Analysis of Endophyte Diversity of Rheum palmatum from Different Production Areas in Gansu Province of China and the Association with Secondary Metabolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. DNA Extraction, Polymerase Chain Reaction (PCR) Amplifcation, and Sequence Processing
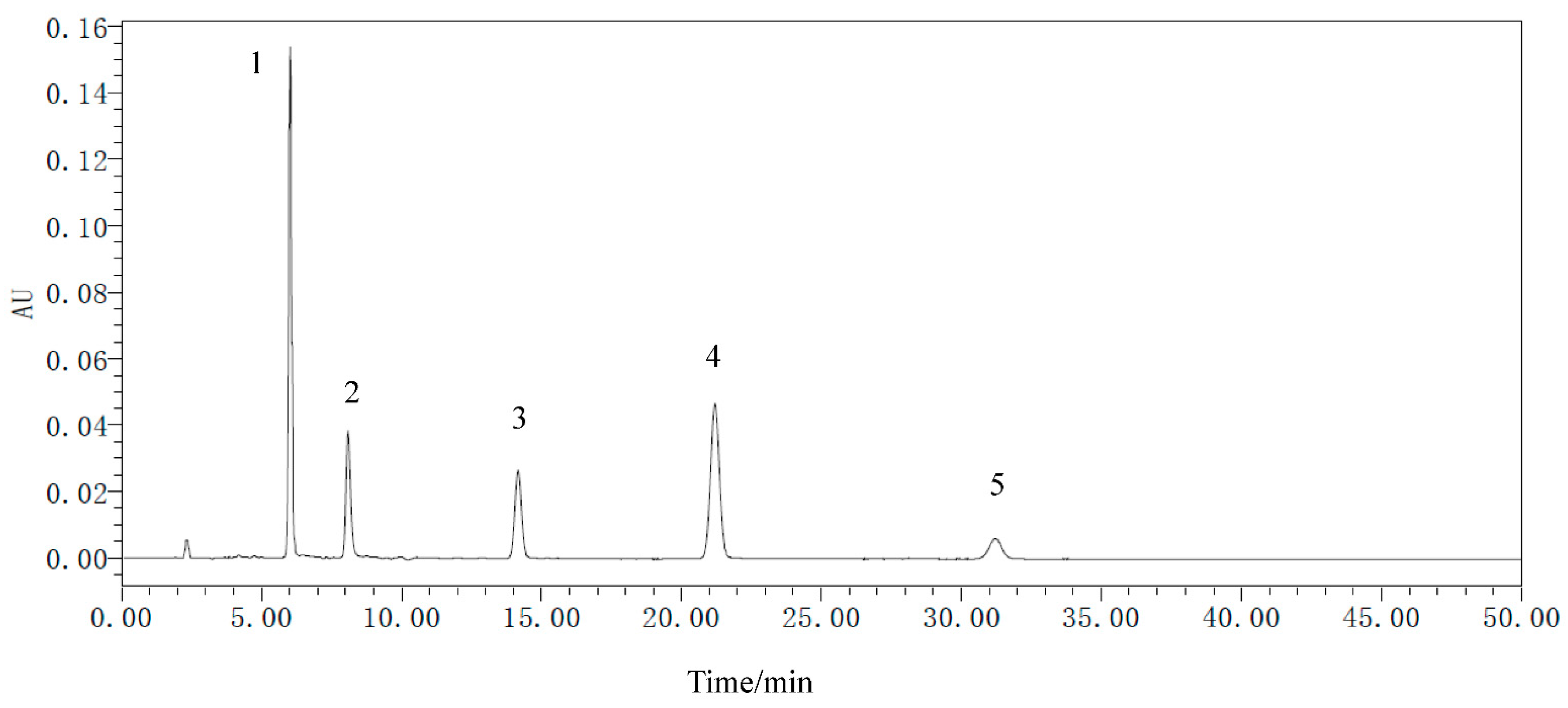
2.3. Metabolites of R. palmatum Quantitative Analysis
2.4. Data Analysis
3. Results
3.1. Surface-Sterilization Efficiency
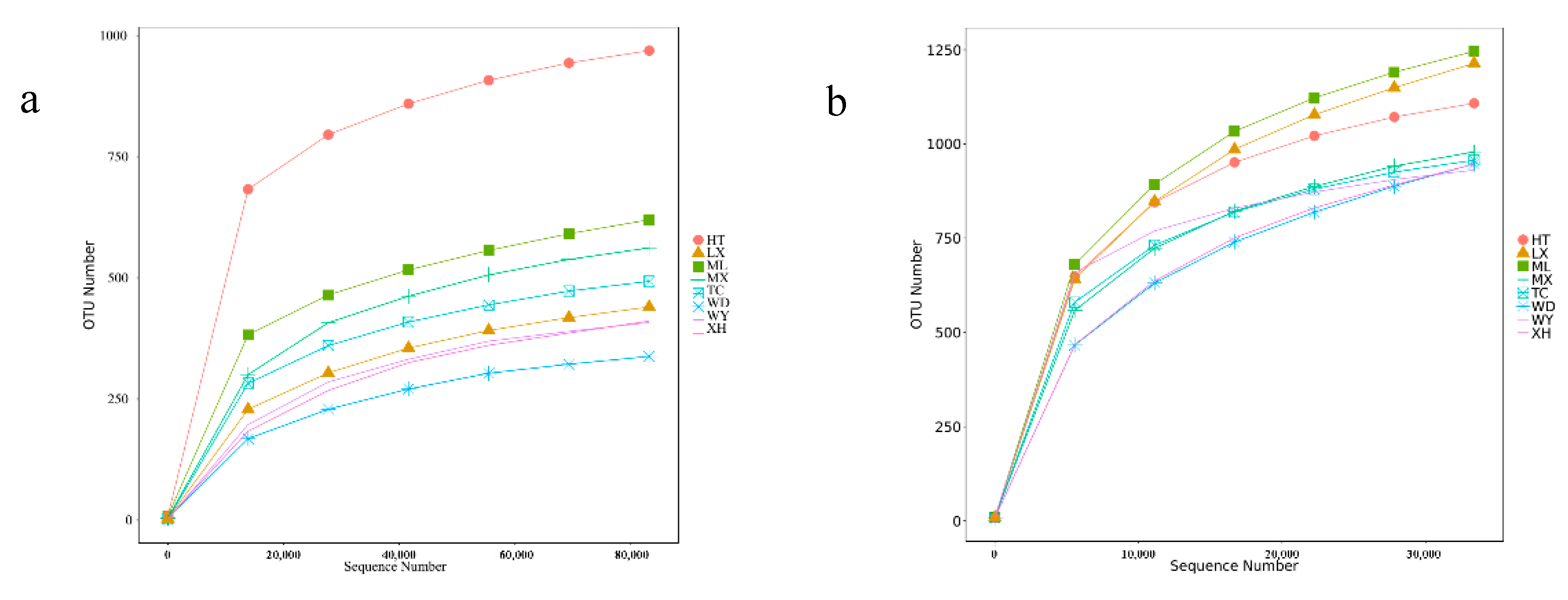
3.2. Analysis of Sequencing Data and Alpha Diversity
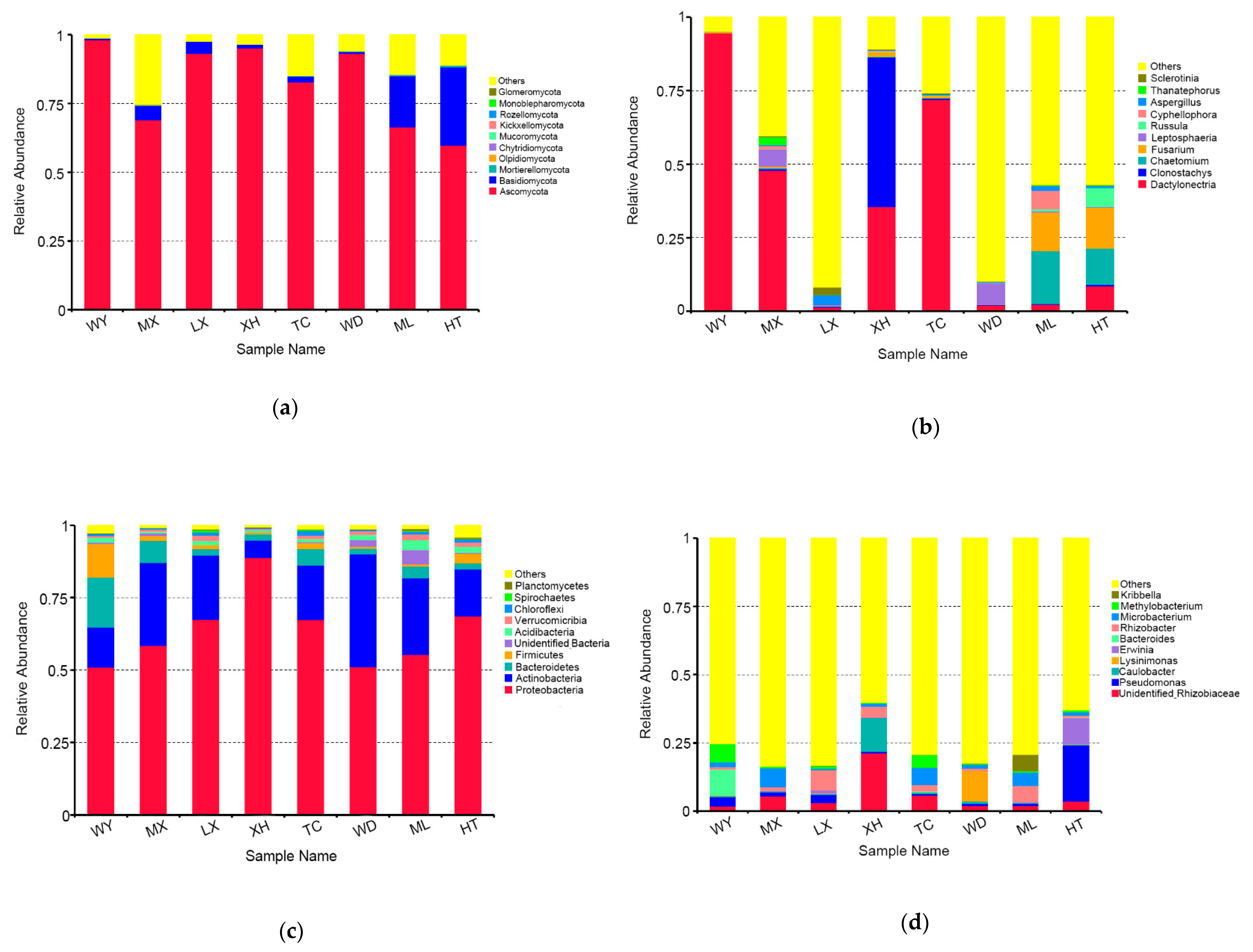
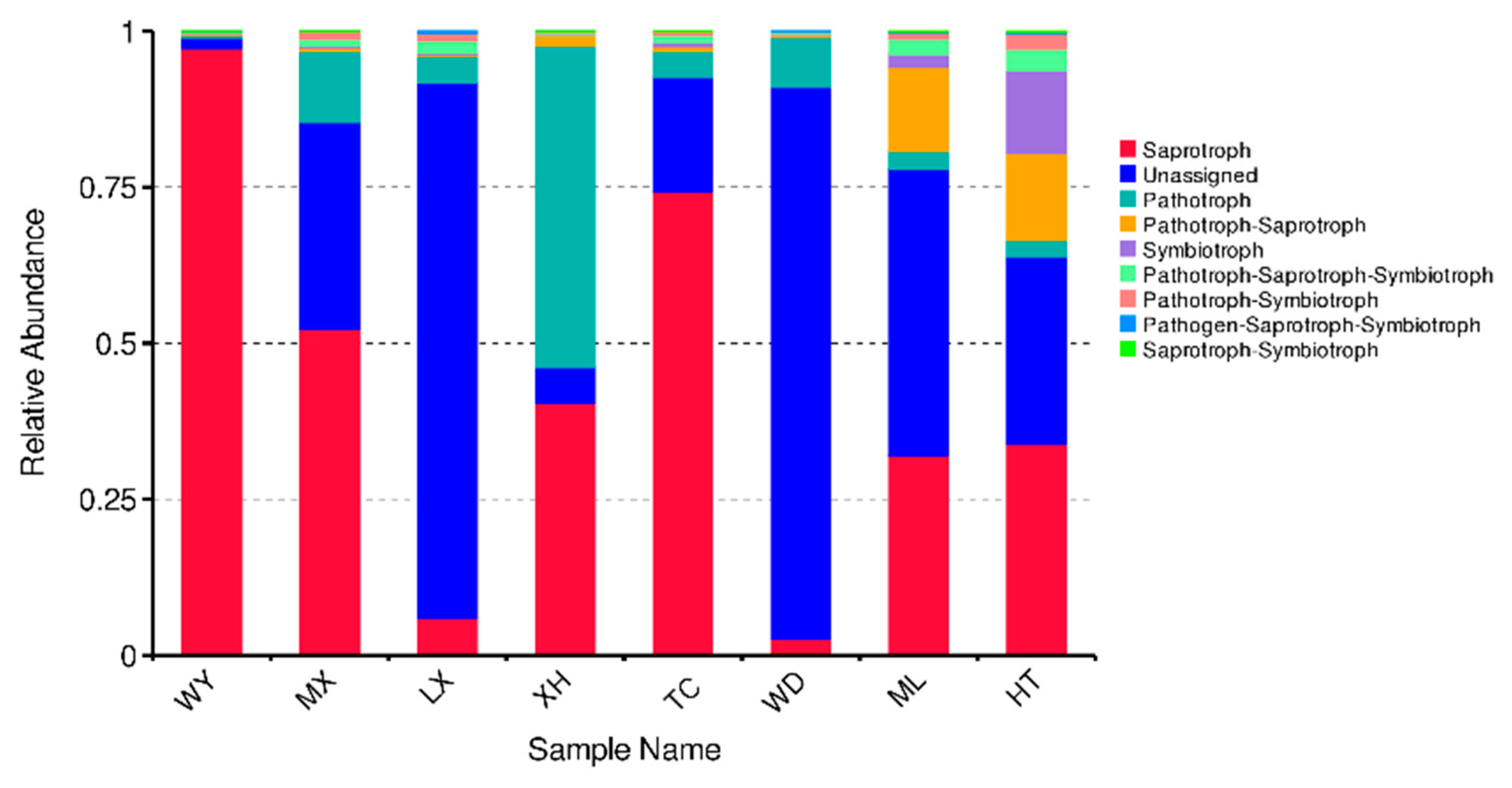
3.3. Community Composition
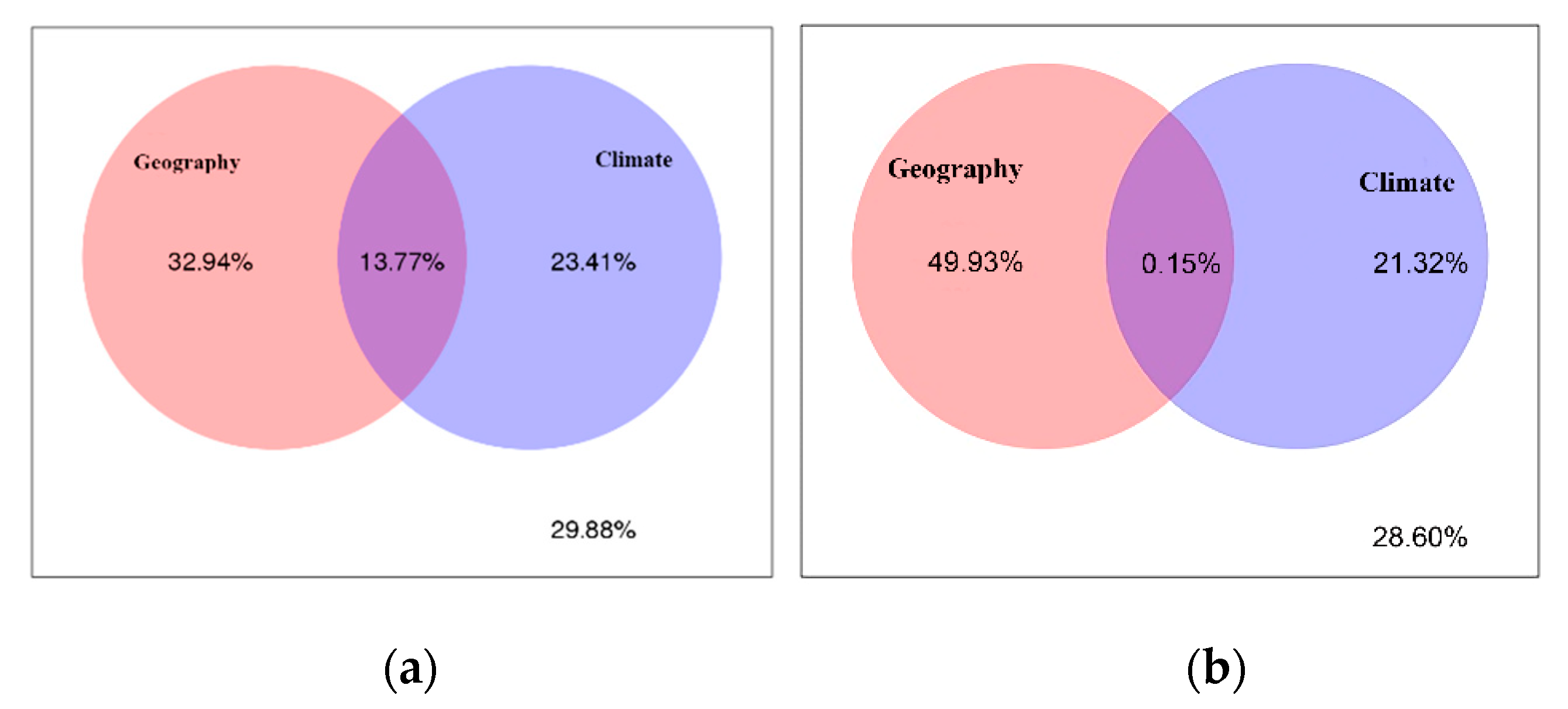
3.4. Effects of Climate and Space on Fungal Endophytic Community
3.5. Correlation Analysis between Endophytes Diversity and Metabolites of R. palmatum
3.6. PICRUSt and FUNGuild Functional Prediction Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wani, Z.A.; Mirza, D.N.; Arora, P.; Riyaz-Ul-Hassan, S. Molecular phylogeny, diversity, community structure, and plant growth promoting properties of fungal endophytes associated with the corms of saffron plant: An insight into the microbiome of Crocus sativus Linn. Fungal Biol. 2016, 120, 1509–1524. [Google Scholar] [CrossRef]
- Compant, S.; Saikkonen, K.; Mitter, B.; Campisano, A.; Mercado-Blanco, J. Editorial special issue: Soil, plants and endophytes. Plant Soil 2016, 405, 1–11. [Google Scholar] [CrossRef]
