Assessment of the Impact of Temperature on Biofilm Composition with a Laboratory Heat Exchanger Module
Abstract
1. Introduction
2. Materials and Methods
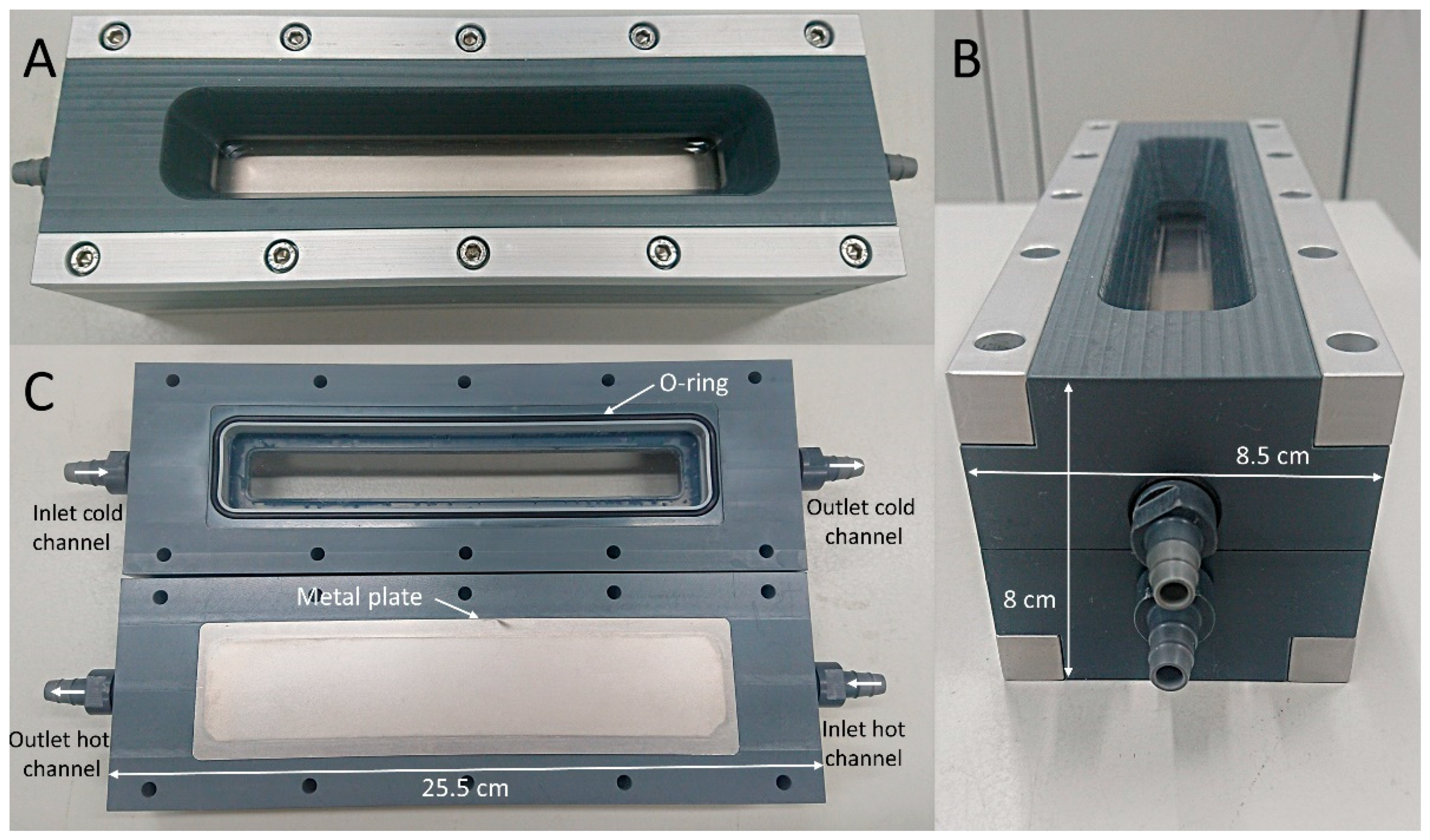
2.1. Laboratory Heat Exchanger Module
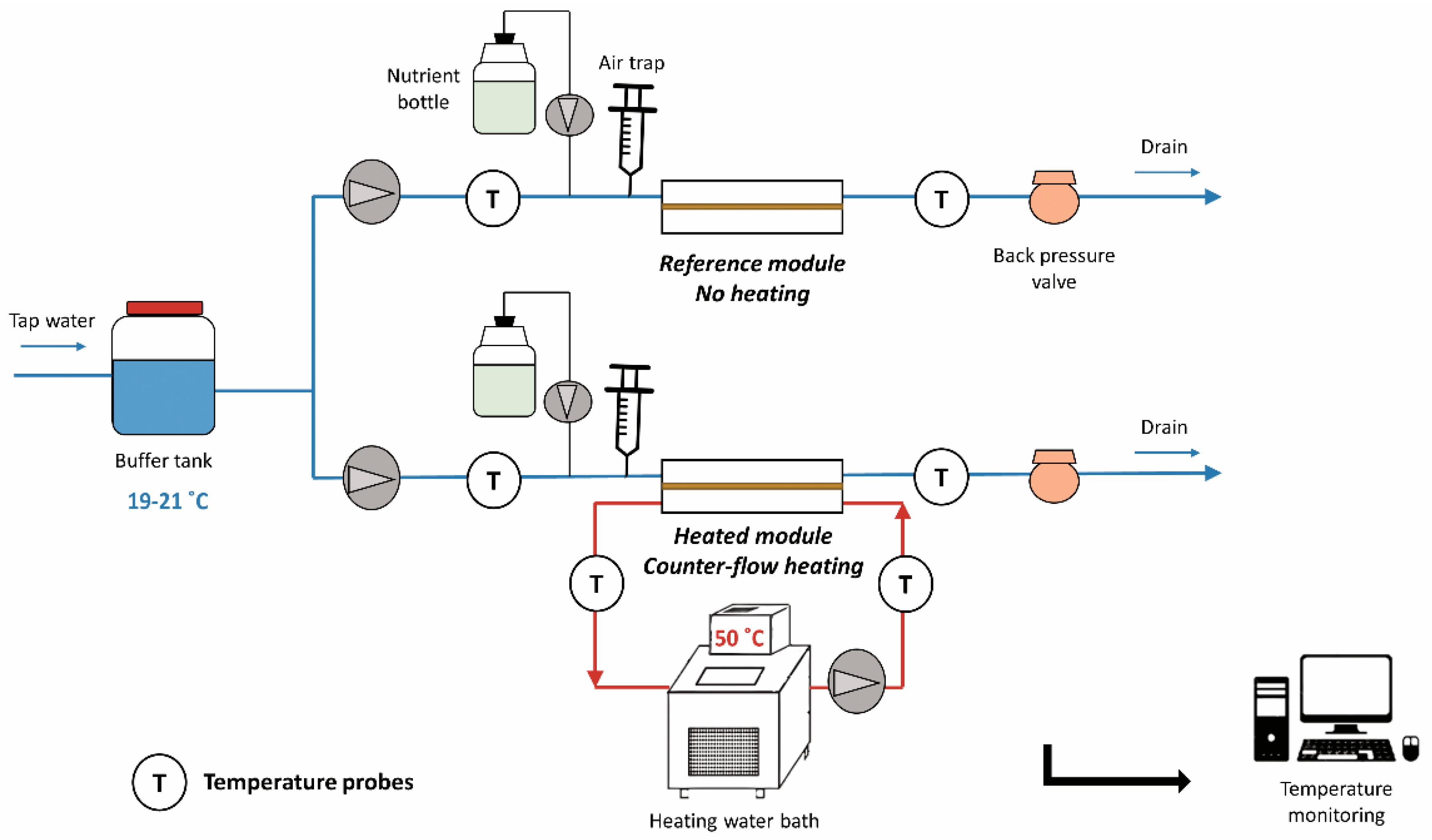
2.2. Experimental Design
2.3. Temperature Monitoring
2.4. Biofilm Collection
2.5. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.6. EPS Extraction
