Abstract
This review outlines the known cellular pathways and mechanisms involved in Drosophila age-dependent immunity to pathogenic microorganisms such as bacteria and fungi. We discuss the implication of host signaling pathways such as the Toll, Immune Deficiency (IMD), Janus kinase signal transducer and activator of transcription (JAK/STAT), and Insulin/Insulin Growth Factor/Target of Rapamycin (IIS/TOR) on immune function with aging. Additionally, we review the effects that factors such as sexual dimorphism, environmental stress, and cellular physiology exert on age-dependent immunity in Drosophila. We discuss potential tradeoffs between heightened immune function and longevity in the absence of infection, and we provide detailed tables outlining the various assays and pathogens used in the cited studies, as well as the age, sex, and strains of Drosophila used. We also discuss the overlapping effects these pathways and mechanisms have on one another. We highlight the great utility of Drosophila as a model organism and the importance of a greater focus on age-dependent antiviral immunity for future studies.
1. Introduction
The common fruit fly, Drosophila melanogaster, with its short lifespan, low cost of culture, and potent conserved innate immune defenses against a variety of microorganisms, serves as an excellent model for investigating the consequences of immunosenescence, a conserved process characterized by the progressive decline of the immune system’s function with age [1,2,3,4]. In humans, immunosenescence is associated with a decreased ability to defend against infections, resulting in significant morbidity and mortality among the elderly [5,6,7,8]. Despite considerable progress made towards our understanding of immunosenescence, the genetic and molecular mechanisms underlying age-dependent responses to immune challenges are areas of ongoing research. Specifically, the interplay between aging and innate immunity, which represents the first line of defense against microbial invaders, is less well understood, and often falls behind studies of the aging adaptive immune system [9]. Individuals aged 65 and older currently outnumber children under 5 globally [10] and, thus, a deeper understanding of the mechanisms underlying immunosenescence has become exceedingly important. A vast repertoire of genetic and genomic tools is available to Drosophila researchers, and the high degree of genetic homology between Drosophila and humans [11] makes the modeling of human diseases and immunosenescence in Drosophila highly translatable [12].
Drosophila lack an adaptive immune response but do have conserved pathways underlying innate immunity, which, in mammalian systems, play a key role in the age-dependent response to infections [13,14]. The Toll and Immune deficiency (IMD) pathways in Drosophila are nuclear factor kappa B (NF-κB) pathways with similarities to mammalian Toll-like receptor/interleukin (IL)-1 receptor and tumor necrosis factor receptor (TNFR) pathways, respectively. In response to fungal and bacterial infection, activation of Toll and IMD pathways leads to the transcription of downstream antimicrobial peptides (AMPs) genes (reviewed in [15]). Notably, the expression of several AMP genes increases with age [16,17,18]. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, which, in mammals, is the main signaling downstream of cytokines and their receptors [19], is involved in response to Drosophila C Virus (DCV) infection in Drosophila [20]. This pathway is also activated in response to bacterial pathogens and is especially important in maintaining homeostasis in the gut, where dysbiosis exacerbates with age [21,22,23,24,25]. The Insulin/Insulin Growth Factor signaling pathway (IIS) which, together with the Target of Rapamycin (TOR) signaling pathway forms the IIS/TOR network, regulates autophagy, detoxification, and protein synthesis in Drosophila. Perhaps most importantly, the IIS/TOR pathway is key to lifespan determination in Drosophila (reviewed in [26]), and it has a linked function in Drosophila immunity due to its interaction with the Toll, IMD, and JAK/STAT pathways in Drosophila [27,28,29,30,31]. The Forkhead box, sub-group O transcription factor in Drosophila (dFOXO), a downstream target of the IIS/TOR pathway, binds to the promoter region of the AMP gene Drosomycin and induces its expression in response to starvation [27]. In Drosophila, dFOXO is required for defense against viral pathogens such as the Cricket paralysis virus (CrPV) and the Flock House virus (FHV) [32], and it plays a role in intestinal immunity to the bacterial pathogen Serratia marcescens (S. marcescens) [33].
In addition to these distinct cellular signaling pathways (Figure 1), other factors such as sexual dimorphism, environmental stress, and phagocytic efficacy affect Drosophila immunity in an age-dependent fashion. This review will focus on these key pathways and factors, as well as the relevant research assays involved in the study of age-dependent immune responses in Drosophila.
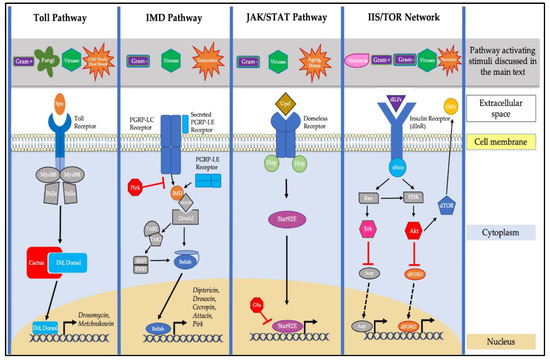
Figure 1.
Pathways in Drosophila involved in aging innate immunity. The Toll pathway is traditionally activated by Gram-positive bacteria and fungi, and it is also implicated in the response to viral infection, heat shock, and cold shock stresses. Toll activation, via its ligand Spätzle (Spz), leads the degradation of the Cactus inhibitor and subsequent nuclear localization of the NF-κB transcription factors Dif and Dorsal. This leads to the transcription of antimicrobial genes such as Drosomycin and Metchnikowin. The Immune deficiency (IMD) pathway is primarily activated by Gram-negative bacteria, and is also implicated in the response to viral infection and desiccation stress. Activation of the IMD pathway leads to the nuclear localization of the NF-κB transcription factor Relish and subsequent transcription of genes, including Diptericin, Drosocin, Cecropin, Attacin, and Pirk. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is activated via Unpaired (Upd) cytokine detection in response to viral and bacterial infection, aging, and stress. This leads to the nuclear localization of the Drosophila Stat92E factor and subsequent activation of transcription. The Drosophila Insulin/Insulin Growth Factor/Target of Rapamycin (IIS/TOR) signaling network regulates growth and nutrition via binding of insulin-like peptides (dILPs) to the insulin receptor (dInR). There is a singular insulin substrate, Chico, that induces two downstream signaling cascades, one via the Akt1 kinase and the other via the extracellular signal-regulated kinase (ERK). The IIS/TOR network is impacted by viral (dFOXO specifically), Gram-positive, and Gram-negative bacterial infections, as well as starvation stress. Activation of dInR subsequently affects the activity of the Target of Rapamycin (dTOR) factor, as well as the dFOXO transcription factor.