- Park, Y.-H.; Mishra, R.C.; Yoon, S.; Kim, H.; Park, C.; Seo, S.-T.; Bae, H. Endophytic Trichoderma citrinoviride isolated from mountain-cultivated ginseng (Panax ginseng) has great potential as a biocontrol agent against ginseng pathogens. J. Ginseng Res. 2019, 43, 408–420. [Google Scholar] [CrossRef] [PubMed]
- González-Teuber, M.; Urzúa, A.; Morales, A.; Ibáñez, C.; Bascuñán-Godoy, L. Benefits of a root fungal endophyte on physiological processes and growth of the vulnerable legume tree Prosopis chilensis (Fabaceae). J. Plant Ecol. 2018, 12, 264–271. [Google Scholar] [CrossRef]
- Kusari, P.; Kusari, S.; Lamshöft, M.; Sezgin, S.; Spiteller, M.; Kayser, O. Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 7173–7183. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wu, H.; Yin, Z.; Lian, M.; Yin, C. Endophytic Bacteria Isolated from Panax ginseng Improves Ginsenoside Accumulation in Adventitious Ginseng Root Culture. Molecules 2017, 22, 837. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Hassan, S.E.-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, P.; Li, Y.B.; Liu, T.C.; Zhang, L.; Zhou, Y.H. Natural deep eutectic solvents as new green solvents to extract anthraquinones from Rheum palmatum L. RSC Adv. 2018, 8, 15069–15077. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Hsieh, M.-J.; Hsieh, Y.-S.; Chang, Y.-C.; Chen, P.-N.; Yang, S.-F.; Yih-Shou, H.; Chou, Y.-E.; Lin, C.-W. Antimetastatic effects of Rheum palmatum L. extract on oral cancer cells. Environ. Toxicol. 2017, 32, 2287–2294. [Google Scholar] [CrossRef]