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Polysaccharide Quantification
2.9. Protein Quantification
3. Results
3.1. Operating Parameters
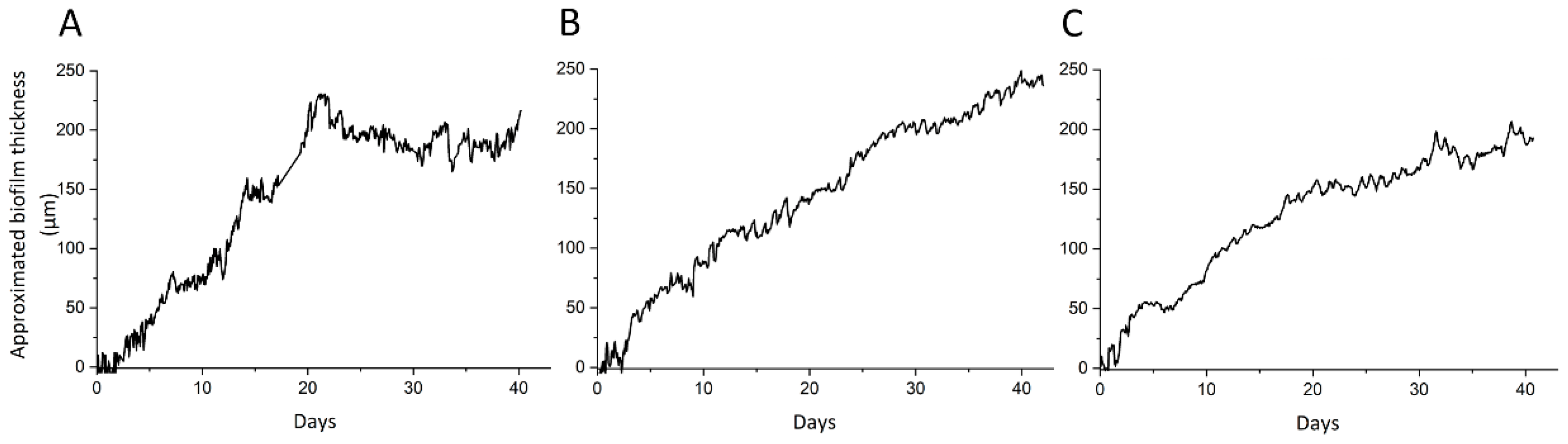
3.2. Monitoring of Biofilm Growth in the Heated Module
3.3. Biofilm Characterization
3.3.1. Bacterial Community Structure along the Heat Exchanger
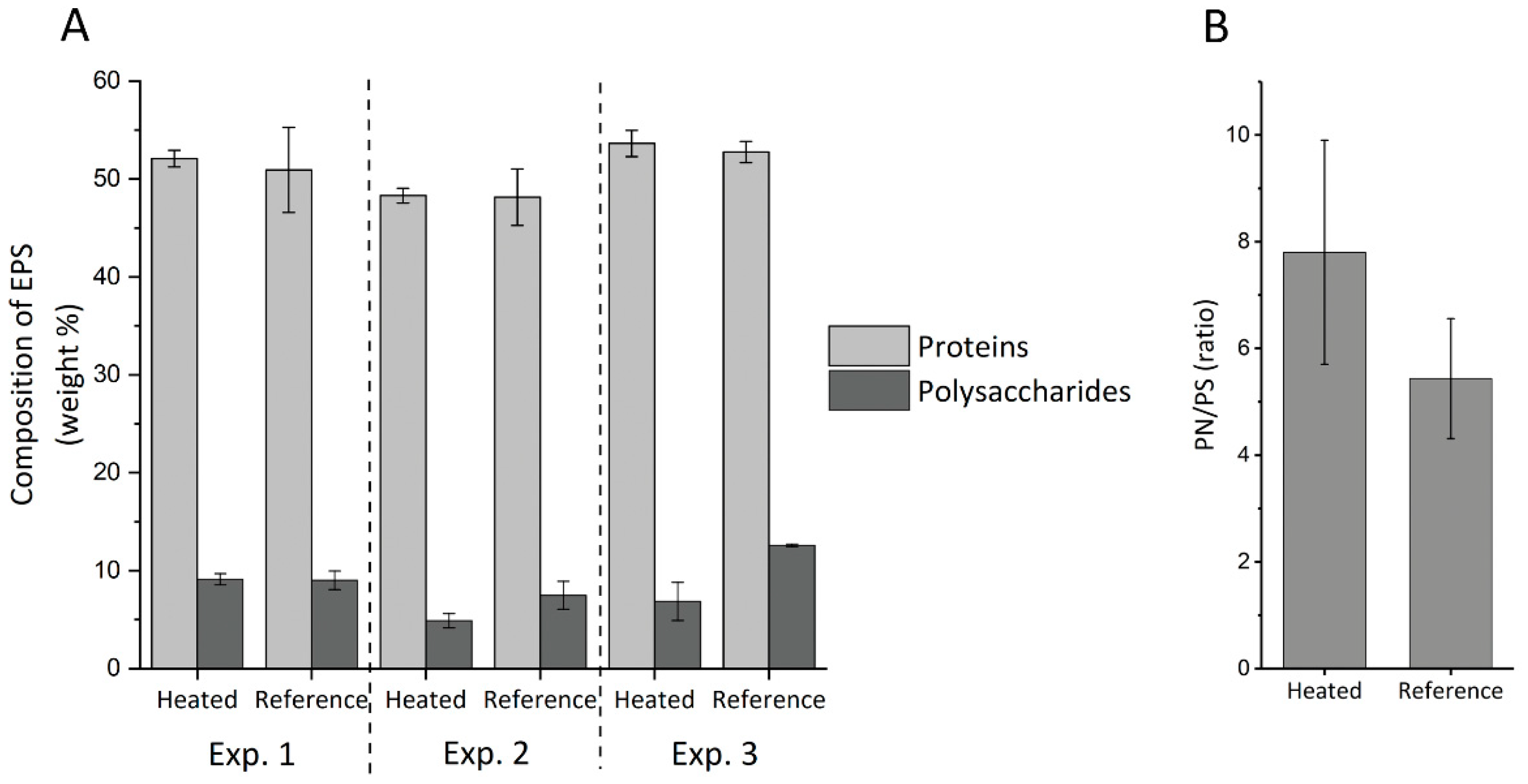
3.3.2. EPS Amounts
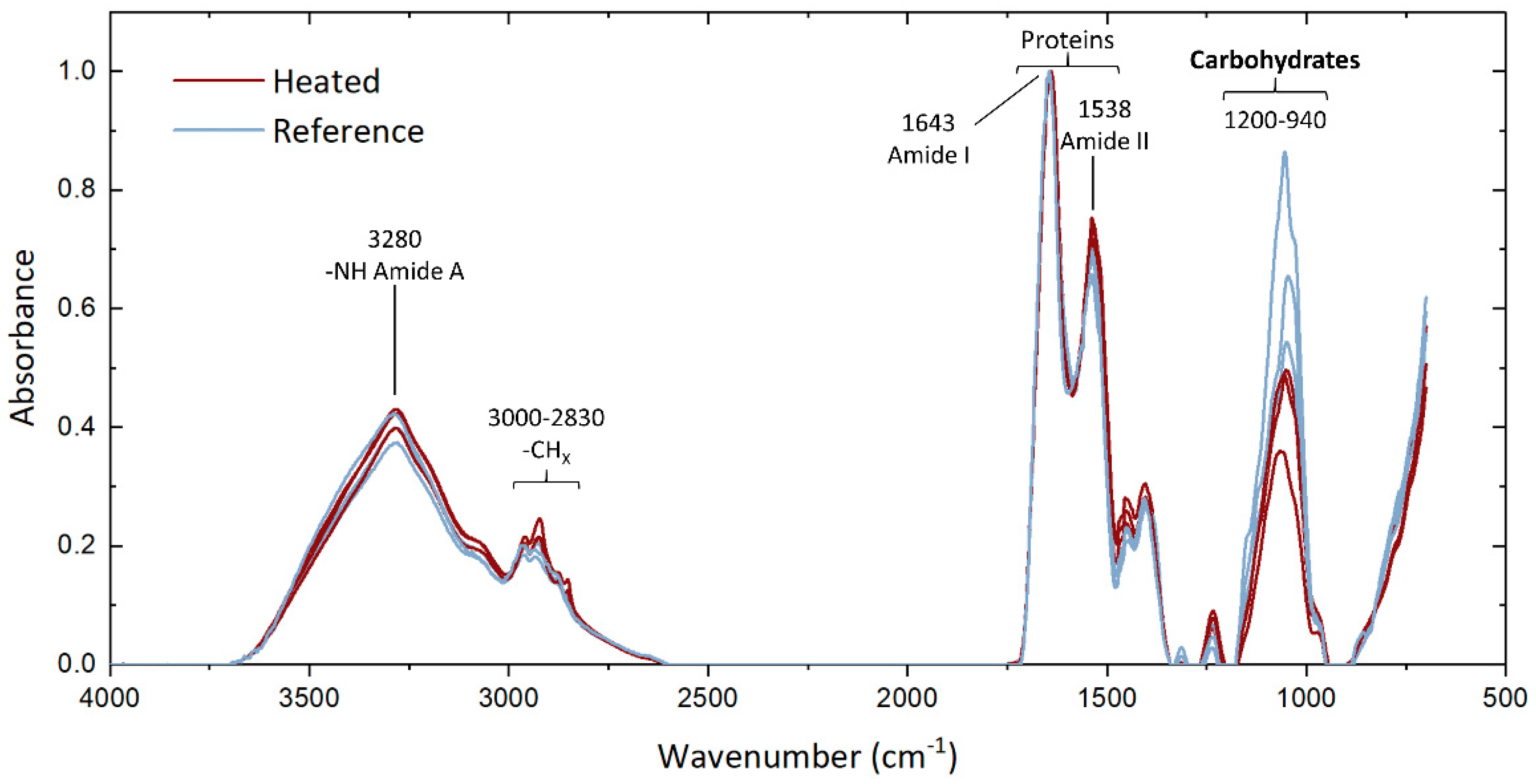
3.3.3. EPS Characterization
4. Discussion
4.1. Impact of Temperature on Biofilm Composition
4.2. Advantages and Limitations of the Heat Exchanger Laboratory Module
4.3. Potential Applications in Future Research
5. Conclusions
- the developed plate heat exchanger was able to monitor online biofilm growth by measuring its resistance to heat transfer;
- the laboratory module is suitable for a large range of applications related to the effect of a thermal field, such as bacterial community identification through the height of the biofilm, composition and morphology of EPS at various temperatures, or microbial corrosion investigations.
- comparable amounts of biofilm and accumulated EPS formed in the non-heated and heated systems over the 40-day experiments;
- differences in proteins-to-polysaccharides ratio in extracellular polymeric substances caused by the thermal field, with a lower production of polysaccharides at elevated temperature;
- differences in biofilm bacterial groups resulting from the temperature change at the surface of the plate.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, G.B.; Pring, E.; Osborn, P.D. Cooling Towers: Principles and Practice; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- European Commission. Reference Document on the Application of Best Available Techniques to Industrial Cooling Systems. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/cvs_bref_1201.pdf (accessed on 1 January 2021).
