Cracking the Challenge of Antimicrobial Drug Resistance with CRISPR/Cas9, Nanotechnology and Other Strategies in ESKAPE Pathogens
Abstract
1. Introduction
2. Drivers of Antimicrobial Resistance
3. Global Dissemination of Antibiotic Resistance
4. Emerging Resistance–Development of Resistant Strains
5. ESKAPE, Healthcare Concomitant Bugs–Bad Bugs with No Drugs
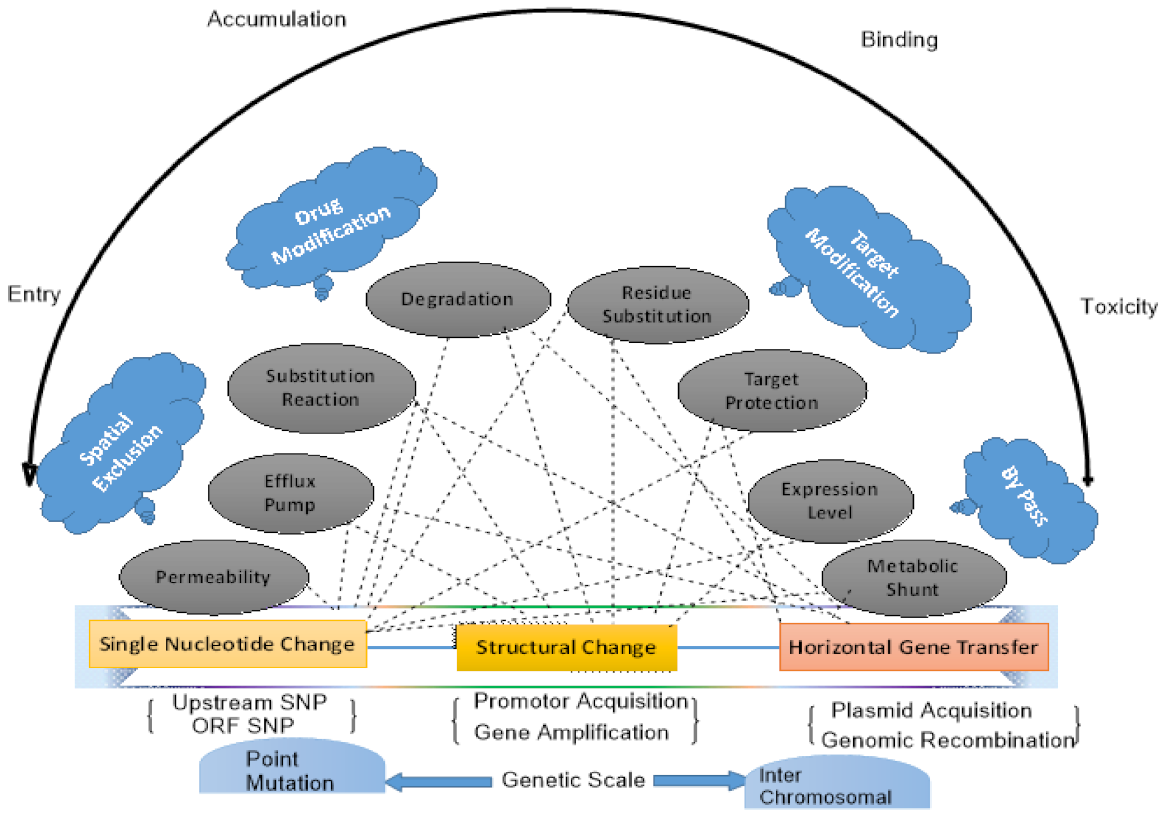
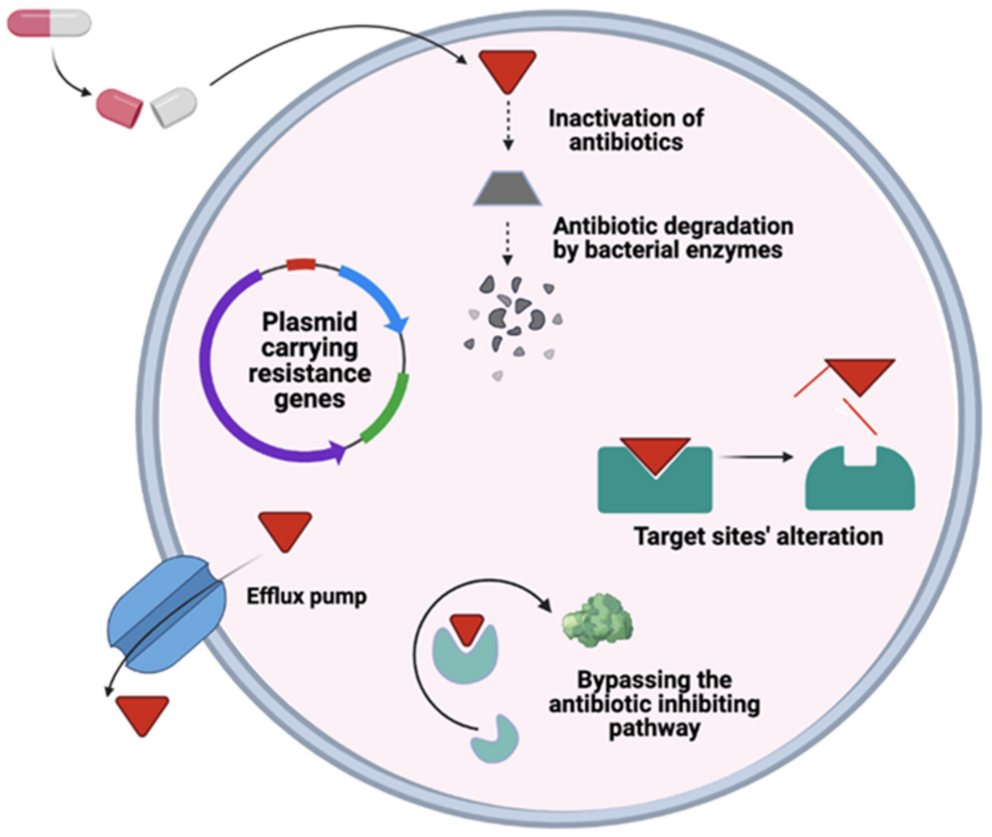
6. General Mechanism of Antimicrobial Resistance
7. Alternative Mechanisms for Combating Multidrug Resistance in ESKAPE Pathogens
7.1. CRISPR-Cas9
7.2. Nanotechnology and Nanoparticles to Combat Multidrug Resistance
8. Host-Directed Therapies
Promoting Bacterial Clearance through Modulating Host’s Inflammatory Responses Regulating PRR Signaling Pathways
9. Vaccine Development
10. Inhibition of Quorum Sensing
11. Other Molecular Mechanisms
11.1. Next-Generation Sequencing and Antimicrobial Peptide Prediction
11.2. Prediction of Antimicrobial Peptide from DNA/RNA Library: Antimicrobial Peptides Search Tools
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species |
| AMP | Antimicrobial peptides |
| AMU | Antimicrobial usage |
| ARGs | Antimicrobial resistant genes |
| ESBL | Extended-spectrum b-lactamases |
| HAIs | Hospital-acquired infections |
| Hib | Haemophilus influenzae type b |
| HGT | Horizontal gene transfer |
| IPD | Invasive pneumococcal disease |
| MDR | Multidrug resistant |
| MRSA | Methicillin-resistance Staphylococcus aureus |
| NLRs | NOD-like receptors |
| PRRs | Recognition receptors |
| TLRs | Toll-like receptors |
| WGS | Whole genome sequencing |
| XDR | Extensively drug-resistant |
References
- Lekshmi, M.; Ammini, P.; Kumar, S.; Varela, M.F. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms 2017, 5, 11. [Google Scholar] [CrossRef]
- Price, N.; Klein, J.L. Infectious Diseases and Emergencies; Oxford University Press (OUP): Oxford, UK, 2016. [Google Scholar]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Henninger, A. Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clin. Microbiol. Infect. 2003, 9, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.E.; Martínez, M.A.; Rodríguez, M.A.S.; I Wertheimer, A. The determinants of the antibiotic resistance process. Infect. Drug Resist. 2009, 2, 1–11. [Google Scholar]
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control. Hosp. Epidemiol. 2010, 31, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Genet. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J. Multistate point-prevalence survey of health care–associated infections. N. Eng. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. Balancing the drug-resistance equation. Trends Microbiol. 1994, 2, 341–342. [Google Scholar] [CrossRef]
- Levy, S. From Tragedy the Antibiotic Era is Born. The Antibiotic Paradox: How the Misuse of Antibiotics Destroys Their Curative Povers, 2nd ed.; Perseus Publishing: Cambridge, MA, USA, 2002; pp. 1–14. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Voorhees, E.M.; Hersh, W.R. Overview of the TREC 2012 Medical Records Track; NIST Publications: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dzink-Fox, J.L.; Chen, M.; Levy, S.B. Genetic characterization of highly fluoroquinolone-resistant clinical Escherichia coli strains from China: Role ofacrR mutations. Antimicrob. Agents Chemother. 2001, 45, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother. 2002, 49, 25–30. [Google Scholar] [CrossRef]