2. Pathways Affecting Age-Dependent Immunity in Drosophila
2.1. The Role of the Toll and IMD Pathways in Drosophila Age-Dependent Immunity
Drosophila have an inducible antimicrobial immune response that combats pathogenic infections with bacteria and fungi. This response is characterized by the induction of antimicrobial peptides (AMPs) [34], which is controlled by the nuclear factor-κB (NF-κB) family of transcription factors (TFs). NF-κB TFs such as Dorsal (dl), Dif, and Relish are activated through two separate signaling cascades: The Toll and IMD pathways [35,36]. The Toll pathway is primarily activated in response to Gram-positive bacterial and fungal pathogens [35,37], while the IMD pathway is mostly activated in response to Gram-negative bacteria [38,39,40,41]. These conserved innate immune pathways undergo age-dependent changes in function and gene expression. For example, Drosophila naturally display increased expression of AMP genes with age [16,17,18,42,43]. Elevated AMP expression typically allows for a more persistent induction of innate immune responses following infection with bacterial pathogens [18]. Some may hypothesize that heightened AMP expression in older individuals allows aged Drosophila to respond more efficiently to infection, but studies suggest the opposite. Older flies exposed to both live and killed bacteria (mixture of Escherichia coli (E.coli) and Micrococcus luteus (M. luteus)) induce less Diptericin, an AMP primarily induced via the IMD pathway, after a heat-killed bacterial jab than younger flies treated simultaneously. Despite the progressive upregulation of AMPs with age, the intrinsic capacity of older flies to effectively induce AMPs and defend against infection is shown to decline with age in Drosophila [18].
Age-dependent decline in immune function is conserved across species [3], but there are studies in which aged Drosophila display a lower bacterial load than younger Drosophila after infection. For example, Khan and Prasad [44] found that, following S. marcescens infection, a 13-day-old (aged) LH laboratory population of Drosophila displayed lower bacterial loads than younger flies (3 and 8 days old, respectively). However, this study did not include infection survival assays, and studies that do include infection survival assays with long-lived (~80 days median survival) Drosophila, such as chico null mutants, have found that survival to bacterial infection is not linked to AMP gene expression [45]. Additionally, there are several examples linking overactivation of Drosophila NF-κB pathways and over-expression of AMPs with reduction in lifespan [16,46,47]. Kounatidis and colleagues found that brain-specific knockdown of Relish increased Drosophila lifespan, while overexpression of the AMP genes AttacinC, Drosocin and CecropinA1 in neural tissue reduced lifespan [16]. Badinloo and colleagues showed that ubiquitous overexpression of the AMPs AttacinA, Metchnikowin, CecropinA1 and Defensin resulted in reduced lifespan [47]. In another example, Fabian and colleagues found that Drosophila longevity was increased following ubiquitous RNAi knockdown of the genes encoding for Toll (Tl) receptor and its ligand Spätzle (spz). Drosophila longevity decreased ~50% following ubiquitous RNAi knockdown of the Toll pathway inhibitor cactus (resulting in overactivation of Toll signaling). The same study also found that long-lived (median lifespan of 62.5–72 days) Drosophila caught in a peach orchard in Michigan displayed increased expression of the AMP genes Drosomycin, AttacinA, and Diptericin at a young age (5–6 days old), and decreased expression of these AMP genes at an older age (25–26 days old) following Erwinia carotovora carotovora (Ecc15) infection, in comparison to a control line [46].
Thus, it is apparent that NF-κB signaling, the ability to defend against infection, and longevity are interconnected in Drosophila. The importance of NF-κB signaling at older age from the Fabian et al., [46] study is in support of earlier findings showing that RelE20 and RelE38 Relish null mutations completely eliminate the improved ability of flies over-expressing the intracellular receptor PGRP-LE to defend against Pseudomonas aeruginosa (P. aeruginosa) infection [48]. Additionally, although fat body-specific over-expression of PGRP-LE offered enhanced pathogen resistance in both young (7 day-old) and older (40 day-old) animals, this chronic activation of NF-κB signaling resulted in a shorter lifespan in the absence of infection in comparison to Drosophila carrying a Relish null mutation [48].
All of these findings suggest a tradeoff between pathways involved in immune signaling and longevity in Drosophila. Findings from Sinam and colleagues [49] build upon this trend. The authors compared the lifespan and response to bacterial and fungal pathogens of two Cytorace lines derived from hybridization of Drosophila nasuta nasuta and Drosophila nasuta albomicans, and which were at different stages of evolutionary divergence. They showed that Cytorace-9 flies failing to upregulate expression of the Cecropin gene were more susceptible to infection with the fungal pathogens Beauveria bassiana (B. bassiana) and Metarhizium anisopliae (Table 1) in comparison to Cytorace-3 flies, which upregulate Cecropin expression after infection. Cytorace-9 flies also showed increased lifespan in the absence of infection when compared to Cytorace-3 flies. While increased AMP gene expression may offer increased pathogen resistance, especially at a younger age, this heightened immune signaling negatively impacts lifespan in the absence of infection. However, given that survival to bacterial infection was shown to not be linked to AMP gene expression in long-lived chico Drosophila mutants [45], this tradeoff between immune signaling and longevity in Drosophila is likely more complex than current research suggests [49]. Further research is necessary to fully elucidate the complex nature of the age-dependent increase of AMP expression in Drosophila and how it affects lifespan and response to infection.

Table 1.
Studies implicating the Toll and IMD pathways in age-dependent immunity.
2.2. The Role of the JAK/STAT Pathway in Drosophila Age-Dependent Immunity
The Janus kinase (JAK) signal transducer and activator of transcription (STAT) signaling pathway is induced by the cytokines of the Unpaired family, such as Unpaired 1 (Upd1), Unpaired 2 (Upd2), and Unpaired 3 (Upd3), in response to cellular stress and damage [19,50,51]. The JAK/STAT signaling pathway plays an important role in Drosophila adult midgut homeostasis, ensuring continuous renewal of this organ throughout the animal’s lifespan [51]. The Toll pathway does not function in the Drosophila gut [52], and the JAK/STAT pathway contributes to gut antimicrobial response through the induction of Drosomycin-like peptides [24]. Given that dysfunction of the intestinal barrier is a hallmark of aging in Drosophila [53], and that a functional intestinal barrier during aging is critical to maintaining lifespan [54], it is thus understandable how JAK/STAT signaling may be involved in age-dependent immunity in Drosophila.