- Chen, T.; Yang, X.; Wang, N.; Li, H.; Zhao, J.; Li, Y. Separation of six compounds including twon-butyrophenone isomers and two stibene isomers fromRheum tanguticumMaxim by recycling high speed counter-current chromatography and preparative high-performance liquid chromatography. J. Sep. Sci. 2018, 41, 3660–3668. [Google Scholar] [CrossRef]
- Asanuma, M.; Zhu, S.; Okura, N.; Cai, S.-Q.; Yoshimatsu, K.; Komatsu, K. Genetic polymorphism of Japanese cultivated Rheum species in the internal transcribed spacer region of nuclear ribosomal DNA. J. Nat. Med. 2019, 73, 541–554. [Google Scholar] [CrossRef]
- Abdo, M.T.; El-Ahmady, S.H.; Gad, H.A. Quality control and long-term stability study of ginger from different geographical origins using chemometrics. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wei, H.; Li, J.; Fan, R.; Xu, M.; Chen, X.; Wang, Z. Geographical Distribution and Environmental Correlates of Eleutherosides and Isofraxidin in Eleutherococcus senticosus from Natural Populations in Forests at Northeast China. Forests 2019, 10, 872. [Google Scholar] [CrossRef]
- Arhancet, G.B.; Woodard, S.S.; Dietz, J.D.; Garland, D.J.; Wagner, G.M.; Iyanar, K.; Collins, J.T.; Blinn, J.R.; Numann, R.E.; Hu, X.; et al. Stereochemical Requirements for the Mineralocorticoid Receptor Antagonist Activity of Dihydropyridines. J. Med. Chem. 2010, 53, 4300–4304. [Google Scholar] [CrossRef]