- Schneiders, T.; Amyes, S.; Levy, S. Role of AcrR and RamA in fluoroquinolone resistance in clinical Klebsiella pneumoniae isolates from Singapore. Antimicrob. Agents Chemother. 2003, 47, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. Mechanisms of fluoroquinolone resistance: An update 1994–1998. Drugs 1999, 58, 11–18. [Google Scholar] [CrossRef]
- Hiramatsu, K. Vancomycin resistance in staphylococci. Drug Resist. Updat. 1998, 1, 135–150. [Google Scholar] [CrossRef]
- Weigel, L.M.; Clewell, D.B.; Gill, S.R.; Clark, N.C.; McDougal, L.K.; Flannagan, S.E.; Kolonay, J.F.; Shetty, J.; Killgore, G.E.; Tenover, F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003, 302, 1569–1571. [Google Scholar] [CrossRef]
- Tenover, F.C.; Weigel, L.M.; Appelbaum, P.C.; McDougal, L.K.; Chaitram, J.; McAllister, S.; Clark, N.; Killgore, G.; O’Hara, C.M.; Jevitt, L.; et al. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 2004, 48, 275–280. [Google Scholar] [CrossRef]
- Lipsitch, M.; Samore, M.H. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg. Infect. Dis. 2002, 8, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.; Edwards, R. Insights into antibiotic resistance through metagenomic approaches. Futur. Microbiol. 2012, 7, 73–89. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P. Antibiotic-resistance genes in waste water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Micobiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, F.; Udikovic-Kolic, N.; Andrew, S.; Handelsman, J. Diverse antibiotic resistance genes in dairy cow manure. mBio 2014, 5, e01017-13. [Google Scholar] [CrossRef]
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarcón, Ó.; et al. The microbiome of uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Ahmadi, S.; Patel, S.; Gibson, M.K.; Wang, B.; Ndao, I.M.; Deych, E.; Shannon, W.D.; Tarr, P.I.; Warner, B.B.; et al. Gut resistome development in healthy twin pairs in the first year of life. Microbiome 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Donato, J.J.; Moe, L.A.; Converse, B.J.; Smart, K.D.; Berklein, F.C.; McManus, P.S.; Handelsman, J. Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl. Environ. Microbiol. 2010, 76, 4396–4401. [Google Scholar] [CrossRef]
- Perron, G.G.; Whyte, L.; Turnbaugh, P.J.; Goordial, J.; Hanage, W.P.; Dantas, G.; Desai, M.M. Functional characterization of bacteria isolated from ancient arctic soil exposes diverse resistance mechanisms to modern antibiotics. PLoS ONE 2015, 10, e0069533. [Google Scholar] [CrossRef] [PubMed]
- Parsley, L.C.; Consuegra, E.J.; Kakirde, K.S.; Land, A.M.; Harper, W.F.; Liles, M.R. Identification of diverse antimicrobial resistance determinants carried on bacterial, plasmid, or viral metagenomes from an activated sludge microbial assemblage. Appl. Environ. Microbiol. 2010, 76, 3753–3757. [Google Scholar] [CrossRef]
- Hawkey, P.M.; Jones, A.M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 2009, 64 (Suppl. S1), i3–i10. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Coque, T.M. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 2006, 9, 466–475. [Google Scholar] [CrossRef]
- Poirel, L.; Liard, A.; Rodriguez-Martinez, J.-M.; Nordmann, P. Vibrionaceae as a possible source of Qnr-like quinolone resistance determinants. J. Antimicrob. Chemother. 2005, 56, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.N.Q.; Thuong, T.C.; Khuong, H.D.; Nga, T.V.T.; Thompson, C.; Campbell, J.I.; De Jong, M.; Farrar, J.J.; Schultsz, C.; Van Doorn, H.R.; et al. The co-selection of fluoroquinolone resistance genes in the gut flora of vietnamese children. PLoS ONE 2012, 7, e42919. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef]
