Cutaneous Leishmaniasis in Algeria; Highlight on the Focus of M’Sila
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Study Design
2.2. Entomological Sampling
2.3. Rodent Capture
2.4. Patient: Skin Wound Sampling Collection
2.5. Parasitological Diagnosis
2.6. Molecular Diagnostic and Typing
2.7. Sequencing and Phylogeny of Chosen Isolates
2.8. Multilocus Enzymatic Electrophoresis (MLEE) Typing
2.9. Data Management and Statistical Analysis
2.10. Ethical Considerations
3. Results
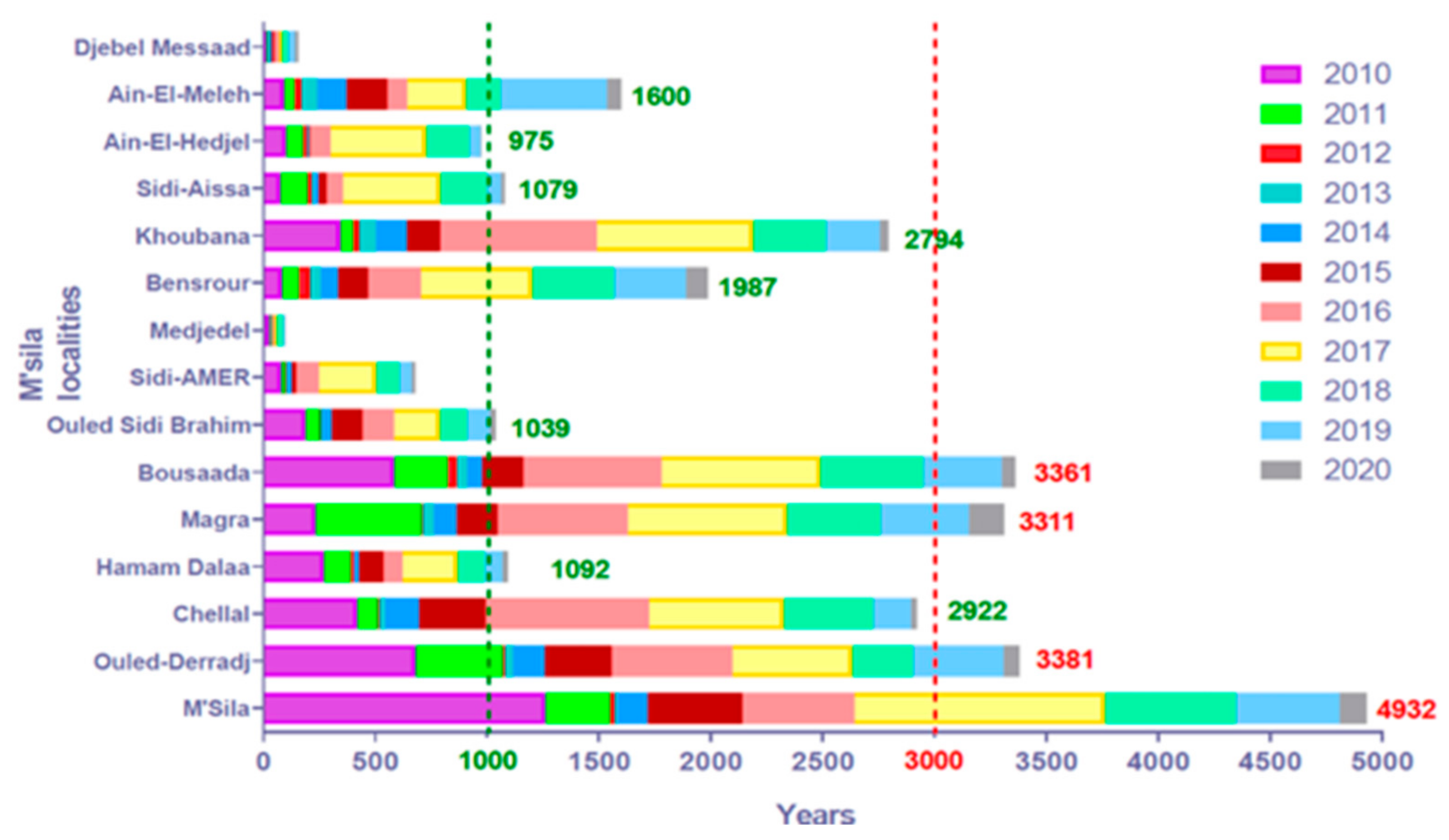
3.1. Dynamics of Cutaneous Leishmaniasis in the Province of M’Sila Compared to the National Epidemiological Status of the Disease
3.2. Cutaneous Leishmaniasis in the M’Sila Province
3.2.1. Lesion Characteristics, Patient Stratification, and Environmental Features
3.2.2. Leishmania Detection and Identification in Patients and Reservoirs
3.3. Entomological Survey in the Province of M’Sila
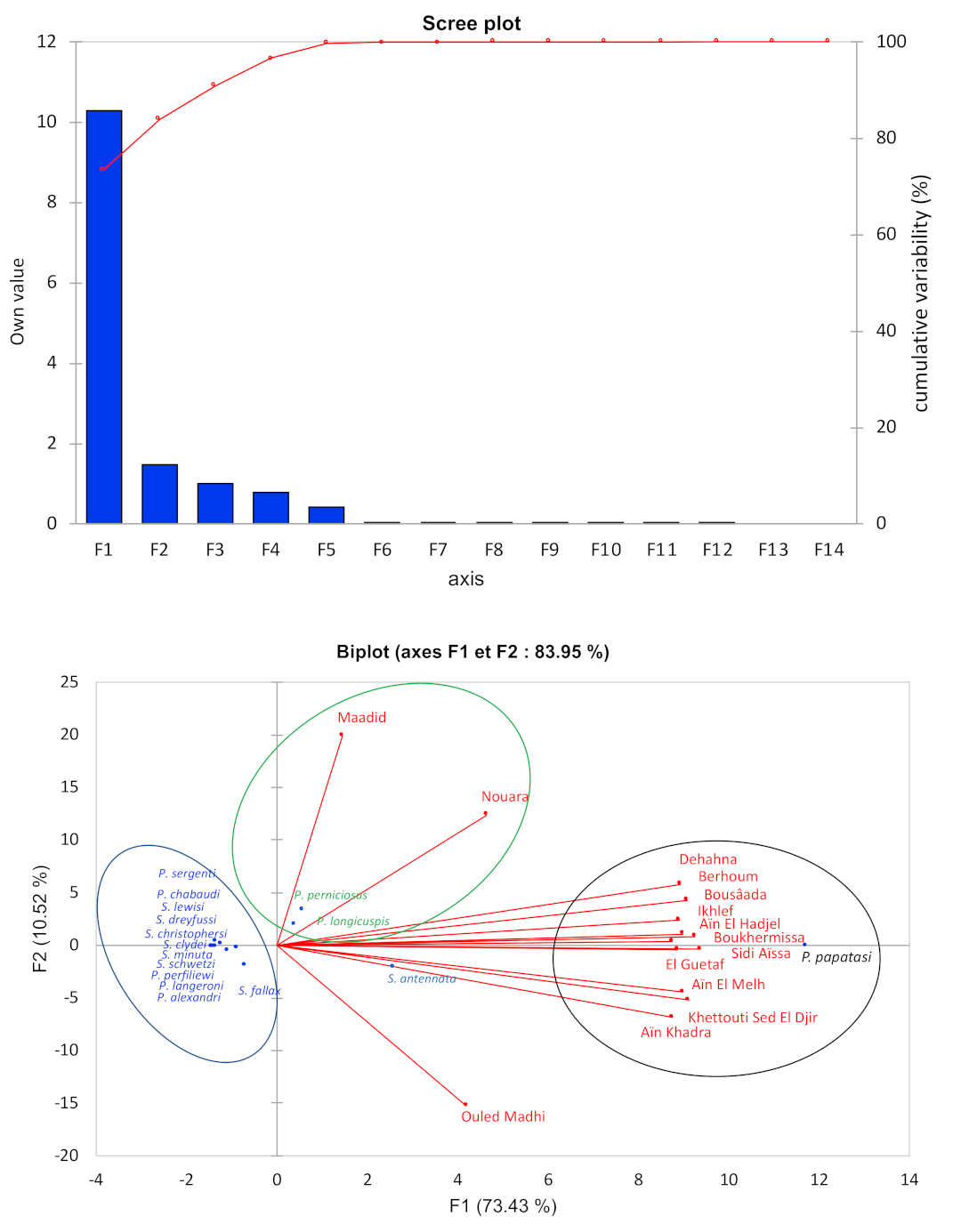
3.3.1. Diversity and Dynamic of Sandfly Populations
3.3.2. Biotope and Distribution of Sandflies in the Province of M’Sila
3.4. Survey of the Reservoir Hosts of Cutaneous Leishmaniasis
4. Discussion
4.1. Epidemiology of Leishmaniases in Algeria and M’Sila
4.2. Leishmania Species and Clinical Presentation in M’Sila
4.3. Sandfly Diversity and Dynamics in Algeria and M’Sila
4.4. Ecology, Diversity, and Medical Importance of Sandflies in M’Sila
4.5. Rodents and Reservoirs in the M’Sila Province
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Belazzoug, S. Outbreak of cutaneous leishmaniasis in the M’Sila region (Algeria). Bulletin de la Societe de Pathologie Exotique et de ses Filiales 1982, 75, 497–504. [Google Scholar] [PubMed]
- Rioux, J.A.; Lanotte, G.; Petter, F.; Dereure, J.; Akalay, O.; Pratlong, F.; Velez, I.D.; Fikri, N.B.; Maazoun, R.; Deniau, M. Les leishmanioses Cutanées du Bassin Méditerranéen Occidental. De L’identification Enzymatique à L’analyse éco-Epidémiologique. L’exemple de Trois ‘Foyers’, Tunisien, Marocain et Français; Colloque International CNRS/INSERM, Institut Méditerranéen d’Etudes Epidemiologiques et Ecologiques: Montpellier, France, 1986; pp. 365–395. [Google Scholar]
- Boudrissa, A.; Cherif, K.; Kherrachi, I.; Benbetka, S.; Bouiba, L.; Boubidi, S.C.; Benikhlef, R.; Arrar, L.; Hamrioui, B.; Harrat, Z. Spread of Leishmania major to the north of Algeria. Bulletin de la Societe de Pathologie Exotique (1990) 2012, 105, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Aoun, K.; Bouratbine, A. Cutaneous Leishmaniasis in North Africa: A review. Parasite 2014, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Eddaikra, N.; Ait-Oudhia, K.; Kherrachi, I.; Oury, B.; Moulti-Mati, F.; Benikhlef, R.; Harrat, Z.; Sereno, D. Antimony susceptibility of Leishmania isolates collected over a 30-year period in Algeria. PLoS Negl. Trop. Dis. 2018, 12, e0006310. [Google Scholar] [CrossRef]