- Liu, J.; Abdelfattah, A.; Norelli, J.; Burchard, E.; Schena, L.; Droby, S.; Wisniewski, M. Apple endophytic microbiota of different rootstock/scion combinations suggests a genotype-specific influence. Microbiome 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Pharmacopoeia Committee of China (Ed.) Chinese Pharmacopoeia; Chemical Industry Publishing House: Beijing, China, 2015; pp. 23–25. [Google Scholar]
- Chen, C.; Fu, Z.; Zhou, W.; Chen, Q.; Wang, C.; Xu, L.; Wang, Z.; Zhang, H. Ionic liquid-immobilized NaY zeolite-based matrix solid phase dispersion for the extraction of active constituents in Rheum palmatum L. Microchem. J. 2020, 152, 104245. [Google Scholar] [CrossRef]
- Penton, C.R.; Gupta, V.V.S.R.; Yu, J.; Tiedje, J.M. Size Matters: Assessing Optimum Soil Sample Size for Fungal and Bacterial Community Structure Analyses Using High Throughput Sequencing of rRNA Gene Amplicons. Front. Microbiol. 2016, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control. 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L. Improvement for unweighted pair group method with arithmetic mean and its application. J. Beijing Univ. Technol. 2007, 33, 1333–1339. [Google Scholar]
- Zhang, F.; Xu, X.; Wang, G.; Wu, B.; Xiao, Y. Medicago sativa and soil microbiome responses to Trichoderma as a biofertilizer in alkaline-saline soils. Appl. Soil Ecol. 2020, 153, 103573. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, W.; Shao, Y.; Li, Y.-J.; Lin, L.-A.; Zhang, Y.-J.; Han, H.; Chen, Z.-J. Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Arch. Microbiol. 2020, 202, 1157–1171. [Google Scholar] [CrossRef]