Intestinal epithelial renewal is a key defense against oral bacterial infection in Drosophila, and the JAK/STAT pathway is required for bacterially induced stem cell proliferation in response to infection, stress, or damage [21,22,24,54]. Salazar and colleagues [54] studied the effects of altered expression of the septate junction-specific protein Snakeskin (Ssk), in combination with the JAK/STAT pathway, and observed significant effects on intestinal barrier dysfunction, dysbiosis, lifespan, and gut morphology (Table 2). Decreased expression of intestinal Ssk resulted in increased barrier dysfunction, reduced lifespan, elevated expression levels of the AMP genes Diptericin, Drosocin, Drosomycin, and Metchnikowin, and increased upd3 mRNA levels. Restoration of Ssk expression completely reversed age-related intestinal barrier dysfunction, protected against S. marcescens infection, and increased lifespan. Thus, there is a correlation between increased upd3 expression, an activator of the JAK/STAT pathway, and junctional protein mislocalization before detectable intestinal barrier failure. This also aligns with previous studies demonstrating elevated upd3 expression in fly mutants for the human CD36 homologue croquemort (crqKO), which is involved in microbial phagocytosis and clearance. crqKO mutants are short-lived and exhibit premature aging associated with gut hyperplasia [55]. This suggests that proper regulation of the JAK/STAT pathway is central in preventing age-dependent gut dysfunction and protecting against infection.

Table 2.
Studies implicating the JAK/STAT pathway in age-dependent immunity.
In addition to its role in age-dependent intestinal barrier homeostasis and defense against oral bacterial infection, the JAK/STAT pathway functions in antiviral immunity, particularly in response to infection with the RNA viruses Drosophila C Virus (DCV) and Cricket paralysis virus (CrPV) [20,56], as well as the DNA virus Invertebrate iridescent Virus 6 (IIV-6) [57]. In Drosophila, the histone H3 lysine 9 methyltransferase G9a was shown to negatively regulate JAK/STAT pathway activation in response to RNA virus infection in order to limit immunopathology caused by hyperactivation of this pathway [58]. Although G9a mutants appear to have increased lifespan in comparison to respective wild-type controls, these flies succumb faster to infection with the RNA viruses FHV, CrPV, DCV and Drosophila X virus (DXV) [58]. Fabian and colleagues [46] identified upd3 as a candidate gene for longevity in Drosophila and infected long-lived (median lifespan of 62.5–72 days) wild-caught Drosophila from a Michigan peach orchard at 5–6 days of age with DCV. Flies of this long-lived strain injected with DCV survived significantly longer than random-bred control lines of the same population, suggesting that prolonged lifespan may be significantly linked to improved realized antiviral immune response. These findings further complicate the relationship between aging and immunity and the fitness tradeoffs that may arise from favoring expression of genes important for extending lifespan or for defense against infection. Maintaining epithelial junctions may be critical to prolonging lifespan and defending against infection with age, but further research is needed to fully understand the complexity of these systems and what fitness tradeoffs come as a result of age-dependent changes in JAK/STAT signaling.
2.3. The Role of the IIS/TOR Network in Drosophila Age-Dependent Immunity
The Insulin/Insulin Growth Factor (IIS) signaling pathway and its linked Target of Rapamycin (TOR) signaling pathway are vital nutritional systems that regulate growth in Drosophila [59]. Drosophila have one Insulin receptor (dInR), a singular insulin receptor substrate (chico), one downstream dFOXO transcription factor, and eight dInR ligands: insulin-like peptides (dILPs) 1–8 [60,61]. Activation of dInR induces two possible downstream signaling cascades, one via the kinase Akt1 and the other via the mitogen activated protein (MAP) kinase Rolled, also known as extracellular signal-regulated kinase (ERK). Akt1 negatively regulates dFOXO and positively regulates TOR [62,63]. Rolled (ERK) activation is implicated in cellular growth and insulin sensitivity [64,65].
IIS signaling pathway functionality is directly linked to longevity and immunity in Drosophila. The Toll, IMD, and JAK/STAT pathways interact with the IIS signaling pathway in the Drosophila fat body to regulate metabolism, growth, and immunity. dFOXO regulates AMP gene expression [27], and the Toll NF-κB transcription factor Dif can inhibit insulin signaling [28]. Additionally, IIS and subsequent Rolled/MAPK induction can activate the fly poor Imd response upon knock-in (Pirk), a gene encoding for a negative regulator of the IMD pathway [64,66,67,68,69]. Furthermore, the Drosophila fat body signals with insulin producing cells (IPCs) located in the Drosophila brain to communicate nutrient status and the release of insulin-like peptides (dILPs). This is accomplished via secretion of another cytokine from the Unpaired family, Unpaired 2 (Upd2), a ligand for the JAK/STAT pathway, when Drosophila are in a fed state [70]. This all emphasizes the possible role of the IIS signaling pathway in age-dependent immunity, given that the Toll, IMD, and JAK/STAT pathways have significant roles in Drosophila aging and immunity that have already been discussed.
Reduced IIS signaling extends lifespan [71,72], slows or delays age-dependent organ degeneration [73], and improves climbing ability in Drosophila [74]. A study from Ueda and colleagues found that increased expression of the miRNA miR-305, which usually decreases with age, accelerates aging phenotypes in Drosophila. Notably, mRNAs for insulin-like peptides also increased, along with miR-305 expression, further emphasizing the link between reduced IIS/TOR signaling and increased lifespan [75]. Growth-Blocking Peptides (GBPs) are produced as a direct result of TOR signaling. Reduced GBP expression reduces Drosophila growth rate and body size [76]. Genetic analyses from Sung and Shears [77] have linked a previously uncharacterized G Protein Coupled Receptor (GPCR), Methuselah-like receptor-10 (Mthl10), as a binding protein for GBP in Drosophila. As in other studies where reduced IIS/TOR signaling increased lifespan, Mthl10 knockdown in Drosophila also increased lifespan. This increased lifespan as a result of Mthl10 knockdown also affected pathogen defense against infection. Mthl10 knockdown increased mortality in adult Drosophila after infection with M. luteus [77]. This suggests a possible fitness tradeoff between increased longevity and age-dependent resistance to infection via IIS/TOR signaling similar to the tradeoffs seen in the Toll and IMD pathways.