- Kholoud, K.; Bounoua, L.; Sereno, D.; El Hidan, M.; Messouli, M. Emerging and Re-Emerging Leishmaniases in the Mediterranean Area: What Can Be Learned from a Retrospective Review Analysis of the Situation in Morocco during 1990 to 2010? Microorganisms 2020, 8, 1511. [Google Scholar] [CrossRef]
- INSP. Relevé Epidémiologique Mensuel. Available online: http://insp.dz/index.php/Non-categorise/rem.html (accessed on 28 May 2020).
- Hamel, H. Étude Comparée des Boutons d’Alep et de Biskra, History of Natural Sciences and Medicine; Medical Library. Seidel collection; Hachette Livre: Paris, France, 1860; p. 31. [Google Scholar]
- Parrot, L.; Foley, H. Epidemiology of Oriental Sore in Algeria. Bulletin de la Societe de Pathologie Exotique (1990) 1925, 18, 639–641. [Google Scholar]
- INSP. Situation Epidémiologique de L’année 2010 sur la Base des cas Déclarés à l’INSP. Available online: http://www.insp.dz/images/PDF/Epidemio/rem_2010.pdf (accessed on 28 May 2020).
- Harrat, Z.; Pratlong, F.; Belazzoug, S.; Dereure, J.; Deniau, M.; Rioux, J.A.; Belkaid, M.; Dedet, J.P. Leishmania infantum and L. major in Algeria. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 625–629. [Google Scholar] [CrossRef]
- Belazzoug, S. Isolation of Leishmania major Yakimoff & Schokhor, 1914 from Psammomys obesus Gretzschmar, 1828 (Rodentia: Gerbillidae) in Algeria. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 876. [Google Scholar] [CrossRef]
- Belazzoug, S. Découverte d’un Meriones shawi (Rongeur, Gerbillidé) naturellement infesté par Leishmania dans le nouveau foyer de leishmaniose cutanée de Ksar Chellala (Algérie). Bulletin de la Société de Pathologie Exotique 1986, 79, 630–633. [Google Scholar]
- Izri, M.; Belazzoug, S.; Pratlong, F.; Rioux, J.A. Isolement de Leishmania major chez Phlebotomus papatasi à Biskra (Algérie): Fin d’une épopée écoépidémiologique. Annales de Parasitologie Humaine et Comparee 1992, 67, 31–32. [Google Scholar] [CrossRef]
- Mili, M.; Boutabba, H.; Boutabba, S.-D. La nature urbaine: Dégradation quantitative et qualitative des espaces verts urbains, cas de la ville steppique de M’Sila, Algérie. urbe. Revista Brasileira de Gestão Urbana 2019, 11, 11. [Google Scholar] [CrossRef]
- Despois, J. Le Hodna (Algérie). Revue de géographie alpine. Publications de la Faculté des Lettres d’Alger; Presses Universitaires de France: Paris, France, 1953; p. 409. [Google Scholar]
- INSP. Situation Epidémiologique de L’année 2005 sur la Base des cas Déclarés à l’INSP. Available online: http://insp.dz/images/PDF/Epidemio/rem_2005.pdf (accessed on 28 May 2020).