- Chi, W.-C.; Chen, W.; He, C.-C.; Guo, S.-Y.; Cha, H.-J.; Tsang, L.M.; Ho, T.W.; Pang, K.-L. A highly diverse fungal community associated with leaves of the mangrove plant Acanthus ilicifolius var. xiamenensis revealed by isolation and metabarcoding analyses. PeerJ 2019, 7, e7293. [Google Scholar] [CrossRef]
- Dong, L.; Cheng, R.; Xiao, L.; Wei, F.; Wei, G.; Xu, J.; Wang, Y.; Guo, X.; Chen, Z.; Chen, S. Diversity and composition of bacterial endophytes among plant parts of Panax notoginseng. Chin. Med. 2018, 13, 41. [Google Scholar] [CrossRef]
- Urumbil, S.K.; Kumar, M.A. Diversity Analysis of Endophytic Bacterial Microflora in Emilia sonchifolia (Linn.) DC on Illumina Mi Seq Platforms. J. Pure Appl. Microbiol. 2020, 14, 679–687. [Google Scholar] [CrossRef]
- Varanda, C.M.R.; Oliveira, M.; Materatski, P.; Landum, M.; Clara, M.I.E.; Félix, M.D.R. Fungal endophytic communities associated to the phyllosphere of grapevine cultivars under different types of management. Fungal Biol. 2016, 120, 1525–1536. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.D.; Arnold, A.E. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H.; Liu, L.; Lin, J.; Tang, K. A review: Recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 2010, 86, 1707–1717. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-Derived Bioactive Compounds Produced by Endophytic Fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef]