- Summers, A.O. Generally overlooked fundamentals of bacterial genetics and ecology. Clin. Infect. Dis. 2002, 34 (Suppl. S3), S85–S92. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, M.S.; Heir, E.; Leegaard, T.; Wiger, K.; Holck, A. Frequency of disinfectant resistance genes and genetic linkage with β-lactamase transposon Tn552 among clinical staphylococci. Antimicrob. Agents Chemother. 2002, 46, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.M.; Levy, S.B. The impact of antibiotic use on resistance development and persistence. Drug Resist. Updat. 2000, 3, 303–311. [Google Scholar] [CrossRef]
- Weinstein, R.A. Controlling antimicrobial resistance in hospitals: Infection control and use of antibiotics. Emerg. Infect. Dis. 2001, 7, 188–192. [Google Scholar] [CrossRef]
- Gagliotti, C.; Balode, A.; Baquero, F.; Degener, J.; Grundmann, H.; Gür, D.; Jarlier, V.; Kahlmeter, G.; Monen, J.; Monnet, D.; et al. Escherichia coli and Staphylococcus aureus: Bad news and good news from the European Antimicrobial Resistance Surveillance Network (EARS-Net, formerly EARSS), 2002 to 2009. Eurosurveillance 2011, 16, 19819. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Micrbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in US hospitals, 2002. Pub. Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U. Global antibacterial resistance: The never-ending story. J. Glob. Antimicrob. Resist. 2013, 1, 63–69. [Google Scholar] [CrossRef]
- Penesyan, A.; Gillings, M.; Paulsen, I. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Rather, P.; Hare, R.; Miller, G. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Mol. Biol. Rev. 1993, 57, 138–163. [Google Scholar] [CrossRef]
- Bush, K.; Fisher, J.F. Epidemiological Expansion, Structural Studies, and Clinical Challenges of New β-Lactamases from Gram-Negative Bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The Challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the Antibiotic Resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef]
- Arcilla, M.S.; van Hattem, J.M.; Haverkate, M.R.; Bootsma, M.C.; van Genderen, P.J.; Goorhuis, A.; Grobusch, M.P.; Lashof, A.M.O.; Molhoek, N.; Schultsz, C.; et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): A prospective, multicentre cohort study. Lancet Infect. Dis. 2017, 17, 78–85. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Micobiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Genet. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.-M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Jin, Y.H.; Dunlap, P.E.; McBride, S.J.; Al-Refai, H.; Bushel, P.R.; Freedman, J.H. Global transcriptome and deletome profiles of yeast exposed to transition metals. PLoS Genet. 2008, 4, e1000053. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mat. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Muzammil, S.; Hayat, S.; Fakhar-E-Alam, M.; Aslam, B.; Siddique, M.; Nisar, M.; Saqalein, M.; Atif, M.; Sarwar, A.; Khurshid, A. Nanoantibiotics: Future nanotechnologies to combat antibiotic resistance. Front Biosci. 2018, 10, 352–374. [Google Scholar]
- Jamaran, S.; Zarif, B.R. Synergistic effect of silver nanoparticles with neomycin or gentamicin antibiotics on mastitis-causing Staphylococcus aureus. Open J. Ecol. 2016, 6, 452–459. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomedicine Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Hassan, M.; Ismail, M.; Moharram, A.; Shoreit, A. Synergistic Effect of Biogenic Silver-nanoparticles with β lactam Cefotaxime against Resistant Staphylococcus arlettae AUMC b-163 Isolated from T3A Pharmaceutical Cleanroom, Assiut, Egypt. Am. J. Microbiol. Res. 2016, 4, 132–137. [Google Scholar]