- Boudjemline, F.; Semar, A. Assessment and mapping of desertification sensitivity with MEDALUS model and GIS – Case study: Basin of Hodna, Algeria. J. Water Land Dev. 2018, 36, 17–26. [Google Scholar] [CrossRef]
- Infoclimat. Available online: https://www.infoclimat.fr/observations-meteo/temps-reel/m-sila/60467.html (accessed on 28 July 2020).
- Rioux, J.-A.; Golvan, Y.-J.; Croset, H.; Houin, R.; Juminer, B.; Bain, O.; Tour, S. Ecologie des Leishmanioses dans le sud de la France. 1. - Les Phlébotomes. Annales de Parasitologie Humaine et Comparée 1967, 42, 561–603. [Google Scholar] [CrossRef]
- Abonnenc, E. Les Phlébotomes de la Région Ethiopienne (Diptera, Psychodidae); ORSTOM: Paris, France, 1972; Volume 55, p. 289. [Google Scholar]
- Croset, H.; Rioux, J.A.; Maistre, M.; Bayar, N. Les Phlébotomes de Tunisie (Diptera, Phlebotomidae). Mise au point systématique, chorologique et éthologique. Ann. Parasitol. Hum. Comp. 1978, 53, 711–749. [Google Scholar] [CrossRef]
- Dedet, J.P.; Addadi, K.; Belazzoug, S. Les phlébotomes (Diptera, Psychodidae) d’Algérie. Cahiers-ORSTOM. Entomologie Médicale et Parasitologie 1984, 22, 99–127. [Google Scholar]
- Bernard, J. Clef de détermination des rongeurs de Tunisie. Arch. Inst. Pasteur Tunis 1970, 47, 265–307. [Google Scholar]
- Berrebi, J. The Cultivation of Leishmania. Arch. Inst. Pasteur Tunis. 1936, 25, 89–141. [Google Scholar]
- Chouihi, E.; Amri, F.; Bouslimi, N.; Siala, E.; Selmi, K.; Zallagua, N.; Abdallah, R.B.; Bouratbine, A.; Aoun, K. Les cultures sur milieu NNN dans le diagnostic biologique des leishmanioses Cultures on NNN medium for the diagnosis of leishmaniasis. Pathol. Biol. 2009, 57, 219–224. [Google Scholar] [CrossRef]
- Schonian, G.; Nasereddin, A.; Dinse, N.; Schweynoch, C.; Schallig, H.D.; Presber, W.; Jaffe, C.L. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis. 2003, 47, 349–358. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Manuel Pour la Prise en Charge de la Leishmaniose Cutanée dans la Région OMS de la Méditerranée Orientale; WHO Regional Publications, Eastern Mediterranean Series; WHO: Geneve, Switzerland, 2014; N35: 978-92-9021-996-5. [Google Scholar]
- Belkaid, P.M.; Harrat, Z.; Hamrioui, B.; Thellier, M.; Datry, A.; Danis, M. A simple media for isolation and culture of leishmania. Bulletin de la Societe de Pathologie Exotique (1990) 1996, 89, 276–277. [Google Scholar]
- Mary, C.; Faraut, F.; Lascombe, L.; Dumon, H. Quantification of Leishmania infantum DNA by a Real-Time PCR Assay with High Sensitivity. J. Clin. Microbiol. 2004, 42, 5249–5255. [Google Scholar] [CrossRef] [PubMed]
- Staden Package Home. Available online: http://staden.sourceforge.net (accessed on 15 December 2020).
- Molecular Evolutionary Genetics Analysis. Available online: www.megasoftware.net (accessed on 20 July 2019).
- Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 July 2019).
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2009, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Rioux, J.A.; Lanotte, G.; Serres, E.; Pratlong, F.; Bastien, P.; Perieres, J. Taxonomy ofLeishmania.Use of isoenzymes. Suggestions for a new classification. Annales de Parasitologie Humaine et Comparée 1990, 65, 111–125. [Google Scholar] [CrossRef]
- Gherbi, R.; Bounechada, M.; Latrofa, M.S.; Annoscia, G.; Tarallo, V.D.; Dantas-Torres, F.; Otranto, D. Phlebotomine sand flies and Leishmania species in a focus of cutaneous leishmaniasis in Algeria. PLOS Negl. Trop. Dis. 2020, 14, e0008024. [Google Scholar] [CrossRef]