In addition to its known role in regulating lifespan across numerous species, the IIS/TOR pathway also regulates homeostasis of the Drosophila lymph gland, a larval hematopoietic organ important for the generation of blood cell progenitors [78]. Despite the disappearance of the Drosophila lymph gland after the larval stage of development [79], genetic analysis of Drosophila infected with E. coli at 4 weeks of age still found genes involved with the IIS/TOR pathway to be significantly associated with bacterial clearance at older age [80]. Additionally, mutations in chico, a Drosophila insulin receptor substrate, extended lifespan and increased survival of mutant flies following E. coli and Photorhabdus luminescens (P. luminescens) infection [81]. Following E. coli and P. luminescens infection, chico mutants survived bacterial infection better than control flies, and displayed significantly lower amounts of bacterial cells at 3 and 16 h post-infection. However, chico mutants displayed higher bacterial loads at 30 h post-infection in response to E. coli infection, but not P. luminescens infection. These mutants also showed reduced transcription of the AMP genes Diptericin, CecropinA1, and Drosomycin, although pathogen- and time point post infection-based variation was observed [81]. These results suggest a link to age-dependent immunity for the IIS/TOR pathway.
Furthermore, the downstream target of the IIS/TOR signaling pathways, dFOXO, is essential to both intestinal immunity against S. marcescens and defense against viral pathogens [32,33]. In comparison to wild-type controls, dFOXO mutants die faster after oral infection with S. marcescens, and male dFOXO mutants accumulate higher bacterial loads [33]. dFOXO null mutants are also deficient in fighting off infection from both CrPV and FHV [32]. Additionally, dFOXO activity increases in response to virus infection and activated dFOXO can decrease viral load following infection [32], displaying a role for the linked IIS/TOR pathway in Drosophila innate immunity (Table 3). Interestingly, the number of FOXO-bound genes significantly decreases with age in female Drosophila (comparing 2-week-old and 5-week-old w1118 control flies), and many FOXO-targeted genes have altered transcription levels with age [82]. However, it is also notable that the FOXO transcription factor is not only influenced by the IIS/TOR pathway, and many of the observed age-dependent changes in FOXO DNA binding were independent of the Insulin signaling pathway [82]. With its role both within and independent of the IIS/TOR signaling pathway, FOXO will be a transcription factor of very high interest for future studies investigating age-dependent immunity in Drosophila.

Table 3.
Studies implicating the IIS/TOR network and dFOXO Transcription factor in age-dependent immunity.
The IIS/TOR pathway appears to be significantly involved in age-dependent immunity and the control of lifespan in Drosophila, but further research is needed to understand the complexity of these systems and the fitness tradeoffs that may come with reduced IIS/TOR signaling. Additionally, the FOXO transcription factor, its diverse mechanisms in transcriptional regulation, and its altered targeting and function related to the IIS/TOR pathway with age requires further research into the dynamics of FOXO’s roles in both aging and immunity.
3. Other Factors Affecting Drosophila Age-Dependent Immunity
There are various environmental, genetic, and physiological factors that can affect age-dependent immunity in Drosophila. One of these factors is sex [83,84]. The causes of immunosenescence can vary between sexes. Both males and females become more susceptible to infection with age, but the cause of immunosenescence can vary between sexes. Following infection with the fungus B. bassiana, age-dependent decline in immune function for males results from barrier defense deterioration, while, in females, it results from systemic senescence of immune defenses [85]. However, Khan and Prasad found that sex did not affect bacterial load levels following S. marcescens infection [44]. The true significance of sex in respect to age-dependent immunity in Drosophila specifically is yet to be determined but, given the established profound differences in immune response between males and females [84], it can be expected that future immunosenescence research may display significant sexually dimorphic results.
Environmental factors, which have sexually dimorphic effects, also have a significant impact on age-dependent immunity in Drosophila. Cold stress, subjecting Drosophila to periods of extreme low temperatures, has beneficial effects on aging, lifespan, and resistance to stresses such as severe temperature exposure and B. bassiana infection [86,87,88]. Additionally, these environmental stresses have different effects on males and females. Cold stress in male Drosophila increases longevity when applied before 4 weeks of age, while providing no positive effect in female Drosophila at any age. Meanwhile, cold stress can increase heat resistance ability at 6 weeks of age in both sexes, no matter when the cold stress was applied. This displays that exposure to environmental stress early in life can improve resistance to environmental stress later in life, regardless of sex. Prior exposure to cold stress increased survival to fungal infection (B. bassiana) at 6 weeks of age in both sexes, although males were more significantly affected. These differences in pathogen defense between sexes at old age highlights the significance of both environmental stress and sexual dimorphism on age-dependent immunity [87].
Other environmental factors, such as desiccation, also affect age-dependent immunity in Drosophila. Desiccated flies that were kept in empty vials without food for 2 h displayed an increased susceptibility to Ecc15 infection [89]. In the same study, the authors showed that desiccated flies allowed to recover prior to Ecc15 infection display higher survival rates than flies not desiccated prior to infection. This suggests that being under significant environmental stress during infection decreases resistance, but that exposure to an environmental stress with allowed recovery prior to infection increases resistance. This short desiccation period elevates levels of peptidoglycan recognition protein-LC (PGRP-LC) expression in Malpighian tubules (the equivalent of the kidney) and increases induction of the AMP genes CecropinA2, CecropinC, AttacinD, Diptericin, Defensin, and Metchnikowin [89]. This further emphasizes the impact of environmental factors on age-dependent immunity in Drosophila, and these factors should be accounted for when designing experiments relevant to aging and immunity.
In addition to environmental and physiological factors, the functionality of basic cellular mechanisms can have significant impacts on age-dependent immunity in Drosophila. Findings from Horn and colleagues highlight the effects of Drosophila phagocytic efficiency, which significantly declines with age [90]. Notably, the rate of Escherichia coli engulfment after infection does not decline, but the functional clearance of phagocytic vesicles after engulfment decreases considerably with age. Four lines from the Drosophila Genetic Reference Panel (DGRP) collection [91,92] (lines 359, 389, 437, and 589), with increased or decreased bacterial clearance with age, display the same number of phagocytic events per hemocyte, suggesting that the functional clearance of engulfed bacteria, and not engulfment itself, is an important factor in age-dependent immunity [90] (Table 4).

Table 4.
Studies implicating other factors in age-dependent immunity.