- Cui, J.-L.; Zhang, Y.-Y.; Vijayakumar, V.; Zhang, G.; Wang, M.-L.; Wang, J.-H. Secondary Metabolite Accumulation Associates with Ecological Succession of Endophytic Fungi in Cynomorium songaricum Rupr. J. Agric. Food Chem. 2018, 66, 5499–5509. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tao, Q.; Wu, K.; Li, J.; Tingqiang, L.; Liang, Y.; Yang, X.; Li, T. Structural and functional variability in root-associated bacterial microbiomes of Cd/Zn hyperaccumulator Sedum alfredii. Appl. Microbiol. Biotechnol. 2017, 101, 7961–7976. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Borruso, L.; Brusetti, L.; Crecchio, C.; Cesco, S.; Mimmo, T. The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol. Biochem. 2016, 99, 39–48. [Google Scholar] [CrossRef]
- Pepe-Ranney, C.; Keyser, C.; Trimble, J.K.; Bissinger, B. Surveying the Sweetpotato Rhizosphere, Endophyte, and Surrounding Soil Microbiomes at Two North Carolina Farms Reveals Underpinnings of Sweetpotato Microbiome Community Assembly. Phytobiomes J. 2020, 4, 75–89. [Google Scholar] [CrossRef]
- Martínez-Diz, M.D.P.; Andrés-Sodupe, M.; Bujanda, R.; Díaz-Losada, E.; Eichmeier, A.; Gramaje, D. Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol. 2019, 41, 234–244. [Google Scholar] [CrossRef]




| Location | Altitude (m) | Longitude | Latitude | Mean Annual Precipitation (mm) | Mean Annual Temperature (°C) | Code No. |
|---|---|---|---|---|---|---|
| Weiyuan county, Dingxi city | 2221 | 104°09′30.45″ | 35°04′11.43″ | 507 | 6.8 | WY |
| Min county, Dingxi city | 2489 | 104°02′08.94″ | 34°17′26.24″ | 635 | 5.5 | MX |
