1. Introduction
Exacerbated by climate change [
1], drought, increasing temperatures, and soil salinization threaten agricultural sustainability. Impacts of climate change are already decreasing agricultural productivity globally. For example, in Northern India, where the majority of farmers are small land holders without irrigation, monsoons are the only source of water and have become undependable [
1]. Crop yields have suffered due to drought and high heat conditions that are now commonplace. In Asia and Africa, rice production has declined by 15–20% over the last 20 years due to increased temperatures, drought, and soil salinization [
2,
3,
4,
5]. Moreover, rice is particularly sensitive to nighttime temperatures above 24 °C. It is now common in many locations around the world where nighttime temperatures are reaching 29 °C during the growing season [
2]. High temperatures impact spikelet anthesis and fertility, resulting in a decreased grain yield [
6]. Elevated temperatures also increase the occurrence of rice diseases, resulting in significant economic losses [
7,
8,
9].
By generating abiotic stress tolerant crops, the human impacts of climate change in agriculture could be lessened. For more than 40 years, three approaches have been taken to develop stress tolerant crops: genetic modification, mutational selection, and breeding traits from wild plants that have adapted to high stress habitats [
10]. However, these efforts have had limited field success, presumably because stress tolerance involves genetically complex processes and the ecological/evolutionary mechanisms responsible for plant stress tolerance are still being defined [
11,
12].
All plants in natural ecosystems are thought to be symbiotic with fungal endophytes that reside within the plant [
13]. These intimate associations play a pivotal role in the fitness and survival of plants thriving in stressful habitats. For example, fungal endophytes have been shown to adapt plants in coastal and geothermal habitats to salt and heat stress, respectively [
14]. In addition, fungal endophytes can regulate plant growth, development, and reproduction [
13,
14,
15,
16,
17,
18]. Without their endophytes, plants do not survive in these habitats, indicating that stress adaptation involves symbiotic processes.
Weedy rice is widely distributed in rice-planting areas all over the world, particularly in South and South-east Asia, South and North America, and Southern Europe [
19,
20]. Weedy rice is classified as the same species as commercial rice varieties (
Oryza sativa) and includes both major subspecies Indica and Japonica. In addition, it is common for weedy rice plants to exhibit tolerance to drought, cold temperatures, high salinity, and heavy metal resistance [
21,
22,
23]. Because weedy rice is successfully acclimatized and adapted to thrive in habitats varying in soil types and environmental stresses, weedy rice is considered an important reservoir for genetic variation and has been implemented in breeding programs to improve commercial rice lines for enhanced traits of interest [
22,
24]. Indeed, many useful traits have been bred into commercial rice lines, such as enhanced blast disease-resistance, cold tolerance, and enhanced grain quality [
20,
21]. However, attempts to breed drought, salt, or heat tolerant rice lines have had minimal success [
25].
The objective of this project was to determine if endophytes could be commercially developed and used to generate climate resilient crops capable of tolerating drought, heat, and salt stress. Fungal endophytes were isolated from three habitats imposing abiotic stress on resident plants: drought or salt stress on weedy rice in two locations in South Korea and drought and salt stress on resident plants in the USA. Although the weedy rice endophytes conferred sufficient stress tolerance, the fungal species are known as plant pathogens, making it difficult to obtain regulatory approval for agricultural use, and so were not pursued for commercial development. However, strains of endophytic Trichoderma harzianum isolated from native grasses in the USA were found to confer abiotic stress tolerance to multiple stresses and did not present a regulatory impediment. Due to the extensive use of fungicides on crop seeds, two Trichoderma harzianum isolates AST-TT08 and AST-TS10 were mutagenized with UV light to generate fungicide resistant strains. Three strains with resistance to different fungicides were mixed in equal proportions and applied as a seed treatment for field testing. The three-strain mixture was designated ThSM3a and was field tested on monocot and eudicot crops in North and South America, Australia, Europe, India, and Africa and commercialized as the microbial inoculant BioEnsure®. The most dramatic results were observed with poor small land-holding farmers in Rajasthan, India where it is commonly very hot and dry throughout the summer growing season. The endophytes significantly increased the yields of two staple food crops for the region, namely pearl millet and mung bean. We propose that this symbiotic technology can be used to mitigate impacts of climate change in agriculture and has the potential to break the chain of poverty for poor farmers globally.
2. Materials and Methods
2.1. Isolation and Identification of Endophytes from Weedy Rice
Endophyte isolation: Weedy rice seed lines containing their seed husks were collected (2008) from native arid (N = 7) and coastal (N = 8) habitats from observed high drought or moderate to high salinity (>6000 ppm NaCl, >13 mM NaCl, >5.9 g/L NaCl, EC > 12 dSm−1 NaCl) habitats of South Korea, respectively. Seeds were obtained from Dr. Hak Soo Suh, Wild Crop Germplasm Bank, Yeungnam University, Geongsan, South Korea. Seven drought tolerant weedy rice lines Oryza sativa L. were the Japonica type (2559, 2581, 2604, 2691, 2716, 2733, 2861), six salt tolerant lines (2518, 2582, 2687, 2692, 2693, 2844) were of the Japonica type, and two salt tolerant lines (22024, 22099) were the Indica type. One-hundred seeds with their seed husks of each rice line were surface sterilized by placing seeds in 0.5% (v/v) sodium hypochlorite for five minutes, rinsed in 70% ethanol for 15 s, rinsed with 5–10 volumes of sterile distilled water, and placed on 0.1× potato dextrose agar (1.5% agar) medium (PDA) supplemented with 100 μg/mL of ampicillin and chloramphenicol, and culturable fungi was allowed to grow out of samples at 25–28 °C under a 12 h light regime for 5–10 days. Fungal growth was observed out of the seed husks and the dominant endophytes (present in ≥70% of the seeds analyzed from each habitat) were selected and cultured, with single spores isolated and identified using classic microbiological and DNA sequencing techniques. The dominant endophytes present in weedy rice seeds from arid and high salt habitats was Curvularia brachyspora, and Fusarium asiaticum, respectively.