- Hwang, I.-s.; Hwang, J.H.; Choi, H.; Kim, K.-J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Hari, N.; Thomas, T.K.; Nair, A.J. Comparative Study on the Synergistic Action of Differentially Synthesized Silver Nanoparticles with β-Cephem Antibiotics and Chloramphenicol. J. Nanosci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.; Santra, C.; Dasgupta, A.K.; Karmakar, P. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2007, 2, 614–622. [Google Scholar] [CrossRef]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Q.; Xue, J.; Ding, Y. Selectively enhanced antibacterial effects and ultraviolet activation of antibiotics with ZnO nanorods against Escherichia coli. J. Biomed. Nanotechnol. 2013, 9, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Banoee, M.; Seif, S.; Nazari, Z.E.; Jafari-Fesharaki, P.; Shahverdi, H.R.; Moballegh, A.; Moghaddam, K.M.; Shahverdi, A.R. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J. Biomed. Mat. Res. Part B Appl. Biomater. 2010, 93, 557–561. [Google Scholar] [CrossRef]
- Thati, V.; Roy, A.S.; Ambika Prasad, M.; Shivannavar, C.; Gaddad, S. Nanostructured zinc oxide enhances the activity of antibiotics against Staphylococcus aureus. J. Biosci. Tech. 2010, 1, 64–69. [Google Scholar]
- Roy, A.S.; Parveen, A.; Koppalkar, A.R.; Prasad, M.A. Effect of nano-titanium dioxide with different antibiotics against methicillin-resistant Staphylococcus aureus. J. Biomater. Nanobiotechnol. 2010, 1, 37–41. [Google Scholar] [CrossRef]
- Kooti, M.; Gharineh, S.; Mehrkhah, M.; Shaker, A.; Motamedi, H. Preparation and antibacterial activity of CoFe2O4/SiO2/Ag composite impregnated with streptomycin. Chem. Eng. J. 2015, 259, 34–42. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A. Synthesis and antibacterial activity of cuo nanoparticles suspension induced by laser ablation in liquid. Arab. J. Sci. Eng. 2016, 41, 301–310. [Google Scholar] [CrossRef]
- Patra, J.K.; Ali, M.S.; Oh, I.-G.; Baek, K.-H. Proteasome inhibitory, antioxidant, and synergistic antibacterial and anticandidal activity of green biosynthesized magnetic Fe3O4 nanoparticles using the aqueous extract of corn (Zea mays L.) ear leaves. Artif. Cells Nanomed. Biotechnol. 2017, 45, 349–356. [Google Scholar] [CrossRef]
- Tanna, J.A.; Chaudhary, R.G.; Gandhare, N.V.; Rai, A.R.; Yerpude, S.; Juneja, H.D. Copper nanoparticles catalysed an efficient one-pot multicomponents synthesis of chromenes derivatives and its antibacterial activity. J. Exp. Nanosci. 2016, 11, 884–900. [Google Scholar] [CrossRef]
- Tarjoman, Z.; Ganji, S.M.; Mehrabian, S. Synergistic effects of the bismuth nanoparticles along with antibiotics on PKS positive Klebsiella pneumoniae isolates from colorectal cancer patients; comparison with quinolone antibiotics. M. Res. J. Med. Med. Sci. 2015, 3, 387–393. [Google Scholar]
- Bhande, R.M.; Khobragade, C.N.; Mane, R.S.; Bhande, S. Enhanced synergism of antibiotics with zinc oxide nanoparticles against extended spectrum β-lactamase producers implicated in urinary tract infections. J. Nanopart. Res. 2013, 15, 1413. [Google Scholar] [CrossRef]
- Karlström, Å.; Heston, S.M.; Boyd, K.L.; Tuomanen, E.I.; McCullers, J.A. Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J. Infect. Dis. 2011, 204, 1358–1366. [Google Scholar] [CrossRef]