- Guernaoui, S.; Hamarsheh, O.; Garcia, D.; Fontenille, D.; Sereno, D. Population Genetics of Phlebotomus papatasi from Endemic and Nonendemic Areas for Zoonotic Cutaneous Leishmaniasis in Morocco, as Revealed by Cytochrome Oxidase Gene Subunit I Sequencing. Microorganisms 2020, 8, 1010. [Google Scholar] [CrossRef]
- Randa, G.; Samir, Z.; Hamid, B. Association between climatic changes and leishmaniasis incidence in Biskra district, Algeria. J. Entomol. Zool. Stud. 2017, 5, 43–49. [Google Scholar]
- Pratlong, F.; Dereure, J.; Ravel, C.; Lami, P.; Balard, Y.; Serres, G.; Lanotte, G.; Rioux, J.-A.; Dedet, J.-P. Geographical distribution and epidemiological features of Old World cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop. Med. Int. Health 2009, 14, 1071–1085. [Google Scholar] [CrossRef]
- Mauricio, I.L.; Yeo, M.; Baghaei, M.; Doto, D.; Pratlong, F.; Zemanova, E.; Dedet, J.-P.; Lukes, J.; Miles, M.A. Towards multilocus sequence typing of the Leishmania donovani complex: Resolving genotypes and haplotypes for five polymorphic metabolic enzymes (ASAT, GPI, NH1, NH2, PGD). Int. J. Parasitol. 2006, 36, 757–769. [Google Scholar] [CrossRef]
- Mihoubi, I.; Monbrison, F.D.; Romeuf, N.; Moulahem, T.; Picot, S. Diagnostic delocalise par PCR temps reel de la leishmaniose cutanee sevissant dans le foyer de Constantine (Algerie). Médecine Trop. 2006, 66, 39–43. [Google Scholar]
- Aoun, K.; Ben Abda, I.; Bousslimi, N.; Bettaieb, J.; Siala, E.; Ben Abdallah, R.; Benmously, R.; Bouratbine, A. Comparative characterization of skin lesions observed in the three endemic varieties of cutaneous leishmaniasis in Tunisia. Annales de Dermatologie et de Venereologie 2012, 139, 452–458. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Chamakh-Ayari, R.; Chenik, M.; Chakroun, A.S.; Bahi-Jaber, N.; Aoun, K.; Meddeb-Garnaoui, A. Leishmania major large RAB GTPase is highly immunogenic in individuals immune to cutaneous and visceral leishmaniasis. Parasites Vectors 2017, 10, 1–11. [Google Scholar] [CrossRef][Green Version]
- Berchi, S.; Rioux, J.A.; Belmonte, A.; Russo, J. Un phlébotome nouveau pour l’Algérie, Phlebotomus (Paraphlebotomus) kazeruni. Annales de Parasitologie Humaine et Comparee 1986, 61, 507–508. [Google Scholar] [CrossRef][Green Version]
- Belazzoug, S. The sandflies of Algeria. Parassitologia 1991, 33, 85–87. [Google Scholar]
- Zeroual, S.; Gaouaoui, R.; Boudjlida, H. Diversity and occurrence of phlebotomine sand flies (Diptera: Psychodidae) in the area of Biskra (Middle Eastern of Algeria). J. Entomol. Zool. Stud. 2016, 4, 890–895. [Google Scholar]
- Ramdane, E.; Berchi, S.; Louad, K. Les phlébotomes (Diptera, Pshycodidae), vecteurs d’agents pathogènes responsables de la leishmaniose humaine dans la région de Constantine (Algérie). Entomofauna 2018, 39, 537–555. [Google Scholar]
- Boukraa, S.; Boubidi, S.; Zimmer, J.-Y.; Francis, F.; Haubruge, E.; Alibenali-Lounaci, Z.; Doumandji, S. Surveillance des populations de phlébotomes (Diptera: Psychodidae), vecteurs des agents responsables des leishmanioses dans la région du M’Zab-Ghardaïa (Algérie). Faun. Entomol. 2011, 63, 97–101. [Google Scholar]
- Belazzoug, S.; Mahzoul, D.; Rioux, J.A. The phlebotomus sandflies (Diptera, Psychodidae) of M’Sila and Bou-Saada. Archives de l’Institut Pasteur d’Algerie. Institut Pasteur d’Algerie 1986, 55, 117–124. [Google Scholar]