Fungal endophyte identification: Conidiophore and conidia morphology of the fungal isolates assigned them to the genus
Curvularia and
Fusarium [
26,
27,
28]. Subsequent DNA sequence analysis of the variable ITS1 and ITS2 sequences of rDNA (described below) identified the species as
C. brachyspora, and
F. asiaticum.
DNA extraction, PCR amplification and sequence analysis: Prior to pigment formation, mycelia were harvested from fungi grown in 50 mL liquid cultures (1.0× potato dextrose broth supplemented with 100 μg/mL of ampicillin and chloramphenicol). Cultures were grown with agitation (150–180 rpm) at 25–28 °C with a 12 h fluorescent light regime. Mycelia were harvested by filtering cultures though four layers of sterile gauze and washing the mycelia mass with 3–5 volumes of sterile water. The mycelial mass was drained, and 0.1 g resuspended in 5 mL lysis buffer (150 mM EDTA, 50 mM Tris; pH 8, 2% Sarkosyl) and frozen at −80 °C. Once frozen, samples were exposed to 65 °C for five min and cell debris pelleted at 10,000 rpm for 15 min. Nucleic acids were precipitated from the supernatant with 0.7 volumes of PEG/NaCl (20% polyethylene glycol 8000, 2.5 M NaCl) for 10 min at 25–28 °C, DNA pelleted at 10,000 rpm for 15 min and resuspended in 0.4 mL 10 mM Tris (pH 8). DNA was precipitated with two volumes of 95% ethanol, NaCl to a final concentration of 0.1 M, and pelleted at 10,000 rpm for five min. The pellet was then resuspended in 100 µL of 10 mM Tris (pH 8) and DNA (0.5–2 µL) was used to PCR amplify ITS1, ITS2, and 5.8 s regions of the nuclear ribosomal DNA (nrDNA) repeat using the primer pair ITS1-F (5′-ggaagtaaaagtcgtaacaagg-3′) and ITS4 (5′-tcctccgcttattgatatgc-3′) [
29]. PCR products were sequenced via Sanger sequencing at the University of Washington’s High Throughput Genomics Center. Sequences were analyzed and corrected using Sequencher
TM and aligned to annotated sequences in the NCBI BLAST nucleotide database.
2.2. Generation of Fungal Spores and Plants for Testing
Fungi: C. brachyspora and F. asiaticum were cultured on 0.1× potato dextrose agar (1.5% agar) medium (PDA) supplemented with 100 μg/mL of ampicillin and chloramphenicol, and fungal cultures grown at 25–28 °C with a 12 h fluorescent light regime. After 5–14 days of growth, conidia were harvested from plates by adding 10 mL of sterile water and gently scraping off conidia with a sterile glass slide. The final volume of conidia was adjusted to 100 mL with sterile water, filtered through four layers of sterile cotton cheesecloth gauze. Conidial suspensions were centrifuged at 6000 rpm for 10 min and resuspended in 100 mL sterile deionized water. The final spore suspension and concentration were adjusted to 103–104 conidia/mL depending upon the colonization assay chosen.
Plants: Rice seeds were immersed in 2.5% (v/v) sodium hypochlorite for 24–48 h, rinsed with 5–10 volumes of sterile distilled water, and imbibed in 10–20 volumes of water for 8–12 h. Seeds were germinated on 1% agar water medium plates, maintained at 28–30 °C and exposed to a 12 h fluorescent light regime for 1–2 days when seed germination was first observed to obtain known germinated seeds. Plant seedlings devoid of culturable fungal endophytes were obtained by growth of germinated seeds for an additional 1–2 days on plates. Studies began by choosing seedlings that showed no outgrowth of fungi into the surrounding medium.
2.3. Rice Seedling Growth Response
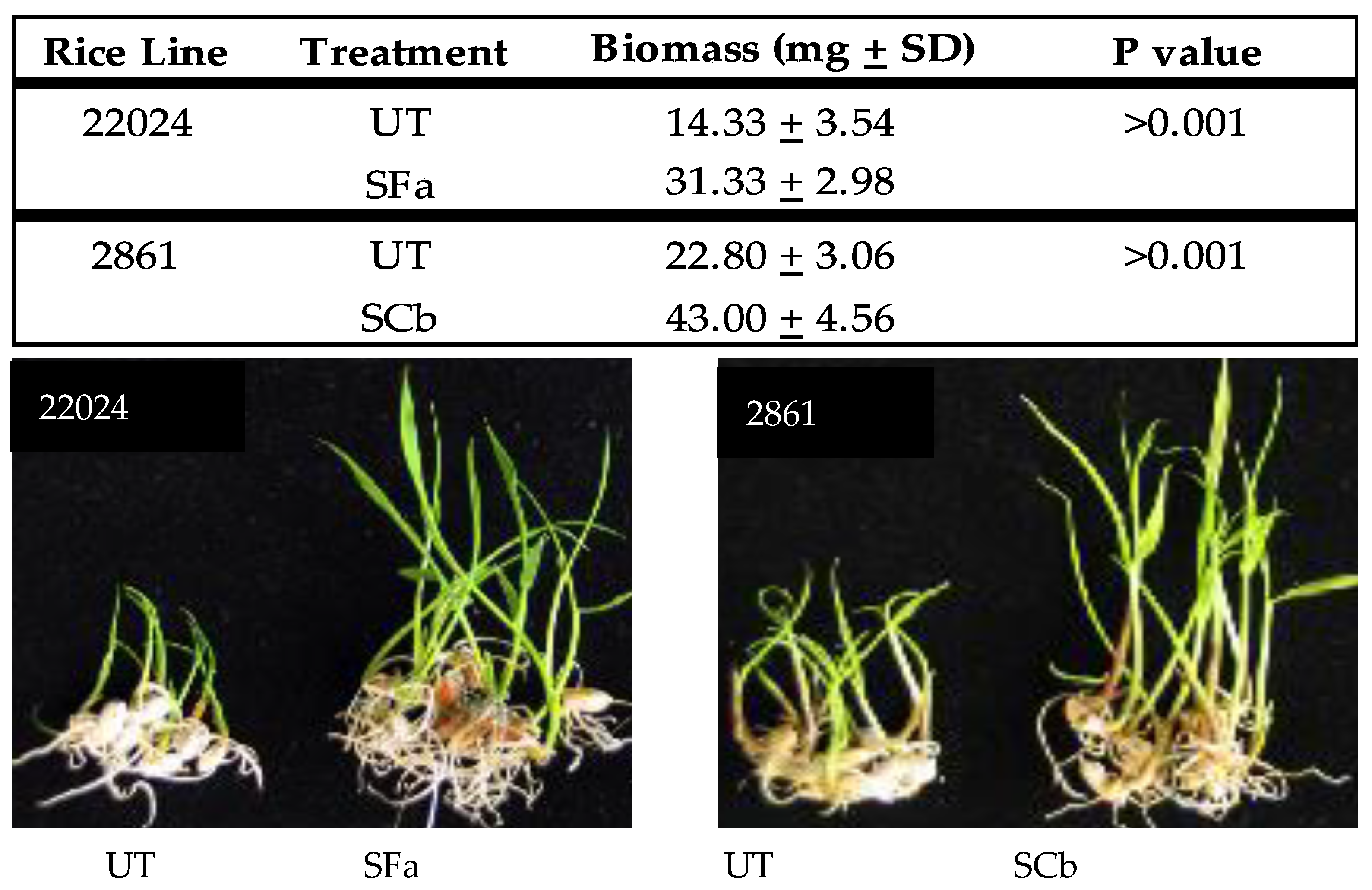
Plant growth response of weedy rice seedlings: Surface sterilized weedy rice seedlings of a salt (22024) and drought (2861) tolerant line were treated with F. asiaticum (SFa) and C. brachyspora (SCb), respectively, by immersion of germinated seedlings into conidial suspensions of 1000 conidia/mL in a plastic bag for 24 h at 28–30 °C with a 12 h fluorescent light regime to generate symbiotic (S) plants. Untreated control plants (UT) were mock inoculated by the immersion of seedlings into sterile distilled water. Plant seedlings were taken out of the conidial suspensions or distilled water and placed into sterile vials containing sterile water and exposed to a 12 h fluorescent light regime for five days at 28–30 °C and wet biomass (mg) weights measured and representative seedlings photographed to demonstrate growth response.