4. Conclusions and Future Directions
There are a variety of cellular pathways and mechanisms, as well as environmental and physiological factors, that affect the ability of aged organisms to respond to infection. Additionally, many of these pathways and factors appear to have tradeoff effects on immune function and lifespan. Toll and IMD pathways activate the NF-κB TFs in Drosophila and subsequent AMP expression [15,34], and Drosophila naturally display higher AMP expression at an older age [16,17,18,42,43,46,47]. The Toll and IMD pathways are critical to survival against bacterial, fungal, and viral infection [35,37,38,93,94,95,96], but overactivation of these pathways has been shown to decrease lifespan in the absence of infection [48]. The JAK/STAT signaling pathway contributes to antimicrobial response and homeostasis in the Drosophila gut [51]. Maintaining a functional intestinal barrier is critical to maintaining lifespan, and dysfunction of the intestinal barrier is a hallmark of aging in Drosophila [53,54]. Intestinal epithelial renewal is vital for protection against oral bacterial infection, and the JAK/STAT pathway is required for this process [21,22,24,54]. In addition to maintaining gut homeostasis and defending against bacterial infection, the JAK/STAT pathway is also important for defense against viruses such as DCV, CrPV and IIV-6 infection [20,56,57]. Upd3 has been identified as a candidate for longevity in Drosophila, and wild-caught long-lived fly strains survive longer than controls following DCV infection [46]. The IIS/TOR signaling pathways regulate growth, longevity, and defense against infection in Drosophila [59]. Reduced IIS/TOR signaling extends lifespan [71,72] and has shown contrasting effects on the ability to respond to bacterial infection in adult Drosophila [80,81]. The IIS/TOR network also interacts with the Toll (Dif), IMD (Pirk), and JAK/STAT (Upd2) pathways [28,69,70], further emphasizing its role in Drosophila age-dependent immunity. dFOXO, a transcription factor downstream of the IIS/TOR signaling pathways, has age-dependent changes in its DNA binding activity [82], and is important for defense against viral pathogens such as FHV and CrPV [32]. There are significant differences in response to infection by male and female Drosophila [84]. Different causes have been identified for the age-dependent decline in anti-fungal immune function between male and female Drosophila [85], but this does not appear universal, as another study found no significant differences in bacterial loads between male and female flies [44]. Environmental stressors such as cold stress and desiccation have positive effects on lifespan and resistance to fungal [86,87] and bacterial infection [89]. Additionally, cellular mechanisms such as phagocytic efficiency significantly decline with age following E. coli infection [90].
The studies discussed in this review illustrate at least some of the aspects the impact of aging exerts on immune function in Drosophila. However, further studies are needed to create a more complete understanding of the age-dependent factors and mechanisms that impact Drosophila immune defenses and the fitness tradeoffs between longevity and the ability to defend against infection. Additionally, given the profound impact of viral pathogens on elderly individuals, as illustrated by the ongoing Coronavirus (COVID-19) global pandemic [97,98,99,100] and the relative lack of focus on age-dependent immunity in response to viral infection, there is a great need for further research investigating the age-dependent mechanisms that defend against viral infection specifically. Drosophila can serve as a great genetic model to investigate the impact of age on innate immune responses to viral infection.
Despite well-characterized pathways involved in Drosophila antiviral immunity, such as antiviral RNAi (reviewed in [101,102]) and cellular processes such as autophagy, apoptosis, and apoptotic body clearance (reviewed in [103]), there is very limited understanding of how these antiviral immune responses are impacted by aging. Interestingly, some of the pathways already outlined in this article, which have been shown to have age-dependent effects on Drosophila immunity in other contexts, such as the JAK/STAT, Toll and IMD pathways, are also important for Drosophila antiviral immunity. The JAK/STAT pathway has been shown to mediate survival to DCV, CrPV, FHV, and DXV infection [20,56,58]. Toll pathway mutants die more rapidly following DXV infection [93], and Toll pathway genes have been shown to be important for resistance to several RNA viruses (DXV, DCV, FHV, CrPV and Nora Virus) [93,94]. IMD pathway mutants are more susceptible to CrPV and Sindbis Virus (SINV) infection [95,96]. Thus, these pathways should be of high interest in future Drosophila immunosenescence research. Additionally, antiviral cellular mechanisms, such as phagocytosis via macrophages, display an age-dependent decline in efficiency [90,104].
Another particular area of future research interest is in the importance of disease tolerance mechanisms in response to viral infection at older age. There are two strategies for organisms to defend against infection: resistance and tolerance. Disease resistance encompasses an organism’s ability to combat and prevent infection by pathogens. Disease tolerance encompasses an organism’s ability to tolerate a given level of infection (pathogen load) and limit negative effects after resistance mechanisms have been compromised (reviewed in [105]). Disease tolerance at older age may be very important for antiviral defense in Drosophila. In a recent study, Sheffield and colleagues found that, despite displaying an increased susceptibility to FHV infection, older (30 day-old) wild-type (OregonR) flies do not accumulate higher virus titers than their younger (5 day-old) counterparts [106]. Intriguingly, a similar pattern has been observed in humans following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for COVID-19. In comparison to younger individuals, older individuals display increased mortality without accumulating a higher virus titer when infected with SARS-CoV-2 [107]. Clearly, there is still much to be discovered regarding immune defenses in both humans and Drosophila, especially regarding the impact of age on these immune defenses. Drosophila can be a powerful tool in our pursuit to elucidate the intricacies of immune defense pathways and their change in efficiency over time. With the availability of advanced genetic and genomic tools in Drosophila, future findings made in this organism could lead to important translational impacts and advancements in healthcare, as well as treatment strategies that lead to improved immune response and increased longevity in aged organisms.
Author Contributions
Writing—original draft preparation, N.S.; writing—review and editing, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Castillo, J.C. Molecular mechanisms of aging and immune system regulation in Drosophila. Int. J. Mol. Sci. 2012, 13, 9826–9844. [Google Scholar] [CrossRef]
- Min, K.J.; Tatar, M. Unraveling the Molecular Mechanism of Immunosenescence in Drosophila. Int. J. Mol. Sci. 2018, 19, 2472. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Arjona, A.; Sapey, E.; Bai, F.; Fikrig, E.; Montgomery, R.R.; Lord, J.M.; Shaw, A.C. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Pawelec, G.; Tarazona, R. Aging and innate immunity. Immunity 2006, 24, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.T. Epidemiology and unique aspects of aging and infectious diseases. Clin. Infect. Dis. 2000, 30, 931–933. [Google Scholar] [CrossRef]
- Crossley, K.B.; Peterson, P.K. Infections in the elderly. Clin. Infect. Dis. 1996, 22, 209–215. [Google Scholar] [CrossRef][Green Version]
- Kline, K.A.; Bowdish, D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs. Population Division World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 21 May 2020).