- Chelbi, I.; Derbali, M.; Al-Ahmadi, Z.; Zaafouri, B.; El Fahem, A.; Zhioua, E. Phenology of Phlebotomus papatasi (Diptera: Psychodidae) relative to the seasonal prevalence of zoonotic cutaneous leishmaniasis in central Tunisia. J. Med Entomol. 2007, 44, 385–388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alten, B.; Maia, C.; Afonso, M.O.; Campino, L.; Jiménez, M.; González, E.; Molina, R.; Bañuls, A.L.; Prudhomme, J.; Vergnes, B.; et al. Seasonal Dynamics of Phlebotomine Sand Fly Species Proven Vectors of Mediterranean Leishmaniasis Caused by Leishmania infantum. PLOS Negl. Trop. Dis. 2016, 10, e0004458. [Google Scholar] [CrossRef] [PubMed]
- Bounoua, L.; Kahime, K.; Houti, L.; Blakey, T.; Ebi, K.L.; Zhang, P.; Imhoff, M.L.; Thome, K.J.; Dudek, C.; Sahabi, S.A.; et al. Linking Climate to Incidence of Zoonotic Cutaneous Leishmaniasis (Lmajor) in Pre-Saharan North Africa. Int. J. Environ. Res. Public Health 2013, 10, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Kholoud, K.; Denis, S.; Lahouari, B.; El Hidan, M.A.; Souad, B. Management of Leishmaniases in the Era of Climate Change in Morocco. Int. J. Environ. Res. Public Health 2018, 15, 1542. [Google Scholar] [CrossRef] [PubMed]
- Parrot, L. Les espèces algériennes du genre Phlebotomus (Psychodidae). Butt. Soc. Hist. nal. Afr. N. 1935, 26, 23–27. [Google Scholar]
- Dereure, J.; Pratlong, F.; Lanotte, G.; Rioux, J.A. La leishmaniose viscérale autochtone au Maroc méridional. Leishmania. Taxonomie et phyllogenèse. Application éco-épidémiologiques. Coll. Int. CNRS/INSERM 1986, 37, 225. [Google Scholar]
- Zhioua, E.; Kaabi, B.; Chelbi, I. Entomological investigations following the spread of visceral leishmaniasis in Tunisia. J. Vector Ecol. 2007, 32, 371–374. [Google Scholar] [CrossRef]
- Berdjanebrouk, Z.; Charrel, R.N.; Bitam, I.; Hamrioui, B.; Izri, A. Record ofPhlebotomus (Transphlebotomus) mascittiiGrassi, 1908 andPhlebotomus (Larroussius) chadliiRioux, Juminer & Gibily, 1966 female in Algeria. Parasite 2011, 18, 337–339. [Google Scholar] [CrossRef]
- Belazzoug, S.; Lanotte, G.; Maazoun, R.; Pratlong, F.; Rioux, J.A. A new enzymatic variant of Leishmania infantum Nicolle, 1908, agent of cutaneous leishmaniasis in northern Algeria. Annales de Parasitologie Humaine et Comparee 1985, 60, 1–3. [Google Scholar] [CrossRef]
- Izri, M.; Belazzoug, S. Phlebotomus (Larroussius) perfiliewi naturally infected with dermotropic Leishmania infantum at Tenes, Algeria. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 399. [Google Scholar] [CrossRef]
- Kallel, K.; Haouas, N.; Pratlong, F.; Kaouech, E.; Belhadj, S.; Anane, S.; Dedet, J.P.; Babba, H.; Chaker, E. Cutaneous leishmaniasis caused by Leishmania infantum MON-24 in Tunisia: Extension of the focus to the center of the country. Bulletin de la Societe de Pathologie Exotique (1990) 2008, 101, 29–31. [Google Scholar]
- Benikhlef, R.; Pratlong, F.; Harrat, Z.; Seridi, N.; Bendali-Braham, S. Leishmaniose viscérale infantile causée par Leishmania infantum zymodème MON-24 en Algérie. Bulletin de la Société de Pathologie Exotique 2001, 94, 14–16. [Google Scholar]
- Moulahem, T.; Fendri, A.H.; Harrat, Z.; Benmezdad, A.; Aissaoui, K.; Ahraou, S.; Addadi, K. Phlebotomus of Constantine: Species captured in an urban apartment. Bulletin de la Société de Pathologie Exotique 1998, 91, 344–345. [Google Scholar]
- Boubidi, S.; Benallal, K.E.; Boudrissa, A.; Bouiba, L.; Bouchareb, B.; Garni, R.; Bouratbine, A.; Ravel, C.; Dvorak, V.; Votypka, J.; et al. Phlebotomus sergenti (Parrot, 1917) identified as Leishmania killicki host in Ghardaïa, south Algeria. Microbes Infect. 2011, 13, 691–696. [Google Scholar] [CrossRef]