2.4. Magenta Box-Experiments of Weedy Rice Endophytes
Plant colonization: Surface-sterilized rice seedlings of weedy rice lines (see
Section 2.2) a commercial USA variety (
Oryza sativa cultivar M206, Japonica type) and a commercial South Korean variety (
Oryza sativa cultivar Dongjin, Japonica type) were generated and transplanted into sterile double-decker magenta boxes (modified magenta boxes to hold soil or sand in upper chamber, and fluid in lower chamber that is wicked-up though a cotton rope [
14]) containing equivalent amounts (380 +/−5 g) of sterile-sand such that nine magenta boxes with six plants/magenta box were produced (N = 54 rice seedlings/rice line or cultivar). The lower chamber was filled with 200 mL of sterile fluid (1× Hoagland’s solution supplemented with 5 mM CaCl
2). The term double-decker magenta box and magenta box will be used interchangeably. After one week, plants were either mock inoculated with water (UT, untreated) or with fungal endophytes (S, symbiotic) by pipetting 1000 fungal conidia at the base of the crowns or stems and plants allowed to grow an additional week. At week two, one magenta box/treatment containing six 2-week-old rice seedlings was randomly selected to assess fungal colonization. Plant seedlings were surface sterilized by immersion into 0.5% (
v/
v) sodium hypochlorite for 20 min and rinsed with 5–10 volumes of sterile distilled water. The imprint technique was utilized to verify adequate surface sterilization of plant tissues was achieved [
30]. Using aseptic techniques, plant tissues were cut into small sections (5 cm) representing the roots and shoots and placed onto 0.1× PDA (1.5% agar) medium supplemented with 100 μg/mL of ampicillin and chloramphenicol. Plates were incubated at 25–28 °C under a 12 h light regime for 7–10 days and assessed for the growth of culturable fungi out of plant tissues. Fungal species were identified using standard microbiological techniques to verify colonization occurred with the appropriate endophyte. Plants for laboratory studies were grown <2 months in double decker magenta boxes (four magenta boxes/treatment with six plants/magenta box = 24 plants) at 28–30 °C with a 12 h fluorescent light regime.
Abiotic Stress
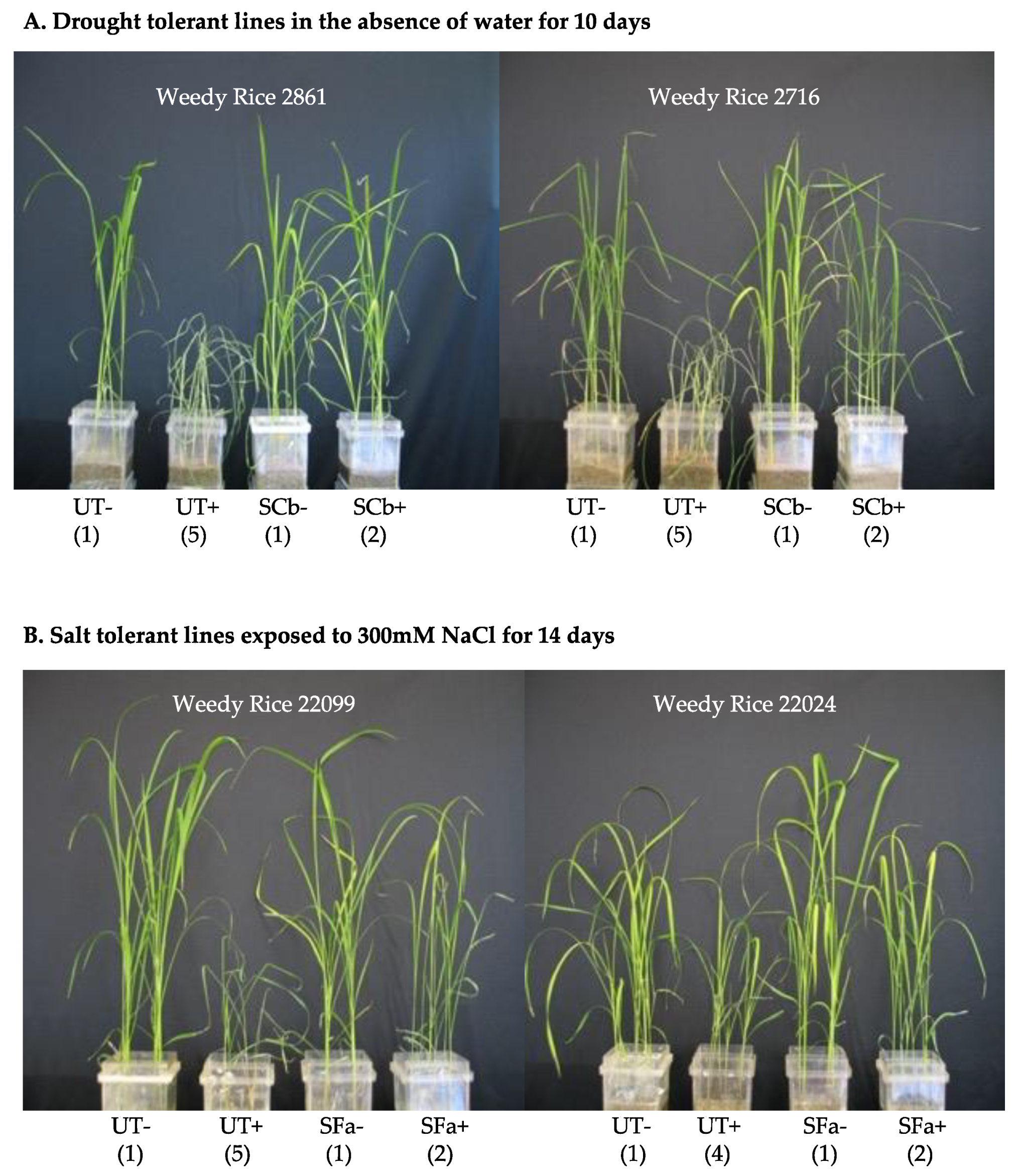
Magenta box-experiments were performed with 2–4 week old plant seedlings. Magenta boxes were randomly moved every 2–3 days to mimic a randomized block design. Rice seedlings were generated that were either untreated (UT) or treated (S) with C. brachyspora (SCb) for drought, F. asiaticum (SFa) for salt stress, and both symbiotic treatments assessed for fluid consumption studies. Plants were assessed for health upon termination of the studies. Plant health was assessed on a 1–5 level with 1 being green and healthy, 2–3 indicating slight to moderate wilting, 4 indicating severe wilting and chlorosis, and 5 indicating wilted and dead. The images in the figures are representative of all replications of each treatment for the study presented.