| Li county, Wudu city | 2282 | 104°52′38.03″ | 34°05′46.48″ | 488.2 | 9.9 | LX |
| Xihe county, Wudu city | 1849 | 105°26′02.39″ | 33°58′42.07″ | 533 | 8.4 | XH |
| Tanchang county, Wudu city | 2186 | 104°13′54.58″ | 34°13′21.42″ | 583.9 | 9.3 | TC |
| Chiba township, Wudu city | 2499 | 104°47′45.95″ | 33°38′37.19″ | 470 | 14.7 | WD |
| Minle county, Zhangye city | 2686 | 100°54′10.44″ | 38°16′44.33″ | 351 | 4.1 | ML |
| Huating county, Pingliang city | 1746 | 106°30′26.28″ | 35°18′22.18″ | 607 | 7.7 | HT |
| Sample | Endophytic Fungi | Endophytic Bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| Effective Tags | Shannon | Chao1 | Goods_Coverage | Effective Tags | Shannon | Chao1 | Goods_Coverage | |
| WY | 90,843 | 0.643 | 443.212 | 0.999 | 59,514 | 7.794 | 1107.833 | 0.995 |
| MX | 90,144 | 3.420 | 630.000 | 0.999 | 78,683 | 7.220 | 1414.852 | 0.992 |
| LX | 96,899 | 1.849 | 525.513 | 0.999 | 93,707 | 7.217 | 1732.563 | 0.989 |
| XH | 93,098 | 2.076 | 465.000 | 0.999 | 86,798 | 5.541 | 1580.968 | 0.990 |
| TC | 87,301 | 2.409 | 559.585 | 0.999 | 63,777 | 7.064 | 1291.762 | 0.993 |
| WD | 89,655 | 1.897 | 392.016 | 0.999 | 57,993 | 6.619 | 2120.333 | 0.988 |
| ML | 82,125 | 5.691 | 757.963 | 0.998 | 88,494 | 7.249 | 1913.009 | 0.988 |
| HT | 77,317 | 6.715 | 1057.724 | 0.998 | 76,213 | 6.871 | 1444.263 | 0.992 |
| Sample | Aloe-Emodin (mg/g) | Rhein (mg/g) | Emodin (mg/g) | Chrysophanol (mg/g) | Physcion (mg/g) |
|---|---|---|---|---|---|
| XH | 0.61 | 1.23 | 1.88 | 5.24 | 1.28 |