- Waterer, G.W.; Somes, G.W.; Wunderink, R.G. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch. Intern. Med. 2001, 161, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.A.; Horcajada, J.P.; Almela, M.; Marco, F.; Soriano, A.; Marco, M.A.; Torres, A.; Mensa, J. Addition of a macrolide to a β-lactam–based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 2003, 36, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Yu, V.L.; Klugman, K.P.; Feldman, C.; Ortqvist, A.; Rello, J.; Morris, A.J.; Luna, C.M.; Snydman, D.R.; Ko, W.C.; et al. Combination Antibiotic Therapy Lowers Mortality among Severely Ill Patients with Pneumococcal Bacteremia. Am. J. Respir. Crit. Care Med. 2004, 170, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Skerry, C.; Scanlon, K.; Rosen, H.; Carbonetti, N.H. Sphingosine-1-phosphate receptor agonism reduces bordetella pertussis-mediated lung pathology. J. Infect. Dis. 2014, 211, 1883–1886. [Google Scholar] [CrossRef][Green Version]
- Fine, P.E. Herd Immunity: History, theory, practice. Epidemiologic Rev. 1993, 15, 265–302. [Google Scholar] [CrossRef]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef]
- Peltola, H.; Rød, T.O.; Jónsdóttir, K.; Bötttger, M.; Coolidge, J.A.S. Life-Threatening Haemophilus influenzae infections in Scandinavia: A Five-country analysis of the incidence and the main clinical and bacteriologic characteristics. Rev. Infect. Dis. 1990, 12, 708–715. [Google Scholar] [CrossRef]
- Tristram, S.; Jacobs, M.R.; Appelbaum, P.C. Antimicrobial resistance in Haemophilus influenzae. Clin. Microbiol. Rev. 2007, 20, 368–389. [Google Scholar] [CrossRef]
- Hoban, D.; Felmingham, D. The PROTEKT surveillance study: Antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis from community-acquired respiratory tract infections. J. Antimicrob. Chemother. 2002, 50 (Suppl. S2), 49–59. [Google Scholar] [CrossRef]
- Peltola, H.; Aavitsland, P.; Hansen, K.G.; Jónsdóttir, K.E.; Nøkleby, H.; Romanus, V. Perspective: A five-country analysis of the impact of four different Haemophilus Influenzae type b conjugates and vaccination strategies in Scandinavia. J. Infect. Dis. 1999, 179, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adam, H.; Richardson, S.; Jamieson, F.; Rawte, P.; Low, D.; Fisman, D. Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: Evidence for herd effects and strain replacement due to Hib vaccination. Vaccine 2010, 28, 4073–4078. [Google Scholar] [CrossRef]
- Hargreaves, R.M.; E Slack, M.P.; Howard, A.J.; Anderson, E.; Ramsay, M.E. Changing patterns of invasive Haemophilus influenzae disease in England and Wales after introduction of the Hib vaccination programme. BMJ 1996, 312, 160–161. [Google Scholar] [CrossRef][Green Version]
- Heilmann, K.P.; Rice, C.L.; Miller, A.L.; Miller, N.J.; Beekmann, S.E.; Pfaller, M.A.; Richter, S.S.; Doern, G.V. Decreasing prevalence of β-lactamase production among respiratory tract isolates of Haemophilus influenzae in the United States. Antimicrob. Agents Chemother. 2005, 49, 2561–2564. [Google Scholar] [CrossRef]
- Hazir, T.; Nisar, Y.B.; Qazi, S.A.; Khan, S.F.; Raza, M.; Zameer, S.; Masood, S.A. Chest radiography in children aged 2–59 months diagnosed with non-severe pneumonia as defined by World Health Organization: Descriptive multicentre study in Pakistan. BMJ 2006, 333, 629. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. Epidemiology, virulence factors and management of the pneumococcus. F1000Research 2016, 5, 2320. [Google Scholar] [CrossRef]
- Hampton, L.M.; Farley, M.M.; Schaffner, W.; Thomas, A.; Reingold, A.; Harrison, L.H.; Lynfield, R.; Bennett, N.M.; Petit, S.; Gershman, K.; et al. Prevention of antibiotic-nonsusceptible streptococcus pneumoniae with conjugate vaccines. J. Infect. Dis. 2011, 205, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, S.M.; Lynfield, R.; Schaffner, W.; Reingold, A.; Miller, L.; Petit, S.; Holtzman, C.; Zansky, S.M.; Thomas, A.; Baumbach, J.; et al. Prevention of Antibiotic-Nonsusceptible Invasive Pneumococcal Disease With the 13-Valent Pneumococcal Conjugate Vaccine. Clin. Infect. Dis. 2016, 62, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Cohen, J.F.; Chalumeau, M.; Levy, C. Impact of pneumococcal conjugate vaccines for children in high-and non–high-income countries. Expert Rev. Vaccines 2017, 16, 625–640. [Google Scholar] [CrossRef]
