and HIV coinfection in Latin America. PLoS Negl. Trop. Dis. 2014, 8, e3136. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Leishmaniose Visceral: Recomendações Clínicas Para Redução da Letalidade. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/leishmaniose_visceral_reducao_letalidade.pdf (accessed on 22 March 2021).
- Romero, G.A.S.; Costa, D.L.; Costa, C.H.N.; de Almeida, R.P.; de Melo, E.V.; de Carvalho, S.F.G.; Rabello, A.; de Carvalho, A.L.; Sousa, A.Q.; Leite, R.D.; et al. Efficacy and safety of available treatments for visceral leishmaniasis in Brazil: A multicenter, randomized, open label trial. PLoS Negl. Trop. Dis. 2017, 11, e0005706. [Google Scholar] [CrossRef]
- Bern, C.; Adler-Moore, J.; Berenguer, J.; Boelaert, M.; den Boer, M.; Davidson, R.N.; Figueras, C.; Gradoni, L.; Kafetzis, D.A.; Ritmeijer, K.; et al. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clinical Infectious Diseases 2006, 43, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Balasegaram, M.; Ritmeijer, K.; Lima, M.A.; Burza, S.; Ortiz Genovese, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin. Emerg. Drugs 2012, 17, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Agrawal, G.; Rai, M.; Makharia, M.K.; Murray, H.W. Treatment of Indian visceral leishmaniasis with single or daily infusions of low dose liposomal amphotericin B: Randomised trial. BMJ (Clin. Res. Ed.) 2001, 323, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Mehta, H.; Suresh, A.V.; Singh, S.P.; Rai, M.; Murray, H.W. Amphotericin B treatment for Indian visceral leishmaniasis: Conventional versus lipid formulations. Clin. Infect. Dis. 2004, 38, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N. Engl. J. Med. 2010, 362, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Rai, M.; Chakravarty, J.; Agarwal, D.; Agrawal, N.; Vaillant, M.; Olliaro, P.; Murray, H.W. New treatment approach in Indian visceral leishmaniasis: Single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin. Infect. Dis. 2008, 47, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.N.; di Martino, L.; Gradoni, L.; Giacchino, R.; Gaeta, G.B.; Pempinello, R.; Scotti, S.; Cascio, A.; Castagnola, E.; Maisto, A.; et al. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome). Clin. Infect. Dis. 1996, 22, 938–943. [Google Scholar] [CrossRef]
- Syriopoulou, V.; Daikos, G.L.; Theodoridou, M.; Pavlopoulou, I.; Manolaki, A.G.; Sereti, E.; Karamboula, A.; Papathanasiou, D.; Krikos, X.; Saroglou, G. Two doses of a lipid formulation of amphotericin B for the treatment of Mediterranean visceral leishmaniasis. Clin. Infect. Dis. 2003, 36, 560–566. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Monti-Rocha, R.; Costa, D.L.; Molina Sesana, A.; Pansini, L.N.N.; Segatto, M.; Mottram, J.C.; Costa, C.H.N.; Carvalho, S.F.G.; Dietze, R. Natural Resistance of Leishmania infantum to Miltefosine Contributes to the Low Efficacy in the Treatment of Visceral Leishmaniasis in Brazil. Am. J. Trop. Med. Hyg. 2019, 101, 789–794. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Crouch, K.; Forrester, S.; Silva, V.C.; Carvalho, S.F.G.; Damasceno, J.D.; Brown, E.; Dickens, N.J.; Costa, D.L.; Costa, C.H.N.; et al. A Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. EBioMedicine 2018, 36, 83–91. [Google Scholar] [CrossRef]