Drought stress: Watering was terminated on 4-week-old UT and SCb plants by decanting off the fluid in the lower chamber of the double-decker magenta box and letting the plant and soils dry out over 10 days. A hydraProbe (Stevens-Vitel Inc., Chantilly, VA, USA) was used to ensure that soil moisture levels were equivalent between treatments when watering was terminated. After 10 days, plants showed symptoms (i.e., UT plants dead or severely wilted) and were re-hydrated by addition of 200 mL sterile water to the upper chamber of the magenta box. Plants were allowed 48 h to recover and assessed for health and photographed.
Salt stress: UT and S -Fa plants were exposed to 50–300 mM NaCl (EC = 5.9–26.0 ds/m) solutions (1× Hoagland’s solution supplemented with NaCl and 5 mM CaCl2; referred to as 50 mM, 100 mM, 150 mM, 200 mM and 300 mM NaCl solutions) in a step-wise manner. Two-week old seedlings were exposed to 50 mM NaCl solution for seven days, at which point the 3-week old plants were then exposed to 100 mM NaCl solution for seven days, followed by exposure to 150 mM and 200 mM NaCl solutions each for seven days, and a final exposure for two weeks to 300 mM NaCl. Salt solutions (200 mL) were filled in the lower chamber of the double-decker magenta boxes as needed. Upon termination of the magenta box studies, plants were assessed for health and photographed.
2.5. Mature Greenhouse Plant Studies
Plants for greenhouse studies were generated first in magenta boxes as described above. An additional 24 plants/treatment (four reps of six plants/pot) of UT, SCb and SFa plants were generated for weedy rice plant lines (N = 15) and the two commercial rice varieties Dongjin and M206. Two-week old rice seedlings were transplanted as a cluster of six plants into 7.5 L pots containing potting soil (Sunshine Mix#4) and grown at 28–30 °C to maturity (an additional 3.5 months) under paddy conditions (saturation of soil).
Drought stress: Seed yields and plant biomass (g) were assessed after four months with greenhouse grown UT and SCb treatments. Studies were conducted with seven weedy rice drought tolerant lines and two commercial rice varieties Dongjin and M206. Plants were grown for 13 weeks and watered with 1× Hoagland’s solution supplemented with 5 mM CaCl2. After 13 weeks, watering was terminated for ≤21 days to induce drought. All control plants were maintained at 28–30 °C and watered throughout the experiment with sterile water or 1×Hoagland’s solution supplemented with 5 mM CaCl2.
Salt stress: Greenhouse grown UT and SFa plants of eight weedy rice salt tolerant lines and two commercial rice varieties (Dongjin and M206) were assessed for salt stress tolerance. After sufficient growth was established, one-month old plants were exposed in a stepwise manner to increasing concentrations of salt solutions (1× Hoagland’s solution supplemented with 5 mM CaCl2 + 50 mM, 100 mM, 150 mM or 200 mM NaCl) every three weeks. Pots were flooded until soil was saturated with the various NaCl solutions to induce salt stress. Upon termination of the studies, plants were assessed for plant biomass and seed yields.
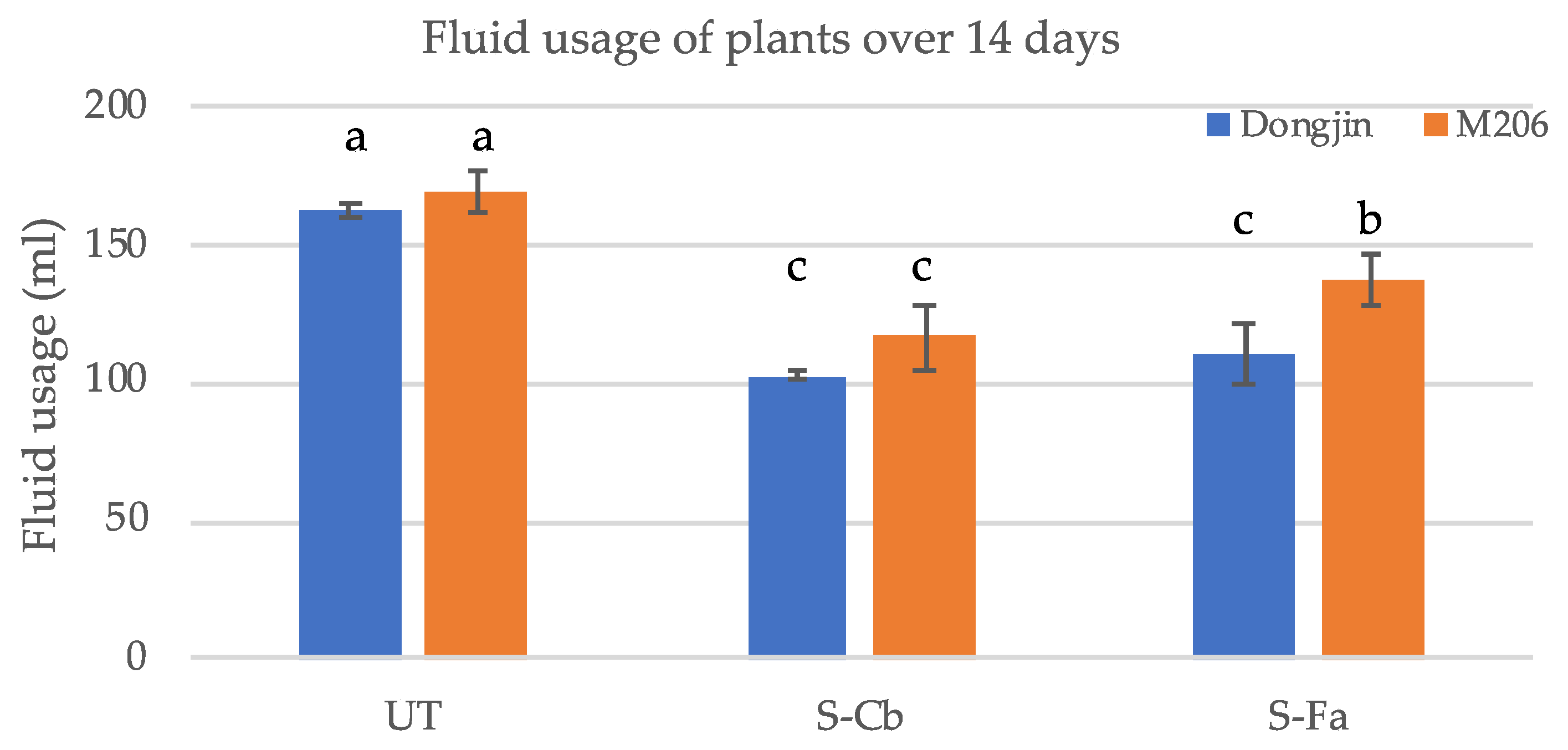
2.6. Fluid Consumption
Four-week-old plants grown in magenta boxes grown in the absence of abiotic stress were measured for the amount of fluid consumed by commercial rice varieties M206 and Dongjin treatments UT, SCb and SFa. Plants were grown (four magenta boxes/treatment with six plants/magenta box = 24 plants) at 28–30 °C with a 12 h fluorescent light regime. At week four, the bottom tier of the double-decker magenta box was removed and 200 mL of 1× Hoagland’s solution supplemented with 5 mM CaCl2 added. After 14 days, the remaining fluid was measured to determine fluid usage. Plants were grown at 28–30 °C under a 12 h fluorescent light regime.
2.7. Development of Symbiotic Technology for Commercialization
Trichoderma harzianum isolates AST-TT08 and AST-TS10 were obtained from bunchgrass species