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Baonza, A.; Grifoni, D. Drosophila Models of Human Disease. Biomed Res. Int. 2018, 2018, 7214974. [Google Scholar] [CrossRef]
- Govind, S. Innate immunity in Drosophila: Pathogens and pathways. Insect. Sci. 2008, 15, 29–43. [Google Scholar] [CrossRef]
- Simell, B.; Vuorela, A.; Ekstrom, N.; Palmu, A.; Reunanen, A.; Meri, S.; Kayhty, H.; Vakevainen, M. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011, 29, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Kounatidis, I.; Chtarbanova, S.; Cao, Y.; Hayne, M.; Jayanth, D.; Ganetzky, B.; Ligoxygakis, P. NF-kappaB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep. 2017, 19, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, S.D.; Macdonald, S.J.; Marguerie, R.; Certa, U.; Stearns, S.C.; Goldstein, D.B.; Partridge, L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol. 2002, 12, 712–723. [Google Scholar] [CrossRef]
- Zerofsky, M.; Harel, E.; Silverman, N.; Tatar, M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 2005, 4, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Cronin, S.J.; Nehme, N.T.; Limmer, S.; Liegeois, S.; Pospisilik, J.A.; Schramek, D.; Leibbrandt, A.; Simoes Rde, M.; Gruber, S.; Puc, U.; et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 2009, 325, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Poidevin, M.; Pradervand, S.; Lemaitre, B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 2009, 5, 200–211. [Google Scholar] [CrossRef]
- Jiang, H.; Patel, P.H.; Kohlmaier, A.; Grenley, M.O.; McEwen, D.G.; Edgar, B.A. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 2009, 137, 1343–1355. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009, 23, 2333–2344. [Google Scholar] [CrossRef]
- Clark, R.I.; Walker, D.W. Role of gut microbiota in aging-related health decline: Insights from invertebrate models. Cell Mol. Life Sci. 2018, 75, 93–101. [Google Scholar] [CrossRef]
- Partridge, L.; Alic, N.; Bjedov, I.; Piper, M.D. Ageing in Drosophila: The role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 2011, 46, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Loch, G.; Beyer, M.; Zinke, I.; Aschenbrenner, A.C.; Carrera, P.; Inhester, T.; Schultze, J.L.; Hoch, M. FOXO-dependent regulation of innate immune homeostasis. Nature 2010, 463, 369–373. [Google Scholar] [CrossRef] [PubMed]
- DiAngelo, J.R.; Bland, M.L.; Bambina, S.; Cherry, S.; Birnbaum, M.J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20853–20858. [Google Scholar] [CrossRef]
- Davoodi, S.; Galenza, A.; Panteluk, A.; Deshpande, R.; Ferguson, M.; Grewal, S.; Foley, E. The Immune Deficiency Pathway Regulates Metabolic Homeostasis in Drosophila. J. Immunol. 2019, 202, 2747–2759. [Google Scholar] [CrossRef] [PubMed]
- Kamareddine, L.; Robins, W.P.; Berkey, C.D.; Mekalanos, J.J.; Watnick, P.I. The Drosophila Immune Deficiency Pathway Modulates Enteroendocrine Function and Host Metabolism. Cell Metab. 2018, 28, 449–462.e445. [Google Scholar] [CrossRef]
- Ahlers, L.R.H.; Trammell, C.E.; Carrell, G.F.; Mackinnon, S.; Torrevillas, B.K.; Chow, C.Y.; Luckhart, S.; Goodman, A.G. Insulin Potentiates JAK/STAT Signaling to Broadly Inhibit Flavivirus Replication in Insect Vectors. Cell Rep. 2019, 29, 1946–1960.e1945. [Google Scholar] [CrossRef]
- Spellberg, M.J.; Marr, M.T., 2nd. FOXO regulates RNA interference in Drosophila and protects from RNA virus infection. Proc. Natl. Acad. Sci. USA 2015, 112, 14587–14592. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Hoffmann, J.; Knop, M.; Li, Y.; Isermann, K.; Roeder, T. Intestinal FoxO signaling is required to survive oral infection in Drosophila. Mucosal. Immunol. 2016, 9, 927–936. [Google Scholar] [CrossRef]
- Strominger, J.L. Animal antimicrobial peptides: Ancient players in innate immunity. J. Immunol. 2009, 182, 6633–6634. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Georgel, P.; Naitza, S.; Kappler, C.; Ferrandon, D.; Zachary, D.; Swimmer, C.; Kopczynski, C.; Duyk, G.; Reichart, J.M.; Hoffmann, J.A. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell 2001, 1, 503–514. [Google Scholar] [CrossRef]
- Rutschmann, S.; Kilinc, A.; Ferrandon, D. Cutting edge: The Toll pathway is required for resistance to Gram-positive bacterial infections in Drosophila. J. Immunol. 2002, 168, 1542–1546. [Google Scholar] [CrossRef]
- Leulier, F.; Rodriguez, A.; Khush, R.S.; Abrams, J.M.; Lemaitre, B. The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO Rep. 2000, 1, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rutschmann, S.; Jung, A.C.; Zhou, R.; Silverman, N.; Hoffmann, J.A.; Ferrandon, D. Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat. Immunol. 2000, 1, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Rutschmann, S.; Jung, A.C.; Hetru, C.; Reichhart, J.M.; Hoffmann, J.A.; Ferrandon, D. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 2000, 12, 569–580. [Google Scholar] [CrossRef]
- Lemaitre, B.; Kromer-Metzger, E.; Michaut, L.; Nicolas, E.; Meister, M.; Georgel, P.; Reichhart, J.M.; Hoffmann, J.A. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl. Acad. Sci. USA 1995, 92, 9465–9469. [Google Scholar] [CrossRef] [PubMed]
- Seroude, L.; Brummel, T.; Kapahi, P.; Benzer, S. Spatio-temporal analysis of gene expression during aging in Drosophila melanogaster. Aging Cell 2002, 1, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Landis, G.N.; Abdueva, D.; Skvortsov, D.; Yang, J.; Rabin, B.E.; Carrick, J.; Tavare, S.; Tower, J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2004, 101, 7663–7668. [Google Scholar] [CrossRef]
- Khan, I.; Prasad, N.G. The aging of the immune response in Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 129–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Libert, S.; Chao, Y.; Zwiener, J.; Pletcher, S.D. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol. Immunol. 2008, 45, 810–817. [Google Scholar] [CrossRef]