- Balamurugan, P.; Hiren Joshi, M.; Rao, T.S. Microbial fouling community analysis of the cooling water system of a nuclear test reactor with emphasis on sulphate reducing bacteria. Biofouling 2011, 27, 967–978. [Google Scholar] [CrossRef]
- Rao, T.S.; Kora, A.J.; Anupkumar, B.; Narasimhan, S.V.; Feser, R. Pitting corrosion of titanium by a freshwater strain of sulphate reducing bacteria (Desulfovibrio vulgaris). Corros. Sci. 2005, 47, 1071–1084. [Google Scholar] [CrossRef]
- Bott, T.R. Fouling of Heat Exchangers; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Melo, L.; Bott, T.; Fletcher, M.; Capdeville, B. Biofilms—Science and Technology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 223. [Google Scholar]
- Characklis, W.G.; Nevimons, M.; Picologlou, B. Influence of fouling biofilms on heat transfer. Heat Transf. Eng. 1981, 3, 23–37. [Google Scholar] [CrossRef]
- Cooksey, K. Extracellular polymers in biofilms. In Biofilms—Science and Technology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 137–147. [Google Scholar]
- Chang, S.; Chen, J.; Shi, L. Using thermal shock to inhibit biofilm formation in the treated sewage source heat pump systems. Appl. Sci. 2017, 7, 343. [Google Scholar] [CrossRef]
- García, S.; Trueba, A.; Vega, L.M.; Madariaga, E. Impact of the surface roughness of AISI 316L stainless steel on biofilm adhesion in a seawater-cooled tubular heat exchanger-condenser. Biofouling 2016, 32, 1185–1193. [Google Scholar] [CrossRef]
- Janknecht, P.; Melo, L.F. Online biofilm monitoring. Rev. Environ. Sci. Biotechnol. 2003, 2, 269–283. [Google Scholar] [CrossRef]
- Tian, L.; Chen, X.D.; Yang, Q.P.; Chen, J.C.; Li, Q.; Shi, L. Effect of silica dioxide particles on the evolution of biofouling by Bacillus subtilis in plate heat exchangers relevant to a heat pump system used with treated sewage. Chem. Eng. J. 2012, 188, 47–56. [Google Scholar] [CrossRef]
- Ahmad, J.I.; Dignum, M.; Liu, G.; Medema, G.; van der Hoek, J.P. Changes in biofilm composition and microbial water quality in drinking water distribution systems by temperature increase induced through thermal energy recovery. Environ. Res. 2021, 194, 110648. [Google Scholar] [CrossRef]
- Farhat, N.M.; Vrouwenvelder, J.S.; Van Loosdrecht, M.C.M.; Bucs, S.S.; Staal, M. Effect of water temperature on biofouling development in reverse osmosis membrane systems. Water Res. 2016, 103, 149–159. [Google Scholar] [CrossRef]
- Obana, N.; Nakamura, K.; Nomura, N. Temperature-regulated heterogeneous extracellular matrix gene expression defines biofilm morphology in Clostridium perfringens. NPJ Biofilms Microbiomes 2020, 6, 29. [Google Scholar] [CrossRef]
- Yang, Q.; Wilson, D.I.; Chen, X.; Shi, L. Experimental investigation of interactions between the temperature field and biofouling in a synthetic treated sewage stream. Biofouling 2013, 29, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zaid, M. A fouling evaluation system for industrial heat transfer equipment subject to fouling. Int. Commun. Heat Mass Transf. 2000, 27, 815–824. [Google Scholar] [CrossRef]
- Ling, A.C.; Lund, D.B. Apparatus for studying fouling of heated surfaces by biological fluids. J. Food Sci. 1978, 43, 390–403. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Xia, L.; Zhang, Y. A biofouling thermal resistance model with a growth term of surface biofilm on heat transfer surface. Int. J. Therm. Sci. 2020, 106699. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Bakker, S.M.; Wessels, L.P.; van Paassen, J.A.M. The Membrane Fouling Simulator as a new tool for biofouling control of spiral-wound membranes. Desalination 2007, 204, 170–174. [Google Scholar] [CrossRef]