Brachypodium sylvaticum and
Muhlenbergia rigens, respectively, thriving in moderate to high arid and saline soil stressed habitats in California, USA. The isolation and identification were done in the same manner as described for weedy rice endophytes (
Section 2.1.) Identification of the conidiophore and conidial morphology of fungal isolates determined the genus as
Trichoderma [
26,
27,
28]. Subsequent PCR based sequence analysis of the variable ITS1 and ITS2 sequences of rDNA (described above) identified the species as
T. harzianum. Conidia were produced by growing fungal cultures on 0.1× potato dextrose agar (1.5% agar) medium (PDA) supplemented with 100 μg/mL of ampicillin and chloramphenicol, and incubated at 25–28 °C with a 12 h light regime. After 7–10 days of growth, conidia were harvested from plates by adding 10 mL of sterile water and gently scraping off conidia with a sterile glass slide. The spore count was found >10
6 conidia/100 mM petri plate. The final spore concentration was adjusted to 10
2–10
8 conidia/mL depending upon the assay chosen for the studies.
2.8. Generating Fungicide Resistant Strains of T. harzianum and Formulating for Field Testing
Conidia of T. harzianum strains were adjusted to 108 conidia/mL, placed in a glass dish, and exposed to 0, 15, 30, 45, 60, and 90 s to UV light until 99.99% lethality was achieved. The UV light was placed 4–6 cm above the spore samples. After irradiation, samples were spread plated onto 0.1× PDA (1.5% agar) medium supplemented with 100 μg/mL of ampicillin and chloramphenicol in the absence or presence of various concentrations of fungicide chemicals commonly used in agriculture. The concentration for each fungicide used for selection was determined by spread plating wildtype conidia on petri plates containing various concentrations of each fungicide and identifying which concentration inhibited fungal growth completely. These levels equated to industry usage rates applied by seed treaters on a per seed basis. UV irradiated and non-irradiated T. harzianum AST-TT08 and AST-TS10 isolate conidia were spread plated on 100 mm media plates containing lethal levels of fungicides (hymexazol, ipconazole, prothioconazole, tebuconazole and thiabendazole). Plates (N = 3 per chemical tested) were incubated at 25–28 °C under a 12 h light regime for 7–10 days. Fungal colonies growing on chemical selection plates were considered to have high (H), moderate (M), or low (L) chemical resistance with 70–100%, 30–69%, and 0–29% conidial viability, respectively. Chemically resistant isolates were maintained for further testing. Phenotype stability on selection medium and plant colonization were criteria used to select candidates for field testing. Obtaining strains with resistance to multiple fungicides required 2–3 successive rounds of mutagenesis. For field testing, conidia from three strains that were collectively resistant to all of the commonly used fungicides were mixed (1:1:1) into a consortium designated ThSM3a.
2.9. Screening T. harzianum Strains for Biopesticidal Activity by Interaction Plating
These studies were performed by inoculating ThSM3a and another fungus onto opposite sides of 90 mm petri plates containing 0.1× PDA according to published protocols [
31]. Antagonism was assessed between a mixture of the fungicide resistant strains (ThSM3a) and either
Aspergillus niger,
Colletotrichum gloeosporioides,
Colletotrichum magna,
Curvularia protuberata,
Curvularia inaequalis,
Alternaria alternata,
Fusarium culmorum,
Penicillium, or
Fusarium spp. Plates were monitored visually for the growth of both fungi to determine if there were antagonistic interactions such as inhibition halos, excessive branching, or swelling of the mycelia hyphae as they grew close to one another. When the dual cultures grew within 1 cm of each other, the interaction zones were monitored microscopically. All assays were repeated three times.