- Fabian, D.K.; Garschall, K.; Klepsatel, P.; Santos-Matos, G.; Sucena, E.; Kapun, M.; Lemaitre, B.; Schlotterer, C.; Arking, R.; Flatt, T. Evolution of longevity improves immunity in Drosophila. Evol. Lett. 2018, 2, 567–579. [Google Scholar] [CrossRef]
- Badinloo, M.; Nguyen, E.; Suh, W.; Alzahrani, F.; Castellanos, J.; Klichko, V.I.; Orr, W.C.; Radyuk, S.N. Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues. Arch. Insect. Biochem. Physiol. 2018, 98, e21464. [Google Scholar] [CrossRef] [PubMed]
- Libert, S.; Chao, Y.; Chu, X.; Pletcher, S.D. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell 2006, 5, 533–543. [Google Scholar] [CrossRef]
- Sinam, Y.M.; Chatterjee, A.; Ranjini, M.S.; Poojari, A.; Nagarajan, A.; Ramachandra, N.B.; Nongthomba, U. A newly evolved Drosophila Cytorace-9 shows trade-off between longevity and immune response. Infect. Genet. Evol. 2016, 44, 1–7. [Google Scholar] [CrossRef]
- Hombria, J.C.; Brown, S.; Hader, S.; Zeidler, M.P. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 2005, 288, 420–433. [Google Scholar] [CrossRef]
- Osman, D.; Buchon, N.; Chakrabarti, S.; Huang, Y.T.; Su, W.C.; Poidevin, M.; Tsai, Y.C.; Lemaitre, B. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. J. Cell Sci. 2012, 125, 5944–5949. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, D.; Jung, A.C.; Criqui, M.; Lemaitre, B.; Uttenweiler-Joseph, S.; Michaut, L.; Reichhart, J.; Hoffmann, J.A. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998, 17, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Resnik-Docampo, M.; Ulgherait, M.; Clark, R.I.; Shirasu-Hiza, M.; Jones, D.L.; Walker, D.W. Intestinal Snakeskin Limits Microbial Dysbiosis during Aging and Promotes Longevity. iScience 2018, 9, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Guillou, A.; Troha, K.; Wang, H.; Franc, N.C.; Buchon, N. The Drosophila CD36 Homologue croquemort Is Required to Maintain Immune and Gut Homeostasis during Development and Aging. PLoS Pathog. 2016, 12, e1005961. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.; Mueller, S.; Goto, A.; Barbier, V.; Paro, S.; Bonnay, F.; Dostert, C.; Troxler, L.; Hetru, C.; Meignin, C.; et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 2013, 190, 650–658. [Google Scholar] [CrossRef]
- West, C.; Silverman, N. p38b and JAK-STAT signaling protect against Invertebrate iridescent virus 6 infection in Drosophila. PLoS Pathog. 2018, 14, e1007020. [Google Scholar] [CrossRef] [PubMed]
- Merkling, S.H.; Bronkhorst, A.W.; Kramer, J.M.; Overheul, G.J.; Schenck, A.; Van Rij, R.P. The epigenetic regulator G9a mediates tolerance to RNA virus infection in Drosophila. PLoS Pathog. 2015, 11, e1004692. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Fridell, Y.W. Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction. Front. Physiol. 2013, 4, 288. [Google Scholar] [CrossRef]
- Gronke, S.; Clarke, D.F.; Broughton, S.; Andrews, T.D.; Partridge, L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 2010, 6, e1000857. [Google Scholar] [CrossRef]
- Nassel, D.R.; Liu, Y.; Luo, J. Insulin/IGF signaling and its regulation in Drosophila. Gen. Comp. Endocrinol. 2015, 221, 255–266. [Google Scholar] [CrossRef]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Dionne, M.S.; Pham, L.N.; Shirasu-Hiza, M.; Schneider, D.S. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 2006, 16, 1977–1985. [Google Scholar] [CrossRef]
- Zhang, W.; Thompson, B.J.; Hietakangas, V.; Cohen, S.M. MAPK/ERK signaling regulates insulin sensitivity to control glucose metabolism in Drosophila. PLoS Genet 2011, 7, e1002429. [Google Scholar] [CrossRef]
- Texada, M.J.; Koyama, T.; Rewitz, K. Regulation of Body Size and Growth Control. Genetics 2020, 216, 269–313. [Google Scholar] [CrossRef]
- Kleino, A.; Myllymaki, H.; Kallio, J.; Vanha-aho, L.M.; Oksanen, K.; Ulvila, J.; Hultmark, D.; Valanne, S.; Ramet, M. Pirk is a negative regulator of the Drosophila Imd pathway. J. Immunol. 2008, 180, 5413–5422. [Google Scholar] [CrossRef]
- Lhocine, N.; Ribeiro, P.S.; Buchon, N.; Wepf, A.; Wilson, R.; Tenev, T.; Lemaitre, B.; Gstaiger, M.; Meier, P.; Leulier, F. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe 2008, 4, 147–158. [Google Scholar] [CrossRef]
- Aggarwal, K.; Rus, F.; Vriesema-Magnuson, C.; Erturk-Hasdemir, D.; Paquette, N.; Silverman, N. Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway. PLoS Pathog. 2008, 4, e1000120. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.; Buechling, T.; Gesellchen, V.; Spirohn, K.; Boettcher, A.L.; Boutros, M. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011, 30, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Perrimon, N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 2012, 151, 123–137. [Google Scholar] [CrossRef]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef]
- Clancy, D.J.; Gems, D.; Hafen, E.; Leevers, S.J.; Partridge, L. Dietary restriction in long-lived dwarf flies. Science 2002, 296, 319. [Google Scholar] [CrossRef]
- Wessells, R.J.; Fitzgerald, E.; Cypser, J.R.; Tatar, M.; Bodmer, R. Insulin regulation of heart function in aging fruit flies. Nat. Genet. 2004, 36, 1275–1281. [Google Scholar] [CrossRef]
- Bai, H.; Post, S.; Kang, P.; Tatar, M. Drosophila Longevity Assurance Conferred by Reduced Insulin Receptor Substrate Chico Partially Requires d4eBP. PLoS ONE 2015, 10, e0134415. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Sato, T.; Ohkawa, Y.; Inoue, Y.H. Identification of miR-305, a microRNA that promotes aging, and its target mRNAs in Drosophila. Genes Cells 2018, 23, 80–93. [Google Scholar] [CrossRef]