- Eickhoff, M.J.; Bassler, B.L. SnapShot: Bacterial quorum sensing. Cell 2018, 174, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.; Kang, Y.C.; Lee, J.-K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.M.; Graeber, T.G.; Krutzik, S.R.; Montoya, D.; Schenk, M.; Lee, D.J.; Komisopoulou, E.; Kelly-Scumpia, K.; Chun, R.; Iyer, S.S. Type I interferon suppresses type II interferon–triggered human anti-mycobacterial responses. Science 2013, 339, 1448–1453. [Google Scholar] [CrossRef]
- Tavares, L.S.; Silva, C.; Souza, V.; Silva, V.; Diniz, C.; Santos, M. Strategies and molecular tools to fight antimicrobial resistance: Resistome, transcriptome, and antimicrobial peptides. Front. Microbiol. 2013, 4, 412. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, C.; Liu, X.; Wu, J.; Yang, H.; Wang, Y.; Li, J.; Yu, H.; Lai, R. Peptidomics and genomics analysis of novel antimicrobial peptides from the frog, Rana nigrovittata. Genomics 2010, 95, 66–71. [Google Scholar] [CrossRef]
- Moreira, R.; Balseiro, P.; Planas, J.V.; Fuste, B.; Beltran, S.; Novoa, B.; Figueras, A. Transcriptomics of in vitro immune-stimulated hemocytes from the Manila clam Ruditapes philippinarum using high-throughput sequencing. PLoS ONE 2012, 7, e35009. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, D.C.; Zou, Z.; Bowen, C.J.; Wasala, N.B.; Madden, R.; Wang, Y.; Kocan, K.; Jiang, H.; Dillwith, J. Pyrosequencing and characterization of immune response genes from the American dog tick, Dermacentor variabilis (L.). Insect Mol. Biol. 2010, 19, 617–630. [Google Scholar] [CrossRef]
- NIHR Global Health Research Unit on Genomic Surveillance of AMR. Whole-genome sequencing as part of national and international surveillance programmes for antimicrobial resistance: A roadmap. BMJ Glob. Health 2020, 5, e002244. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, S.; Chen, H.; Yu, Y.; Ruan, Z. BacWGSTdb 2.0: A one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2020, 49, D644–D650. [Google Scholar] [CrossRef]
- Ruan, Z.; Feng, Y. BacWGSTdb, a database for genotyping and source tracking bacterial pathogens. Nucleic Acids Res. 2016, 44, D682–D687. [Google Scholar] [CrossRef]
- Belarmino, L.; Capriles, P.; Crovella, S.; Dardenne, L.; Benko-Iseppon, A. EST-Database Search of Plant Defensins—An Example Using Sugarcane, a Large and Complex Genome. Curr. Protein Pept. Sci. 2010, 11, 248–254. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2008, 37 (Suppl. S1), D933–D937. [Google Scholar] [CrossRef]
- Bhadra, P.; Yan, J.; Li, J.; Fong, S.; Siu, S.W.I. AmPEP: Sequence-based prediction of antimicrobial peptides using distribution patterns of amino acid properties and random forest. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- HydroCalc. HydroCalc Proteome. Available online: http://gmb.bio.br/hydrocalc/ (accessed on 12 January 2021).
- CAMPR3. Collection of Anti-Microbial Peptides. Available online: http://www.camp.bicnirrh.res.in/ (accessed on 12 January 2021).
- Thomas, S.; Karnik, S.; Barai, R.S.; Jayaraman, V.K.; Idicula-Thomas, S. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2009, 38 (Suppl. S1), D774–D780. [Google Scholar] [CrossRef] [PubMed]
- iAMP-2. iAMP-2L: A web-server for identifying AMP and their functional types. Available online: http://www.jci-bioinfo.cn/iAMP-2L (accessed on 15 January 2021).