| LX | 0.38 | 0.28 | 0.81 | 3.12 | 0.74 |
| TC | 1.38 | 2.33 | 0.94 | 4.16 | 1.58 |
| MX | 0.39 | 0.20 | 1.77 | 6.66 | 1.99 |
| WY | 0.36 | 0.40 | 0.22 | 2.31 | 0.97 |
| WD | 0.96 | 0.38 | 0.53 | 3.16 | 0.99 |
| ML | 0.62 | 2.02 | 3.47 | 4.96 | 1.84 |
| HT | 0.95 | 1.10 | 2.89 | 7.39 | 2.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Jia, L.; Hou, Q.; Zhao, X.; Sun, K. Analysis of Endophyte Diversity of Rheum palmatum from Different Production Areas in Gansu Province of China and the Association with Secondary Metabolite. Microorganisms 2021, 9, 978. https://doi.org/10.3390/microorganisms9050978
Chen D, Jia L, Hou Q, Zhao X, Sun K. Analysis of Endophyte Diversity of Rheum palmatum from Different Production Areas in Gansu Province of China and the Association with Secondary Metabolite. Microorganisms. 2021; 9(5):978. https://doi.org/10.3390/microorganisms9050978
Chicago/Turabian StyleChen, Dawei, Lingyun Jia, Qinzheng Hou, Xiang Zhao, and Kun Sun. 2021. "Analysis of Endophyte Diversity of Rheum palmatum from Different Production Areas in Gansu Province of China and the Association with Secondary Metabolite" Microorganisms 9, no. 5: 978. https://doi.org/10.3390/microorganisms9050978
APA StyleChen, D., Jia, L., Hou, Q., Zhao, X., & Sun, K. (2021). Analysis of Endophyte Diversity of Rheum palmatum from Different Production Areas in Gansu Province of China and the Association with Secondary Metabolite. Microorganisms, 9(5), 978. https://doi.org/10.3390/microorganisms9050978