- Tabbabi, A.; Aoun, K.; Rhim, A.; Bousslimi, N.; Bouratbine, A. First Report on Natural Infection of Phlebotomus sergenti with Leishmania Promastigotes in the Cutaneous Leishmaniasis Focus in Southeastern Tunisia. Am. J. Trop. Med. Hyg. 2011, 85, 646–647. [Google Scholar] [CrossRef][Green Version]
- Harrat, Z.; Boubidi, S.C.; Pratlong, F.; Benikhlef, R.; Selt, B.; Dedet, J.P.; Ravel, C.; Belkaid, M. Description of a dermatropic Leishmania close to L. killicki (Rioux, Lanotte & Pratlong 1986) in Algeria. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 716–720. [Google Scholar] [CrossRef]
- Rioux, J.-A.; Croset, H.; Guy, Y.; Maistre, M. Présence de Phlebotomus (Paraphlebotomus) chahaudi Croset, Abonnenc et Rioux, 1970 en Algérie. Annales de Parasitologie Humaine et Comparée 1970, 45, 875–880. [Google Scholar] [CrossRef][Green Version]
- Rioux, J.A.; Croset, H.; Leger, N.; Benmansour, N.; Soussi, M.C.; Maistre, M.; Jonquet, Y. Présence au Maroc de Phlebotomus bergeroti, Phlebotomus chabaudi, Phlebotomus chadlii et Sergentomyia christophersi. Annales de Parasitologie Humaine et Comparée 1975, 50, 493–506. [Google Scholar] [CrossRef][Green Version]
- Petriščeva, P.A. The natural focality of leishmaniasis in the USSR. Bull. World Health Organ. 1971, 44, 567–576. [Google Scholar]
- Javadian, E.; Nadim, A. Studies on cutaneous leishmaniasis in Khuzestan, Iran. Part II. The status of sandflies. Bulletin de la Societe de Pathologie Exotique et de ses Filiales 1975, 68, 467–471. [Google Scholar]
- Guerbouj, S.; Chemkhi, J.; Kaabi, B.; Rahali, A.; Ben Ismail, R.; Guizani, I. Natural infection of Phlebotomus (Larroussius) langeroni (Diptera: Psychodidae) with Leishmania infantum in Tunisia. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Doha, S.; Shehata, M.G. Leishmania infantum MON-98 Isolated from Naturally Infected Phlebotomus langeroni (Diptera: Psychodidae) in El Agamy, Egypt. J. Med Entomol. 1992, 29, 891–893. [Google Scholar] [CrossRef] [PubMed]
- El Sawaf, B.M.; Beier, J.C.; Hussein, S.M.; Kassem, H.A.; Satter, S.A. Phlebotomus langeroni: A potential vector of kala-azar in the Arab Republic of Egypt. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 421. [Google Scholar] [CrossRef]
- Kowalski, K.; Rzebik-Kowalska, B. Mammals of Algeria. Polish Academy of Sciences, 1st ed.; Institut of Systematics and Evolution of Animals, Ossolineum: Wrocław, Poland, 1991; p. 370. [Google Scholar]
- Sereno, D.; Harrat, Z.; Eddaikra, N. Meta-analysis and discussion on challenges to translate Leishmania drug resistance phenotyping into the clinic. Acta Trop. 2019, 191, 204–211. [Google Scholar] [CrossRef]
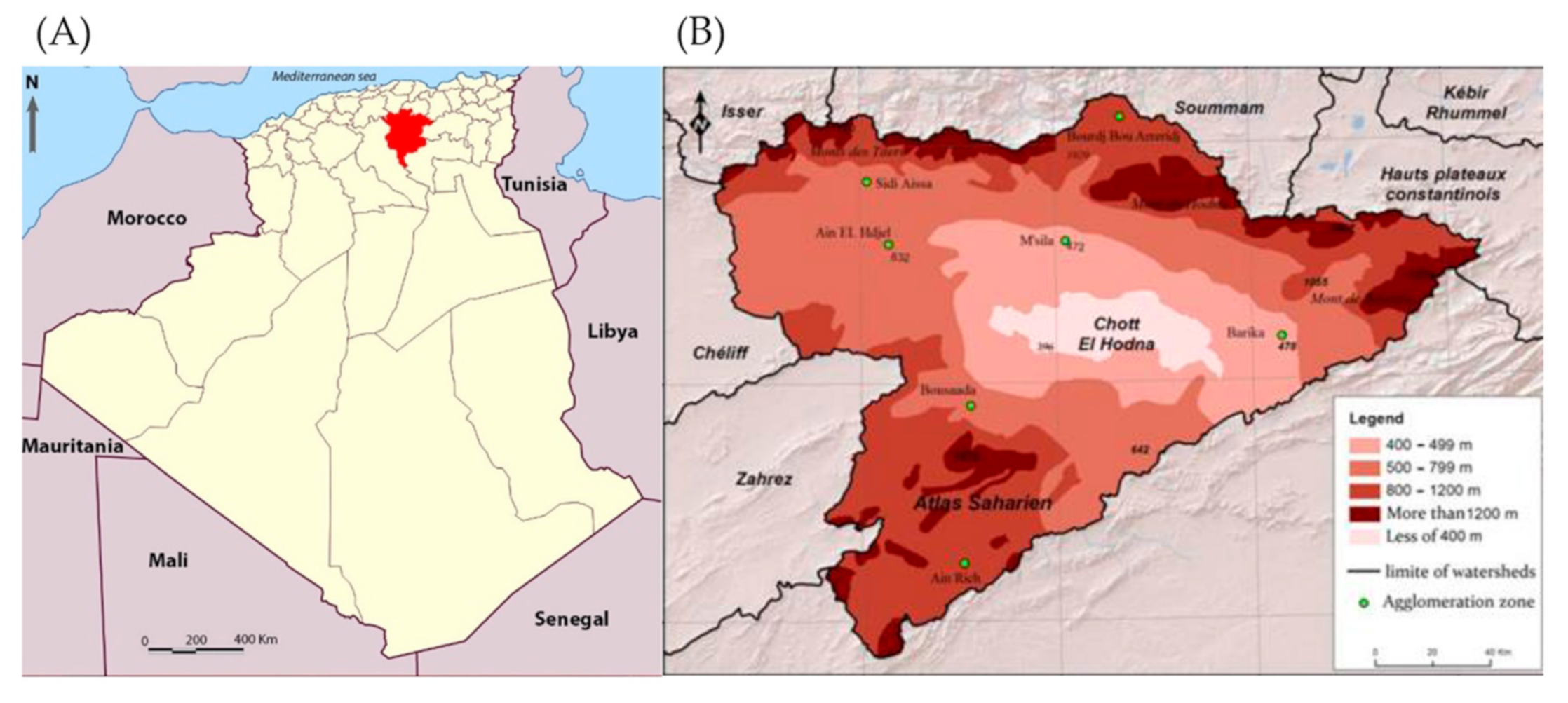



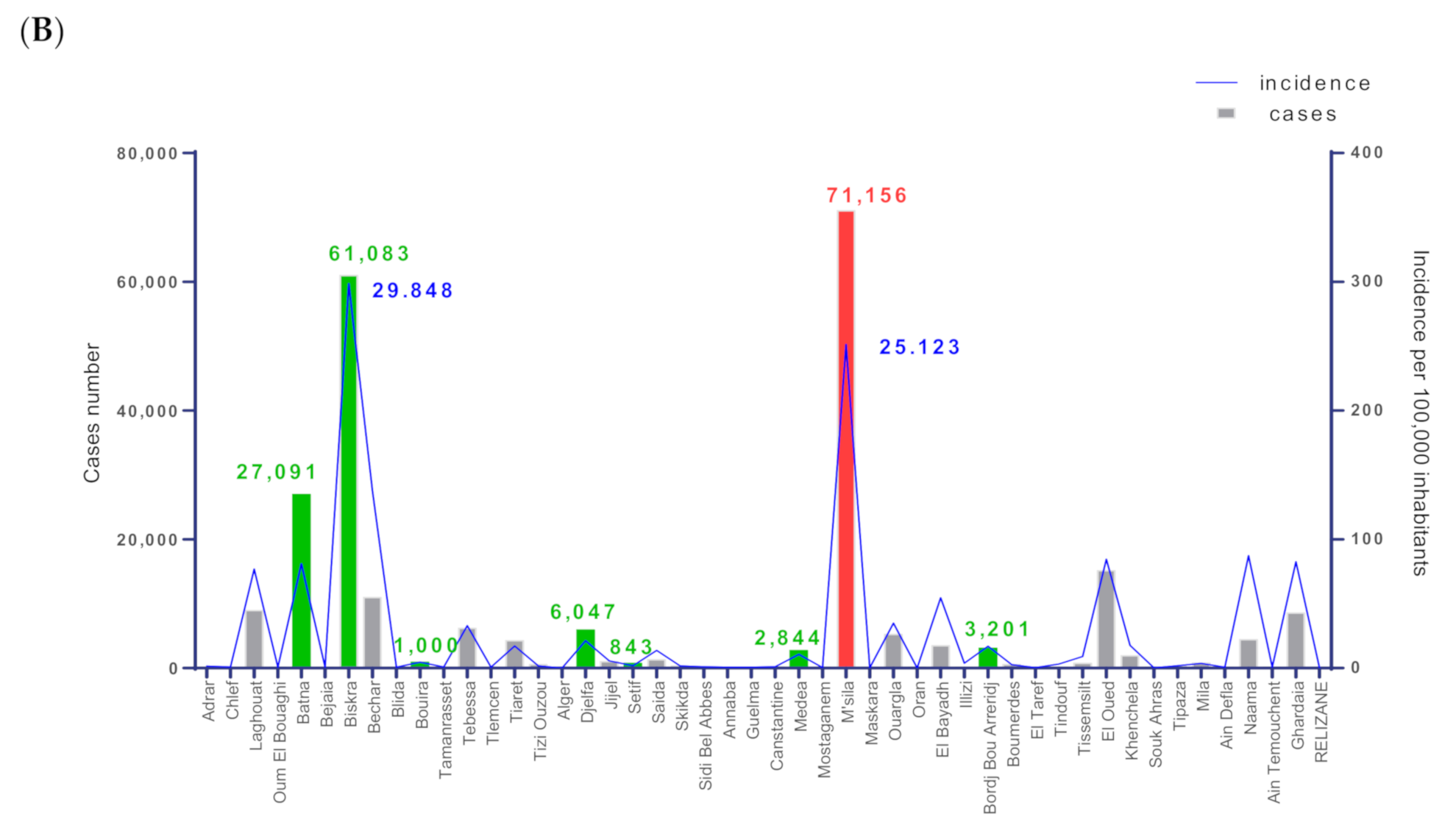
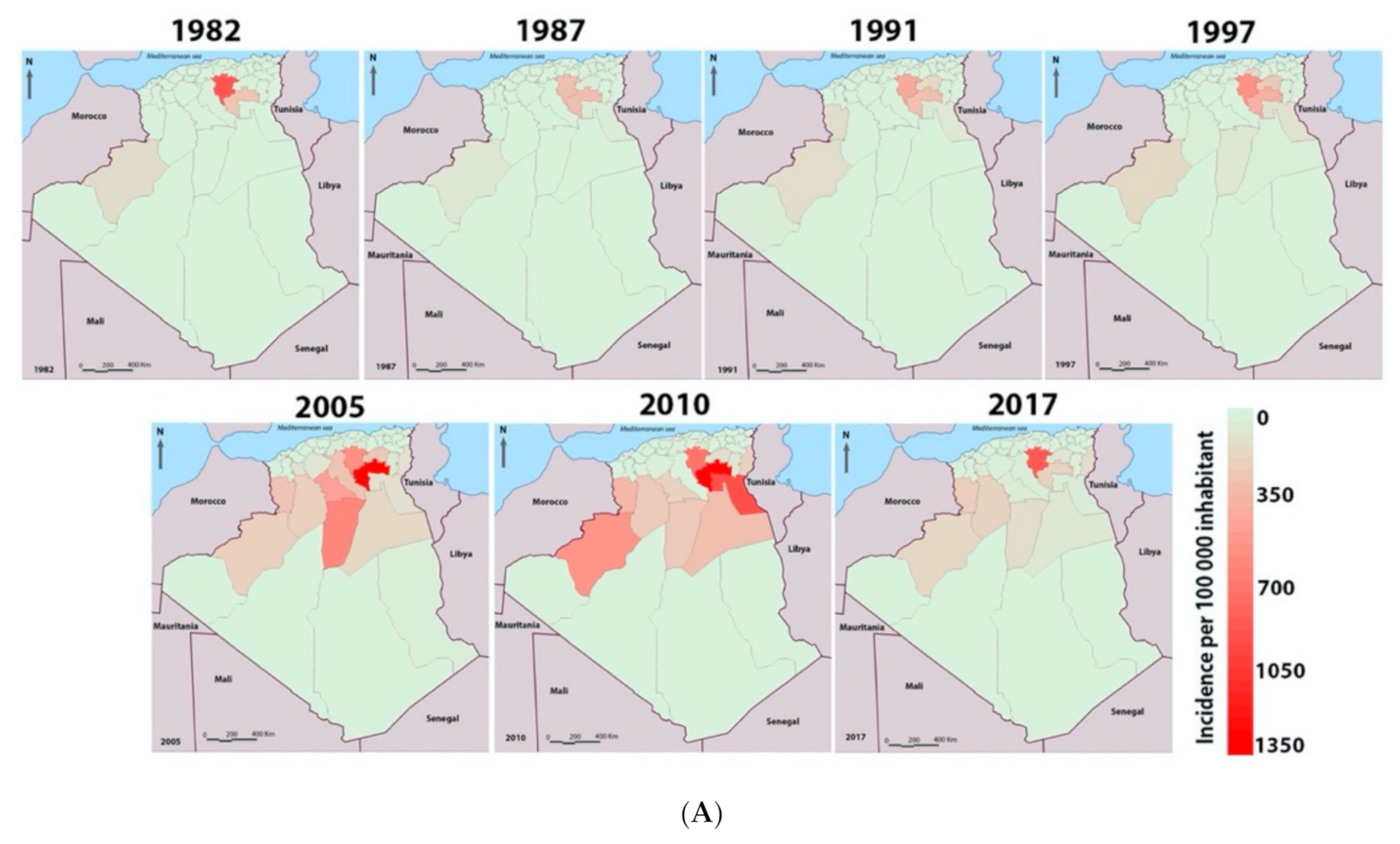
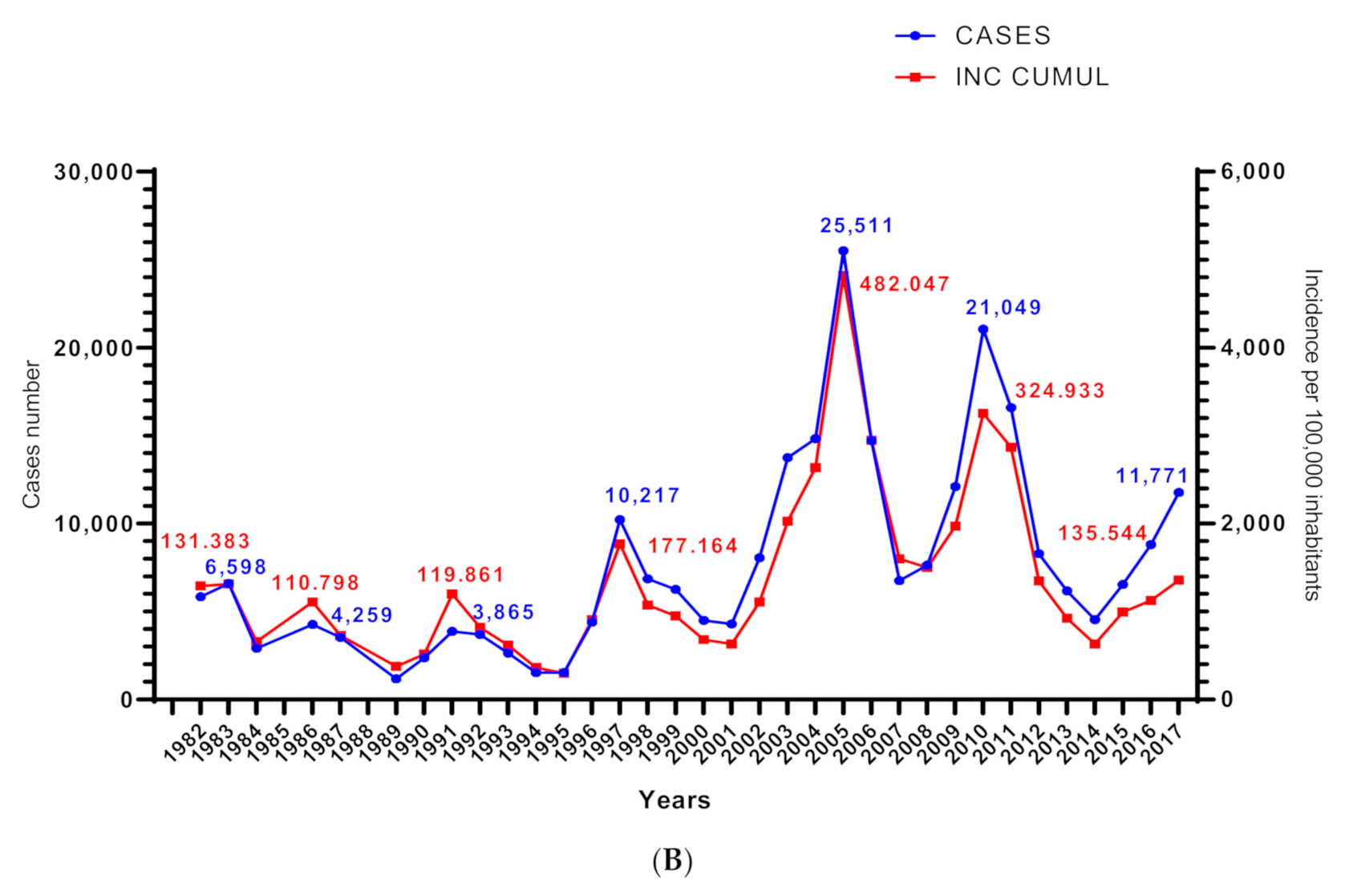





| Districts | Station Name | Geo Reference | Elevation (m) | Description | Specimen | |
|---|---|---|---|---|---|---|
| n | % | |||||
| M‘sila | Naoura | 35°39′ N/04°31′ E | 475 | Rural area. Ovine and bovine population. | 1264 | 11.81 |
| Boukhmissa | 35°43′ N/04°33′ E | 475 | Urban area, with a bovine population. | 173 | 1.62 | |
| Chellal | Ouled Madhi | 35°34′ N/4°30′ E | 415 | Rural area. South of M’Sila, outskirts of Hodna’s chott. Presence of rodent burrows. | 908 | 8.48 |
| Khettouti Sed El Djir | 35°37′ N/4°10′ E | 460 | Rural area. | 82 | 0.77 | |
| Ait Ikhlef | 35°36′ N,4°29′ E | Rural area. | 79 | 0.74 | ||
| Magra | Berrhoum | 35°39′ N/5°2′ E | 596 | Rural area. | 33 | 0.31 |