- Franco, O.L. Peptide promiscuity: An evolutionary concept for plant defense. FEBS Lett. 2011, 585, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, P.; Lin, W.-Z.; Jia, J.-H.; Chou, K.-C. iAMP-2L: A two-level multi-label classifier for identifying antimicrobial peptides and their functional types. Anal. Biochem. 2013, 436, 168–177. [Google Scholar] [CrossRef] [PubMed]


| Bacterial Strain | Gram Staining Type | Resistance Type | Antibiotics | Treatment Option | Resistance Level |
|---|---|---|---|---|---|
| Acinetobacter | Negative | Multidrug | Ceftazidime, aminoglycoside, fluoroquinolones, carbapenems | Carbapenems, b-Lactamase inhibitors, Tigecycline, Aminoglycosides, Polymyxin therapy, Synergy, and combination therapy | High level |
| E. coli | Negative | Multidrug | Cephalosporins (ESBL-producers), fluoroquinolones, aminoglycosides | GyrB/ParE programme, EV-035 | High level |
| K. pneumoniae | Negative | Multidrug | Cephalosporins (ESBL-producers), fluoroquinolones, aminoglycosides, carbapenems | POL7080 and ACHN-975 compounds | High level |
| P. aeruginosa | Negative | Multidrug | Piperacillin/tazobactam, ceftazidime, ciprofloxacin, aminoglycosides, carbapenems | POL7080 and ACHN-975 compounds | High level |
| Enterococcus spp. | Positive | Multidrug | Ampicillin, aminoglycosides, glycopeptides | RX-04 lead series, 50S ribosomal subunit; inhibit translation by stabilizing a distorted mode of P-tRNA binding | High level |
| S. aureus | Positive, | Multidrug | β-lactam antibiotics (except new anti- methicillin-resistant S. aureus cephalosporins), macrolides, fluoroquinolones, aminoglycosides | RX-04 lead series, 50S ribosomal subunit; inhibit translation by stabilizing a distorted mode of P-tRNA binding | High level |
| Resistance | Proposed Mechanism | Examples | Ref. |
|---|---|---|---|
| Inactivation of Drug | Use of hydrolysis or modification | b-lactamase for b-lactam resistance, acetyltransferases for aminoglycoside resistance | [53,54] |
| Alteration of Target | Reduction of binding affinity to the drug by bypassing the drug target | DNA gyrase mutation for fluoroquinolone resistance | [55] |
| Drug influx Reduction | By decreasing permeability | Gram-negative outer membrane | [56] |
| Extrusion of Drug | Efflux pumps | Accessory membrane fusion proteins | [57] |
| Horizontal gene transfer | By resistance determinants from other microorganisms | [58] |
| Nanoparticles (NP) | Mode of Action/Mechanism of Nanoparticles Against ESKAPE Pathogens | Antibiotic Used | Microorganism | Synergic Effects (Antibiotics-Nanoparticles) | Ref. |
|---|---|---|---|---|---|
| AgNPs | Damage the bacterial cell membrane and disrupt the activity of membranous enzymes. Cell wall distraction by cell DNA was condensed to a tension state and could have lost its replicating abilities | Doxycycline | K. pneumoniae | Observed | [68] |
| Gentamicin and Neomycin | S. aureus | AgNPs + Gentamicin showed resistance in 50% strains while AgNPs + Neomycin showed synergy 45% of the strains. | [69] | ||
| E. coli, S. aureus | Observed increase in activity was such that Erythromycin showed 18.9.6%, Kanamycin = 27.9.3%, Chloramphenicol = 18.1.3%, and Ampicillin = 74.8.9% | [69] | |||
| β-Lactam, cefotaxime | E. coli, S. aureus | Synergistic increase in activity was such that 17.2%, 13.5% for E. coli and S. aureus, respectively | [70] | ||
| Ampicillin, chloramphenicol, and kanamycin | S. aureus, E. coli, and P. aeruginosa | Synergistic effects observed | [71] | ||
| Beta-lactam: cephem | S. aureus | Cephalothin and cefazolin showed a 30% increase in activity when used in combination with 20 μg/ mL AgNPs against Micrococcus luteus, and Bacillus subtilis | [72] | ||
| AuNPs | Disturb membrane potential by inhibiting ATPase activities; inhibit the subunit of the ribosome from binding tRNA. Cellular death induced by gold nanoparticles do not include reactive oxygen species-based mechanisms | Ampicillin, streptomycin, and kanamycin | E. coli and S. aureus | 15%, 12%, and 34% increase in inhibition zone for E. coli with A/S/K+Au, respectively; 20%, 109%, and 18% increase in inhibition zone for M. luteus A/S/K+AuNPs, respectively; 12% and 34% increase in inhibition zone for S. aureus with A/ K+AuNPs, respectively | [73] |
| Beta lactams: cefaclor | S. aureus and E. coli | MICs of cefaclor reduced gold nanoparticles were 10 mg/mL and 100 mg/mL for S. aureus and E. coli, respectively | [74] | ||