- Siddiqui, A.; Pinel, I.; Prest, E.; Bucs, S.; van Loosdrecht, M.; Kruithof, J.; Vrouwenvelder, J.S. Application of DBNPA dosage for biofouling control in spiral wound membrane systems. Desalination Water Treat. 2017, 68, 12–22. [Google Scholar] [CrossRef]
- Schloss, P.D.; Hadelsman, J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Opens external link in new window. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.; Lin, Y.M. Extraction of structural extracellular polymeric substances from aerobic granular sludge. J. Vis. Exp. 2016, 115, e54534. [Google Scholar] [CrossRef]
- Pinel, I.S.M.; Kim, L.H.; Proença Borges, V.R.; Farhat, N.M.; Witkamp, G.-J.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Effect of phosphate availability on biofilm formation in cooling towers. Biofouling 2020, 36, 800–815. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Felz, S.; Vermeulen, P.; van Loosdrecht, M.C.; Lin, Y.M. Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Boleij, M.; Seviour, T.; Wong, L.L.; van Loosdrecht, M.C.M.; Lin, Y. Solubilization and characterization of extracellular proteins from anammox granular sludge. Water Res. 2019, 164, 114952. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, H.-Y.; Lv, M.-L.; Kong, Y.; Yu, Y.-W.; Xu, X.-Y. Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM technologies. Bioresour. Technol. 2012, 124, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Whitaker, R.; LeChevallier, M.W.; Liu, W.-T. Drinking water microbiome assembly induced by water stagnation. Isme J. 2018, 12, 1520–1531. [Google Scholar] [CrossRef]
- Prest, E.I.; Weissbrodt, D.G.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Long-term bacterial dynamics in a full-scale drinking water distribution system. PLoS ONE 2016, 11, e0164445. [Google Scholar] [CrossRef] [PubMed]
- Zlatanović, L.; van der Hoek, J.P.; Vreeburg, J.H.G. An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Res. 2017, 123, 761–772. [Google Scholar] [CrossRef]
- Vandermaesen, J.; Lievens, B.; Springael, D. Isolation and identification of culturable bacteria, capable of heterotrophic growth, from rapid sand filters of drinking water treatment plants. Res. Microbiol. 2017, 168, 594–607. [Google Scholar] [CrossRef]
- Manz, W.; Kalmbach, S.; Szewzyk, U.; Incertae Sedis, I. Aquabacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.S.; Hugunin, K.M.; Maute, C.J.; Dysko, R.C. Bacteria from drinking water supply and their fate in gastrointestinal tracts of germ-free mice: A phylogenetic comparison study. Water Res. 2010, 44, 5050–5058. [Google Scholar] [CrossRef]
- Vandamme, P.; Coenye, T. Taxonomy of the genus Cupriavidus: A tale of lost and found. Int. J. Syst. Evol. Microbiol. 2004, 54, 2285–2289. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Kämpfer, P.; De Baere, T.; Falsen, E.; Verschraegen, G. Wautersia gen. nov., a novel genus accommodating the phylogenetic lineage including Ralstonia eutropha and related species, and proposal of Ralstonia [Pseudomonas] syzygii (Roberts et al. 1990) comb. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef]
- Rao, T.S.; Kora, A.J.; Chandramohan, P.; Panigrahi, B.S.; Narasimhan, S.V. Biofouling and microbial corrosion problem in the thermo-fluid heat exchanger and cooling water system of a nuclear test reactor. Biofouling 2009, 25, 581–591. [Google Scholar] [CrossRef]