| Ain El Khadra | 35°32′ N/4°58′ E | 446 | Rural area. | 11 | 0.10 | |
| Dehahna | ND | ND | Rural area. | 60 | 0.56 | |
| Hammam Dalaa | El Guetaf | ND | ND | ND | 64 | 0.60 |
| OuledDerradj | Maadid | 35°55′ N/04°22′ E | 1000 | Rural and semi-arid areas. | 301 | 2.83 |
| Ain El Hadjel | Ain El Hadjel | 35°40′ N/03°52′ E | 500 | Steppe with farming | 2773 | 25.90 |
| Sidi Aissa | Sidi Aissa | 36°30′ N/04°17′ E | 1003 | Rural area. | 50 | 0.47 |
| Ain El Melh | Ain El Melh | 34°50′ N/04°9′ E | 948 | Rural area. | 20 | 0.19 |
| Bou Saâda | Boussaâda | 35°12′ N-04°12′ E | ND | Palm grove and Oued. Presence of goats, chickens, and pigeons | 4888 | 45.66 |
| Locality | Family | Genus/Species | Year | N Tot | N Inv | N Inf | N Isol |
|---|---|---|---|---|---|---|---|
| Chellal, Sidi Aissa, other | Gerbillidae | Psammomys obesus | 1996 | ND | ND | ND | 3 |
| 2003 | ND | 10 | 7 | 4 | |||
| 2004 | 60 | 30 | 5 | 4 | |||
| 2007 | ND | ND | ND | 6 | |||
| 2010 | 223 | 144 | 43 | - | |||
| 2018 | ND | 12 | - | - | |||
| M’Sila, Sidi Aissa, Magra, Ain-El-Hadjel | Gerbillidae | Meriones shawi | 1996 | ND | ND | ND | 1 |
| 2000 | ND | ND | ND | 1 | |||
| 2004 | ND | 10 | 3 | - | |||
| 2008 | 49 | 20 | 6 | - | |||
| 2009 | ND | ND | ND | 2 | |||
| 2012 | 12 | 6 | 1 | - | |||
| 2018 | ND | 1 | - | - | |||
| M’Silasila | Gerbillidae | Meriones lybicus | ND | 13 | 6 | - | - |
| Boussaâda, M’Sila, Ain-El-Hadjel, Khoubana | Gerbillidae | Gerbillus sp. | ND | 46 | ND | - | - |
| 2018 | ND | 3 | - | - | |||
| Boussaâda | Gerbillidae | Ctenodactylus gundi | ND | 12 | 12 | - | - |
| Ain-El-Melh | 2018 | ND | 2 | - | - | ||
| M’Sila, Hamama, Delaa | Dipodidae | Jaculus jaculus | ND | 12 | 6 | - | - |
| Ain-El-Melh, Khettouti, Sed-El-Djir | Dipodidae | Jaculus orientalis | ND | 11 | 4 | - | - |
| Ain-El-Mehl, Boussaâda, Hamama, Dalaa, Khettouti, Sed-El-Djir | Muridae | Rattus ratus | ND | 5 | 5 | - | - |
| Hamama, Dalaa | Macroscelidae | Elephantus rozeti | ND | 2 | 2 | - | - |
| Total | 445 | 273 | 65 | 21 |
| Number of Samples | % | Sensitivity | ||||
|---|---|---|---|---|---|---|
| Test | % | IC | ||||
| Positive Case | Negative Case | Positive Case | Negative Case | |||
| Smears | 44 | 52 | 45.83 | 54.16 | 62 | [50.7–73.3] |
| Culture | 51 | 44 | 53.68 | 46.31 | 72.9 | [62.4–83.3] |
| ITS1-PCR | 54 | 40 | 57.44 | 42.55 | 78.3 | [66.5–88.0] |
| RT-PCR | 69 | 25 | 73.40 | 26.59 | 100 | [94.8–100] |
| Total | 71 | 25 | 73.95 | 26.04 | ||
| District | Station | P. papatasi | P. perniciosus | P. alexandri | P. sergenti | P. chabaudi | P. longicuspis | P. perfiliewi | P. langeroni | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| M’Sila | Nouara | 349 | 125 | 2 | 1 | 0 | 655 | 2 | 0 | 1134 |
| Boukhmissa | 73 | 30 | 0 | 1 | 0 | 9 | 0 | 0 | 113 | |
| Chellal | Ouled Madhi | 178 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 182 |
| Khettouti Sed El Djir | 52 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 54 | |
| Ait Ikhlef | 60 | 5 | 0 | 0 | 2 | 3 | 0 | 0 | 70 | |
| Magra | Berhoum | 23 | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 30 |
| Ain El Khadra | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | |
| Dehahna | 27 | 14 | 0 | 0 | 0 | 6 | 0 | 0 | 47 | |
| Hammam Dalaa | El Guetaf | 28 | 3 | 2 | 0 | 0 | 12 | 1 | 0 | 46 |
| Ouled Derradj | Maadid | 28 | 199 | 3 | 36 | 0 | 29 | 0 | 0 | 295 |
| Ain El Hadjel | Ain El Hadjel | 2525 | 170 | 20 | 27 | 0 | 9 | 4 | 1 | 2756 |
| Sidi Aissa | Sidi Aissa | 39 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 43 |