- Dorlo, T.P.C.; Kip, A.E.; Younis, B.M.; Ellis, S.J.; Alves, F.; Beijnen, J.H.; Njenga, S.; Kirigi, G.; Hailu, A.; Olobo, J.; et al. Visceral leishmaniasis relapse hazard is linked to reduced miltefosine exposure in patients from Eastern Africa: A population pharmacokinetic/pharmacodynamic study. J. Antimicrob. Chemother. 2017, 72, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Huitema, A.D.; Beijnen, J.H.; de Vries, P.J. Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. J. Antimicrob. Chemother. 2012, 56, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Mbui, J.; Olobo, J.; Omollo, R.; Solomos, A.; Kip, A.E.; Kirigi, G.; Sagaki, P.; Kimutai, R.; Were, L.; Omollo, T.; et al. Pharmacokinetics, Safety, and Efficacy of an Allometric Miltefosine Regimen for the Treatment of Visceral Leishmaniasis in Eastern African Children: An Open-label, Phase II Clinical Trial. Clin. Infect. Dis. 2019, 68, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Rijal, S.; Ostyn, B.; de Vries, P.J.; Singh, R.; Bhattarai, N.; Uranw, S.; Dujardin, J.C.; Boelaert, M.; Beijnen, J.H.; et al. Failure of Miltefosine in Visceral Leishmaniasis Is Associated with Low Drug Exposure. J. Infect. Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Espada, C.R.; Ribeiro-Dias, F.; Dorta, M.L.; de Araujo Pereira, L.I.; de Carvalho, E.M.; Machado, P.R.; Yokoyama-Yasunaka, J.K.; Coelho, A.C.; Uliana, S.R. Susceptibility to Miltefosine in Brazilian Clinical Isolates of Leishmania (Viannia) braziliensis. Am. J. Trop. Med. Hyg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde. Leishmaniose Visceral: O Que é, Causas, Sintomas, Tratamento, Diagnóstico e Prevenção. Available online: https://antigo.saude.gov.br/images/pdf/2020/August/25/LV-Gr--ficos-e-Mapas.pdf (accessed on 25 March 2021).
- Zauli-Nascimento, R.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Pereira, L.I.; Pelli de Oliveira, M.A.; Ribeiro-Dias, F.; Dorta, M.L.; Uliana, S.R. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop. Med. Int. Health 2010, 15, 68–76. [Google Scholar] [CrossRef]
- Schwabl, P.; Boité, M.C.; Bussotti, G.; Jacobs, A.; Andersson, B.; Moreira, O.; Freitas-Mesquita, A.L.; Meyer-Fernandes, J.R.; Telleria, E.L.; Traub-Csekö, Y.; et al. Colonization and genetic diversification processes of Leishmania infantum in the Americas. Commun. Biol. 2021, 4, 139. [Google Scholar] [CrossRef]
- Kumar, D.; Kulshrestha, A.; Singh, R.; Salotra, P. In vitro susceptibility of field isolates of Leishmania donovani to Miltefosine and amphotericin B: Correlation with sodium antimony gluconate susceptibility and implications for treatment in areas of endemicity. Antimicrob. Agents Chemother. 2009, 53, 835–838. [Google Scholar] [CrossRef]
- Alvar, J.; Croft, S.; Olliaro, P. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 2006, 61, 223–274. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Bilbe, G.; Blesson, S.; Goyal, V.; Monnerat, S.; Mowbray, C.; Muthoni Ouattara, G.; Pécoul, B.; Rijal, S.; Rode, J.; et al. Recent Development of Visceral Leishmaniasis Treatments: Successes, Pitfalls, and Perspectives. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A.; Rai, M.; Prajapati, V.K.; Singh, A.K.; Ostyn, B.; Boelaert, M.; Dujardin, J.C.; Chakravarty, J. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infect. Dis. 2012, 55, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing failure of miltefosine in the treatment of kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Engel, J.; Sindermann, H.; Fischer, C.; Junge, K.; Bryceson, A.; Berman, J. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 2002, 347, 1739–1746. [Google Scholar] [CrossRef]
- Faral-Tello, P.; Greif, G.; Satragno, D.; Basmadjián, Y.; Robello, C. Leishmania infantum isolates exhibit high infectivity and reduced susceptibility to amphotericin B. RSC Med. Chem. 2020, 11, 913–918. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).