- Baek, Y.; Yu, J.; Kim, S.-H.; Lee, S.; Yoon, J. Effect of surface properties of reverse osmosis membranes on biofouling occurrence under filtration conditions. J. Membr. Sci. 2011, 382, 91–99. [Google Scholar] [CrossRef]
- Chamberland, J.; Messier, T.; Dugat-Bony, E.; Lessard, M.-H.; Labrie, S.; Doyen, A.; Pouliot, Y. Influence of feed temperature to biofouling of ultrafiltration membrane during skim milk processing. Int. Dairy J. 2019, 93, 99–105. [Google Scholar] [CrossRef]
- Casey, E.; Glennon, B.; Hamer, G. Biofilm development in a membrane-aerated biofilm reactor: Effect of flow velocity on performance. Biotechnol. Bioeng. 2000, 67, 476–486. [Google Scholar] [CrossRef]
- Paul, E.; Ochoa, J.C.; Pechaud, Y.; Liu, Y.; Liné, A. Effect of shear stress and growth conditions on detachment and physical properties of biofilms. Water Res. 2012, 46, 5499–5508. [Google Scholar] [CrossRef] [PubMed]
- Almstrand, R.; Daims, H.; Persson, F.; Sörensson, F.; Hermansson, M. New methods for analysis of spatial distribution and coaggregation of microbial populations in complex biofilms. Appl. Environ. Microbiol. 2013, 79, 5978–5987. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Diffusion in biofilms. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Recupido, F.; Toscano, G.; Tatè, R.; Petala, M.; Caserta, S.; Karapantsios, T.D.; Guido, S. The role of flow in bacterial biofilm morphology and wetting properties. Colloids Surf. B Biointerfaces 2020, 192, 111047. [Google Scholar] [CrossRef]
- Wagner, M.; Taherzadeh, D.; Haisch, C.; Horn, H. Investigation of the mesoscale structure and volumetric features of biofilms using optical coherence tomography. Biotechnol. Bioeng. 2010, 107, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Microbiological-influenced corrosion failure of a heat exchanger tube of a fertilizer plant. J. Fail. Anal. Prev. 2014, 14, 314–317. [Google Scholar] [CrossRef]
- Little, B.J.; Hinks, J.; Blackwood, D.J. Microbially influenced corrosion: Towards an interdisciplinary perspective on mechanisms. Int. Biodeterior. Biodegrad. 2020, 154, 105062. [Google Scholar] [CrossRef]

| Module | Temperature Hot Channel (°C) | Temperature Cold Channel (°C) | Flow Rate Hot Channel (L/h) | Flow Rate Cold Channel (L/h) | Heat Loss (%) | ||
|---|---|---|---|---|---|---|---|
| Inlet | Outlet | Inlet | Outlet | ||||
| Reference | r.t. * | r.t. * | 20.0 ± 1.4 | 20.1 ± 1.2 | 0 | 11.4 | approx. 0 |
| Heated | 50.4 ± 0.2 | 49.3 ± 0.2 | 20.1 ± 1.3 | 27.3 ± 1.3 | 108 | 11.4 | 27 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinel, I.; Biškauskaitė, R.; Pal’ová, E.; Vrouwenvelder, H.; van Loosdrecht, M. Assessment of the Impact of Temperature on Biofilm Composition with a Laboratory Heat Exchanger Module. Microorganisms 2021, 9, 1185. https://doi.org/10.3390/microorganisms9061185
Pinel I, Biškauskaitė R, Pal’ová E, Vrouwenvelder H, van Loosdrecht M. Assessment of the Impact of Temperature on Biofilm Composition with a Laboratory Heat Exchanger Module. Microorganisms. 2021; 9(6):1185. https://doi.org/10.3390/microorganisms9061185
Chicago/Turabian StylePinel, Ingrid, Renata Biškauskaitė, Ema Pal’ová, Hans Vrouwenvelder, and Mark van Loosdrecht. 2021. "Assessment of the Impact of Temperature on Biofilm Composition with a Laboratory Heat Exchanger Module" Microorganisms 9, no. 6: 1185. https://doi.org/10.3390/microorganisms9061185
APA StylePinel, I., Biškauskaitė, R., Pal’ová, E., Vrouwenvelder, H., & van Loosdrecht, M. (2021). Assessment of the Impact of Temperature on Biofilm Composition with a Laboratory Heat Exchanger Module. Microorganisms, 9(6), 1185. https://doi.org/10.3390/microorganisms9061185