- Koyama, T.; Mirth, C.K. Growth-Blocking Peptides As Nutrition-Sensitive Signals for Insulin Secretion and Body Size Regulation. PLoS Biol. 2016, 14, e1002392. [Google Scholar] [CrossRef]
- Sung, E.J.; Shears, S.B. A genome-wide dsRNA library screen for Drosophila genes that regulate the GBP/phospholipase C signaling axis that links inflammation to aging. BMC Res. Notes 2018, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Benmimoun, B.; Polesello, C.; Waltzer, L.; Haenlin, M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development 2012, 139, 1713–1717. [Google Scholar] [CrossRef] [PubMed]
- Grigorian, M.; Mandal, L.; Hartenstein, V. Hematopoiesis at the onset of metamorphosis: Terminal differentiation and dissociation of the Drosophila lymph gland. Dev. Genes Evol. 2011, 221, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Felix, T.M.; Hughes, K.A.; Stone, E.A.; Drnevich, J.M.; Leips, J. Age-specific variation in immune response in Drosophila melanogaster has a genetic basis. Genetics 2012, 191, 989–1002. [Google Scholar] [CrossRef]
- McCormack, S.; Yadav, S.; Shokal, U.; Kenney, E.; Cooper, D.; Eleftherianos, I. The insulin receptor substrate Chico regulates antibacterial immune function in Drosophila. Immun. Ageing 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, A.; Wu, X.; Tatar, M.; Liu, N.; Bai, H. Age-Dependent Changes in Transcription Factor FOXO Targeting in Female Drosophila. Front. Genet. 2019, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Troha, K.; Buchon, N. Methods for the study of innate immunity in Drosophila melanogaster. Wiley Interdiscip Rev. Dev. Biol. 2019, 8, e344. [Google Scholar] [CrossRef]
- Belmonte, R.L.; Corbally, M.K.; Duneau, D.F.; Regan, J.C. Sexual Dimorphisms in Innate Immunity and Responses to Infection in Drosophila melanogaster. Front. Immunol. 2019, 10, 3075. [Google Scholar] [CrossRef]
- Kubiak, M.; Tinsley, M.C. Sex-Specific Routes To Immune Senescence In Drosophila melanogaster. Sci. Rep. 2017, 7, 10417. [Google Scholar] [CrossRef]
- Le Bourg, E.; Massou, I.; Gobert, V. Cold stress increases resistance to fungal infection throughout life in Drosophila melanogaster. Biogerontology 2009, 10, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, E. A cold stress applied at various ages can increase resistance to heat and fungal infection in aged Drosophila melanogaster flies. Biogerontology 2011, 12, 185–193. [Google Scholar] [CrossRef]
- Le Bourg, E. The NF-kB like factor DIF has weaker effects on Drosophila melanogaster immune defenses than previously thought. J. Comp. Physiol. B 2011, 181, 741–750. [Google Scholar] [CrossRef]
- Zheng, W.; Rus, F.; Hernandez, A.; Kang, P.; Goldman, W.; Silverman, N.; Tatar, M. Dehydration triggers ecdysone-mediated recognition-protein priming and elevated anti-bacterial immune responses in Drosophila Malpighian tubule renal cells. BMC Biol. 2018, 16, 60. [Google Scholar] [CrossRef]
- Horn, L.; Leips, J.; Starz-Gaiano, M. Phagocytic ability declines with age in adult Drosophila hemocytes. Aging Cell 2014, 13, 719–728. [Google Scholar] [CrossRef]
- Huang, W.; Massouras, A.; Inoue, Y.; Peiffer, J.; Ramia, M.; Tarone, A.M.; Turlapati, L.; Zichner, T.; Zhu, D.; Lyman, R.F.; et al. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 2014, 24, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Mackay, T.F.; Richards, S.; Stone, E.A.; Barbadilla, A.; Ayroles, J.F.; Zhu, D.; Casillas, S.; Han, Y.; Magwire, M.M.; Cridland, J.M.; et al. The Drosophila melanogaster Genetic Reference Panel. Nature 2012, 482, 173–178. [Google Scholar] [CrossRef]
- Zambon, R.A.; Nandakumar, M.; Vakharia, V.N.; Wu, L.P. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in Drosophila. PLoS Pathog. 2014, 10, e1004507. [Google Scholar] [CrossRef] [PubMed]
- Avadhanula, V.; Weasner, B.P.; Hardy, G.G.; Kumar, J.P.; Hardy, R.W. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009, 5, e1000582. [Google Scholar] [CrossRef]
- Costa, A.; Jan, E.; Sarnow, P.; Schneider, D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 2009, 4, e7436. [Google Scholar] [CrossRef]
- Promislow, D.E.L. A Geroscience Perspective on COVID-19 Mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e30–e33. [Google Scholar] [CrossRef]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; CMMID COVID-19 Working Group; Eggo, R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, I.; Cesari, M. Geriatric Syndromes and SARS-Cov-2: More than Just Being Old. J. Frailty Aging 2020, 9, 127–129. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Schneider, J.; Imler, J.L. Sensing and signalling viral infection in drosophila. Dev. Comp. Immunol. 2021, 117, 103985. [Google Scholar] [CrossRef]
- Mussabekova, A.; Daeffler, L.; Imler, J.L. Innate and intrinsic antiviral immunity in Drosophila. Cell Mol. Life Sci. 2017, 74, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, O.; Imler, J.L. Induced antiviral innate immunity in Drosophila. Curr. Opin. Microbiol. 2014, 20, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, D.K.; Bussiere, L.F.; Tinsley, M.C. Senescence of the cellular immune response in Drosophila melanogaster. Exp. Gerontol. 2011, 46, 853–859. [Google Scholar] [CrossRef]
- McCarville, J.L.; Ayres, J.S. Disease tolerance: Concept and mechanisms. Curr. Opin. Immunol. 2018, 50, 88–93. [Google Scholar] [CrossRef]
- Sheffield, L.; Sciambra, N.; Evans, A.; Hagedorn, E.; Goltz, C.; Delfeld, M.; Kuhns, H.; Fierst, J.L.; Chtarbanova, S. Age-dependent impairment of disease tolerance is associated with a robust transcriptional response following RNA virus infection in Drosophila. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Alsamiri, A.D.; Dabbour, A.F.; Alsaeedi, H.; Al-Zalabani, A.H. Association of Viral Load in SARS-CoV-2 Patients With Age and Gender. Front. Med. 2021, 8, 608215. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).