| Ain El Melh | Ain El Melh | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Boussaâda | Boussaâda | 3658 | 4 | 6 | 6 | 210 | 912 | 0 | 0 | 4796 |
| Total | 7059 | 562 | 35 | 72 | 212 | 1637 | 7 | 1 | 9585 |
| District | Station | S. minuta | S. schwetzi | S. fallax | S. antennata | S. dreyfussi | S. lewisi | S. christophersi | S. clydei | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| M’Sila | Nouara | 23 | 51 | 21 | 5 | 30 | 0 | 0 | 0 | 130 |
| Boukhmissa | 5 | 1 | 0 | 53 | 0 | 0 | 0 | 1 | 60 | |
| Chellal | Ouled Madhi | 40 | 67 | 355 | 263 | 1 | 0 | 0 | 0 | 726 |
| Khettouti Sed El Djir | 0 | 0 | 0 | 28 | 0 | 0 | 0 | 0 | 28 | |
| Ait Ikhlef | 4 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 9 | |
| Magra | Berhoum | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| Ain El Khadra | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 5 | |
| Dehahna | 1 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 13 | |
| Hammam Dalaa | El Guetaf | 0 | 1 | 0 | 17 | 0 | 0 | 0 | 0 | 18 |
| Ouled Derradj | Maadid | 4 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 6 |
| Ain El Hadjel | Ain El Hadjel | 16 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 17 |
| Sidi Aissa | Sidi Aissa | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 7 |
| Ain El Melh | Ain El Melh | 2 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 7 |
| Boussaâda | Boussaâda | 59 | 0 | 8 | 6 | 15 | 3 | 1 | 0 | 92 |
| Total | 156 | 122 | 389 | 403 | 46 | 3 | 1 | 1 | 1121 |
| District | Family | Genus/Species | Year | N Inv | N Inf | Infestation Rate (%) | CI |
|---|---|---|---|---|---|---|---|
| Chellal, Sidi Aissa, other | Gerbillidae | Psamomys obesus | 1996 | - | - | - | |
| 2003 | 10 | 7 | 70 | [38.01–91.74] | |||
| 2004 | 30 | 5 | 16.66 | [6.37–33.15] | |||
| 2007 | - | - | - | ||||
| 2010 | 144 | 43 | 29.86 | [22.81–36.34] | |||
| 2018 | 12 | ||||||
| Total | 186 | 55 | 29.57 | [23.34–36.43] | |||
| M’Sila, Sidi Aissa, Magra, Ain-El-Hadjel | Gerbillidae | Meriones shawi | 1996 | - | - | ||
| 2000 | - | - | |||||
| 2004 | 10 | 3 | 30 | [8.26–61.99] | |||
| 2008 | 20 | 6 | 30 | [13.16–52.28] | |||
| 2009 | - | - | |||||
| 2012 | 6 | 1 | 16.66 | [0.83–59.09] | |||
| 2018 | 1 | ||||||
| Total | 37 | 10 | 27.02 | [14.62–42.91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benikhlef, R.; Aoun, K.; Boudrissa, A.; Ben Abid, M.; Cherif, K.; Aissi, W.; Benrekta, S.; Boubidi, S.C.; Späth, G.F.; Bouratbine, A.; et al. Cutaneous Leishmaniasis in Algeria; Highlight on the Focus of M’Sila. Microorganisms 2021, 9, 962. https://doi.org/10.3390/microorganisms9050962
Benikhlef R, Aoun K, Boudrissa A, Ben Abid M, Cherif K, Aissi W, Benrekta S, Boubidi SC, Späth GF, Bouratbine A, et al. Cutaneous Leishmaniasis in Algeria; Highlight on the Focus of M’Sila. Microorganisms. 2021; 9(5):962. https://doi.org/10.3390/microorganisms9050962
Chicago/Turabian StyleBenikhlef, Razika, Karim Aoun, Abdelkarim Boudrissa, Meriem Ben Abid, Kamel Cherif, Wafa Aissi, Souad Benrekta, Said C. Boubidi, Gerald F. Späth, Aïda Bouratbine, and et al. 2021. "Cutaneous Leishmaniasis in Algeria; Highlight on the Focus of M’Sila" Microorganisms 9, no. 5: 962. https://doi.org/10.3390/microorganisms9050962
APA StyleBenikhlef, R., Aoun, K., Boudrissa, A., Ben Abid, M., Cherif, K., Aissi, W., Benrekta, S., Boubidi, S. C., Späth, G. F., Bouratbine, A., Sereno, D., & Harrat, Z. (2021). Cutaneous Leishmaniasis in Algeria; Highlight on the Focus of M’Sila. Microorganisms, 9(5), 962. https://doi.org/10.3390/microorganisms9050962