| ZnONPs | Interactions between reactive oxygen species and membrane proteins result in cell damage. ZnO-NPs disrupt bacterial cell membrane integrity, reduce cell surface hydrophobicity, and down-regulate the transcription of oxidative stress-resistance genes in bacteria | Ceftriaxone | E. coli | Synergistic antibacterial effects against E. coli have been observed by ZnO nanorods with ceftriaxone | [75] |
| Ciprofloxacin | S. aureus and E. coli | Increase in inhibition zones in S. aureus = 27% and 22% in E. coli when ciprofloxacin and ZnONPs were applied in synergism | [76] | ||
| Beta lactams, aminoglycosides, and azolides | S. aureus | The highest increase was observed for penicillin G and amikacin, i.e., 10 mm increase in the zone of inhibition, whereas for clarithromycin, a 2 mm increase had been observed | [77] | ||
| TiO2NPs | Electrostatic interaction between TiO2 NPs and the bacterial cell surface results in suppression of cell division, degradation of the cell wall and cytoplasmic membrane due to the production of reactive oxygen species such as hydroxyl radicals and hydrogen peroxide | Penicillin G, amikacin, cephalexin, cefotaxime | MRSA | 10 mm increase in zone size. TiO2 nanoparticles significantly improved antibiotic efficacy against S. aureus when combined with beta-lactams, cephalosporins, and aminoglycosides | [78] |
| Fe3O4NPs | Generation of reactive oxygen species from the disruption of the electronic transport chains owing to the resilient affinity of the iron-based nanoparticles for the cell membrane. Reactive oxygen species generated by Fe3O4 nanoparticles kill bacteria without harming non-bacterial cells | Streptomycin | S. aureus, E. coli, and P. aeruginosa | Zones of inhibition at concentrations (10, 20, 40, and 80): S. aureus (15 mm, 14 mm, 17 mm, 20 mm), E. coli (12 mm, 14 mm, 15 mm, 17 mm), P. aeruginosa (13 mm, 14 mm, 15 mm, 18 mm) | [79,80,81] |
| Kanamycin and rifampicin | E. coli and S. aureus | Kanamycin formed an inhibition zone against both, whereas rifampicin formed an inhibitory zone against S. aureus only | [81] | ||
| Amoxicillin | E. coli and S. aureus | A total of 9.9% and 8.9% increase in inhibitory effect observed in the presence of Cu NPs for E. coli and S. aureus, respectively | [80] | ||
| CuNPs | Generation of reactive oxygen species, lipid peroxidation, protein oxidation, and DNA degradation. Cu2+ ions released from nanoparticles penetrate bacterial cells and are subsequently oxidized intracellularly | Amikacin, ciprofloxacin, gentamicin, norfloxacin | E. coli, P. aeruginosa, Klebsiella spp. S. aureus | At 60 mg/mL, 18 mm for E. coli, 16 mm for Klebsiella | [82] |
| BiNPs | Production of reactive oxygen species | Ciprofloxacin, norfloxacin, tetracycline, and metronidazole | K. pneumoniae | A synergistic effect was observed between all antibiotics and BiNPs. | [83] |
| Cefotaxime, ampicillin, ceftriaxone, cefepime | E. coli, K. pneumoniae, and P.aeruginosa | Significant decrease in MIC decrease with cefotaxime and ZnO NPs against K. pneumoniae (85.7%), P. aeruginosa (70%), and E. coli (50%) has been observed. Meanwhile, a decrease in MIC due to ZnO NP with other antibiotics has been observed. | [84] | ||
| Norfloxacin, Ofloxacin, and Cephalexin | P. aeruginosa, E. coli | Significant increase in inhibition zone of antibiotics with ZnONPshave been observed against all isolates. | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zohra, T.; Numan, M.; Ikram, A.; Salman, M.; Khan, T.; Din, M.; Salman, M.; Farooq, A.; Amir, A.; Ali, M. Cracking the Challenge of Antimicrobial Drug Resistance with CRISPR/Cas9, Nanotechnology and Other Strategies in ESKAPE Pathogens. Microorganisms 2021, 9, 954. https://doi.org/10.3390/microorganisms9050954
Zohra T, Numan M, Ikram A, Salman M, Khan T, Din M, Salman M, Farooq A, Amir A, Ali M. Cracking the Challenge of Antimicrobial Drug Resistance with CRISPR/Cas9, Nanotechnology and Other Strategies in ESKAPE Pathogens. Microorganisms. 2021; 9(5):954. https://doi.org/10.3390/microorganisms9050954
Chicago/Turabian StyleZohra, Tanzeel, Muhammad Numan, Aamer Ikram, Muhammad Salman, Tariq Khan, Misbahud Din, Muhammad Salman, Ayesha Farooq, Afreenish Amir, and Muhammad Ali. 2021. "Cracking the Challenge of Antimicrobial Drug Resistance with CRISPR/Cas9, Nanotechnology and Other Strategies in ESKAPE Pathogens" Microorganisms 9, no. 5: 954. https://doi.org/10.3390/microorganisms9050954
APA StyleZohra, T., Numan, M., Ikram, A., Salman, M., Khan, T., Din, M., Salman, M., Farooq, A., Amir, A., & Ali, M. (2021). Cracking the Challenge of Antimicrobial Drug Resistance with CRISPR/Cas9, Nanotechnology and Other Strategies in ESKAPE Pathogens. Microorganisms, 9(5), 954. https://doi.org/10.3390/microorganisms9050954