2.10. Field Testing for the Evaluation of ThSM3a
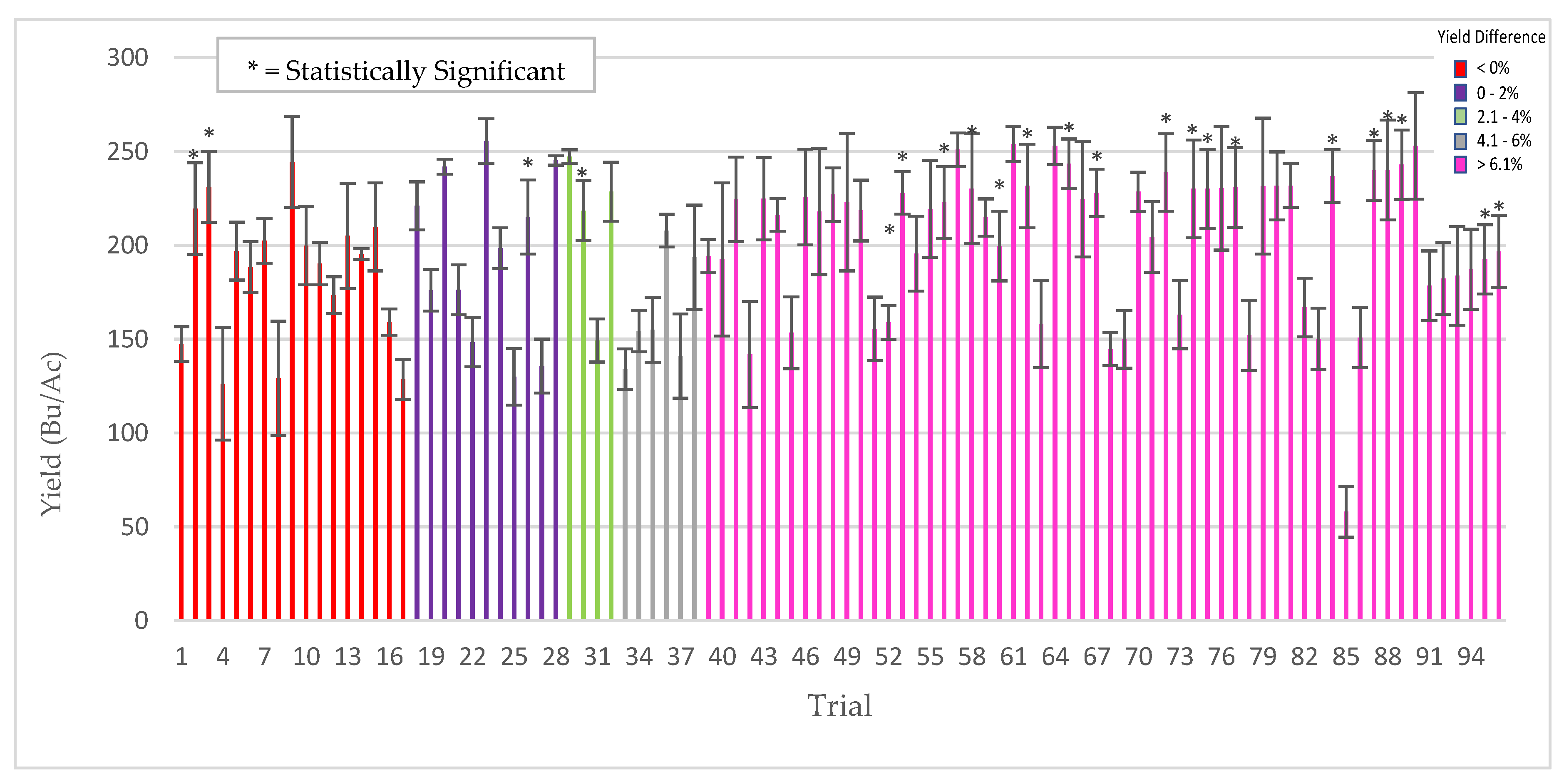
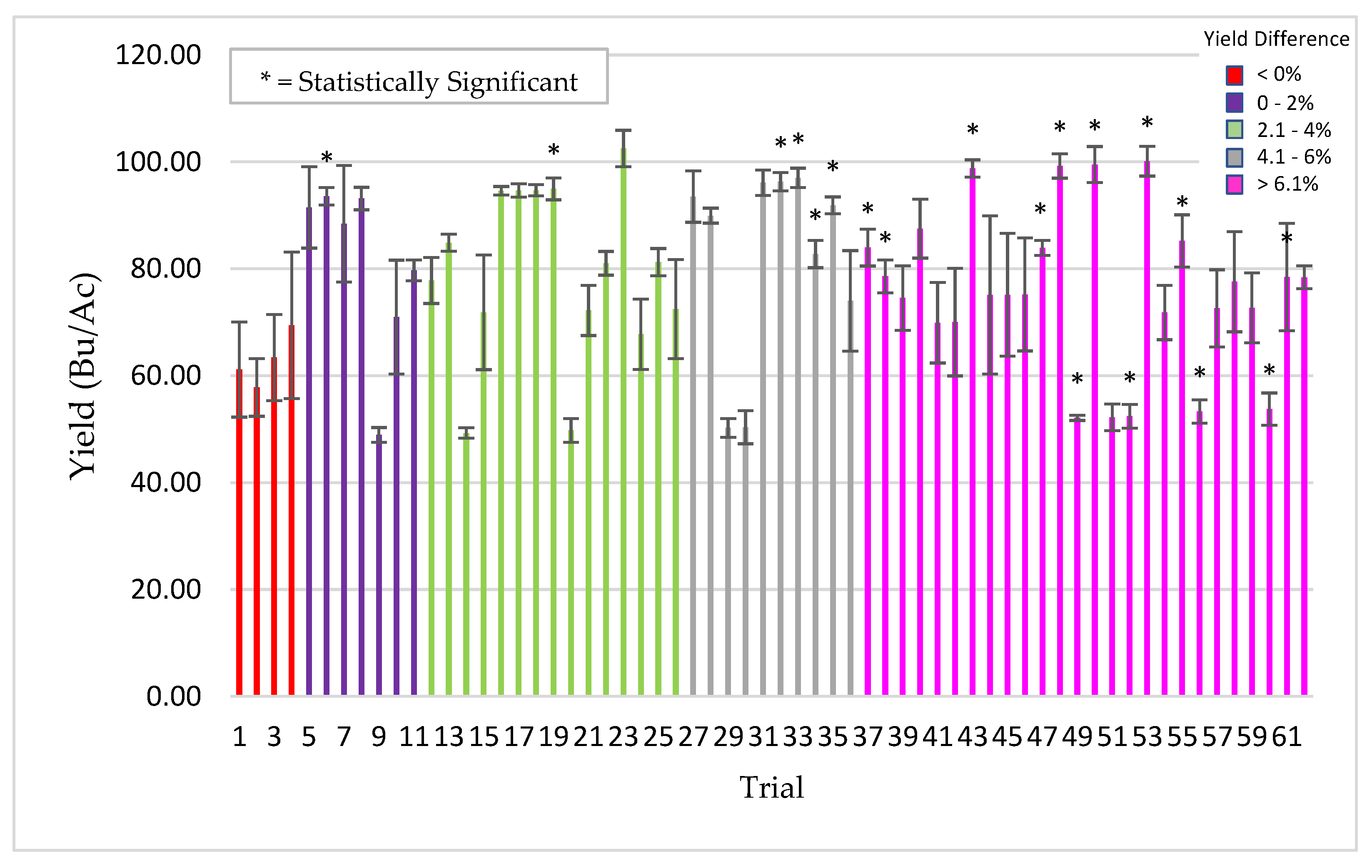
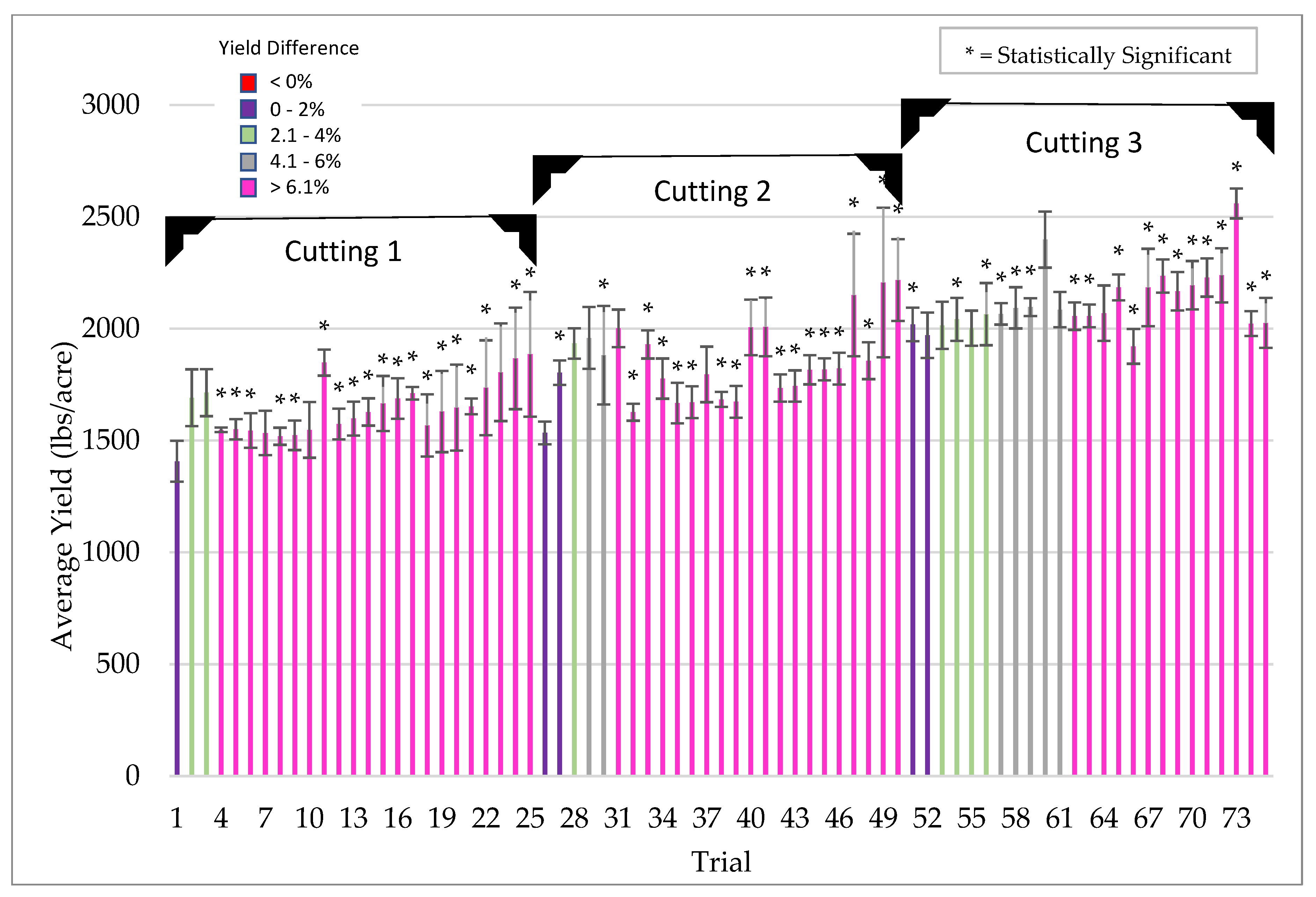
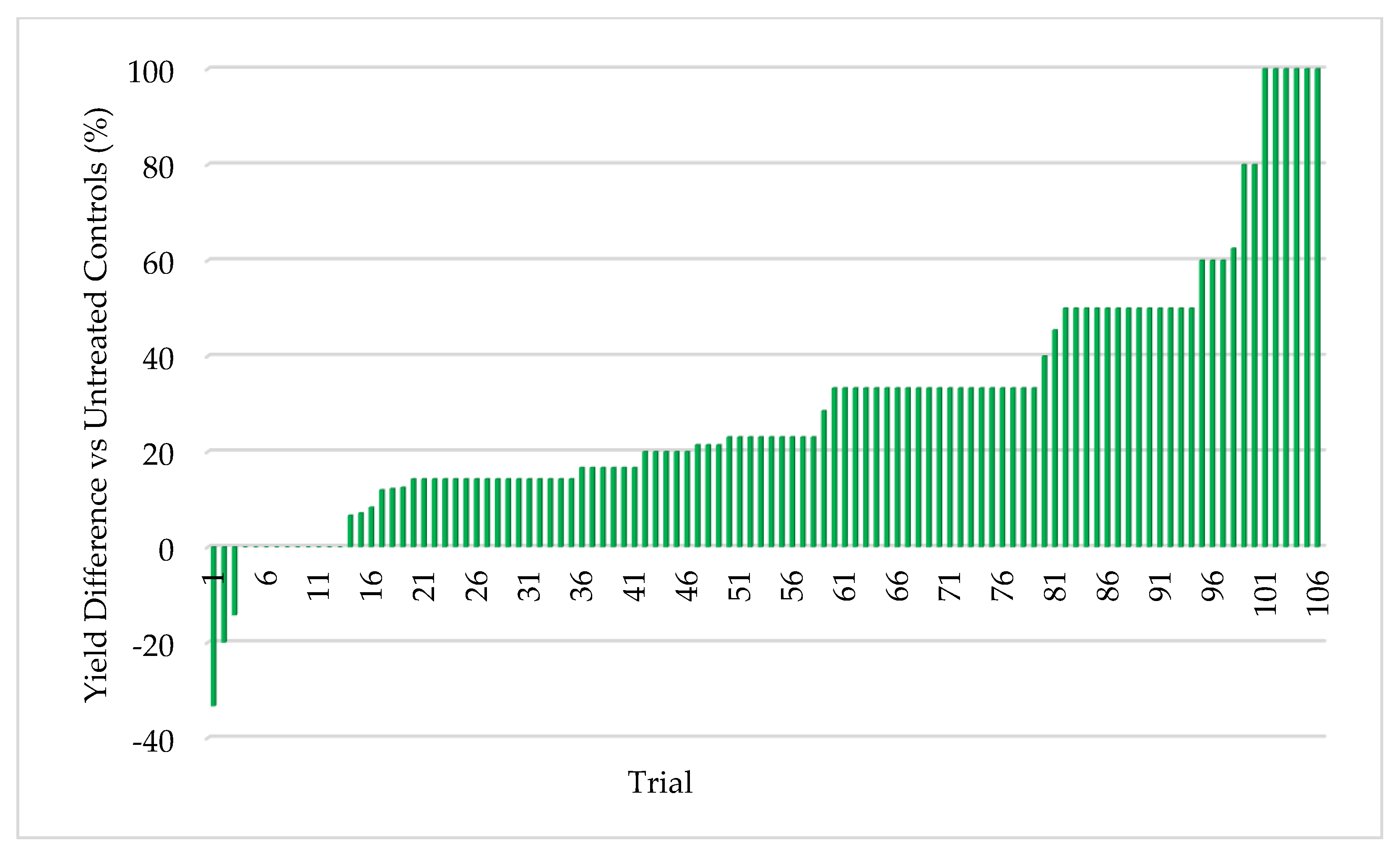
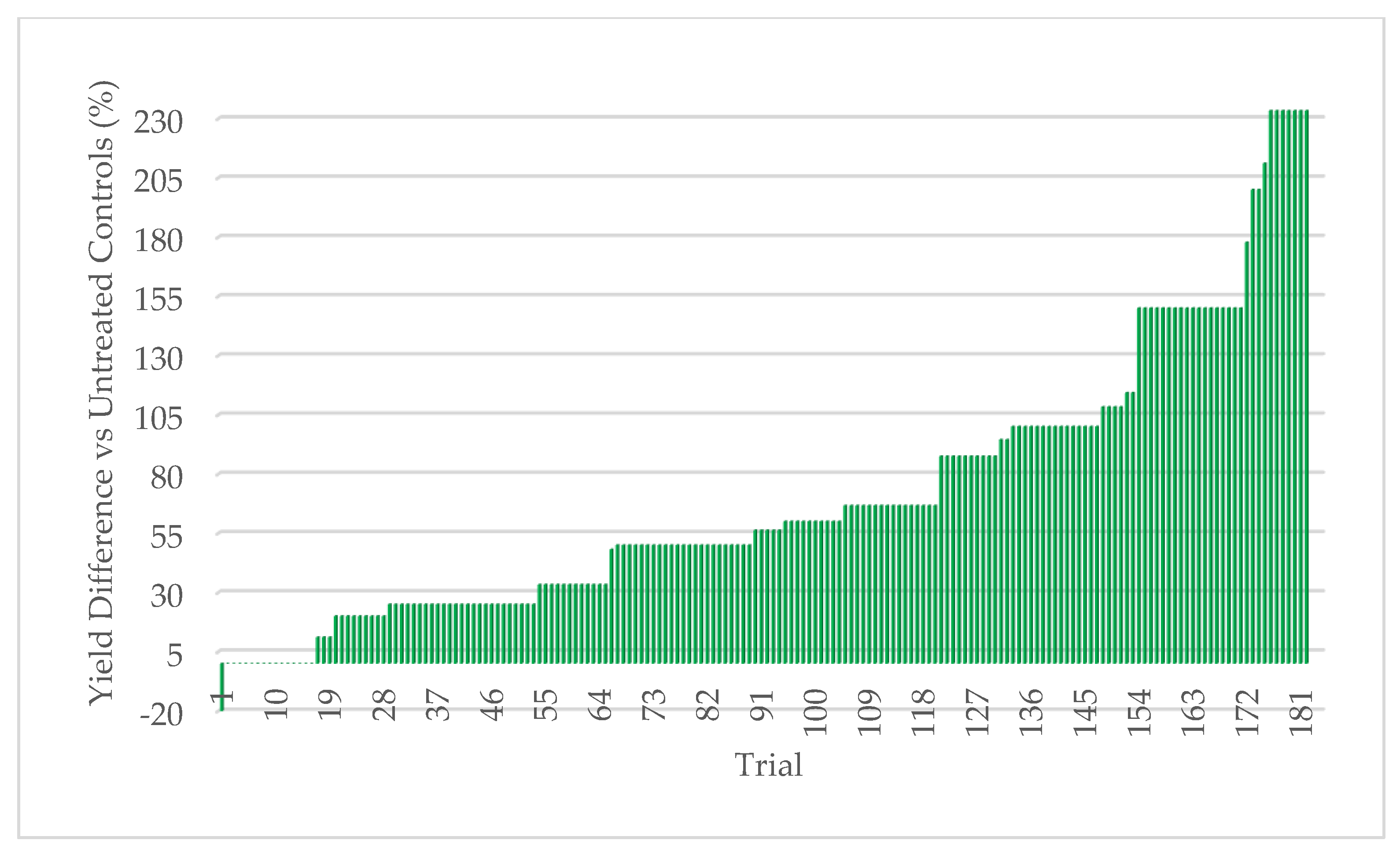
Plants for field testing: Corn (Zea mays), barley (Hordeum vulgare), soybean (Glycine max), rice (Oryza sativa), cotton (Gossypium hirsutum), pearl millet (Cenchus americanus, commonly known as the synonym Pennisetum glaucum) and mung bean (Vigna radiata) of various commercial varieties/hybrids were used for field testing in the USA, Argentina, Uruguay, Australia and India. All of the field trials were conducted by independent cooperators [Farmers, seed companies, agricultural cooperatives, universities, and contract research organizations (CRO’s)]. Standard seeding rates were used for crop planting as befitting the region and country. Seeds were treated with ThSM3a using standard industry rotary seed treaters in all countries with the exception in India, where small manual and electric rotary drums were fabricated for spray application of seeds. The application dose of ThSM3a was dependent upon the size of the seed. Large (corn, soy, cotton), medium (mung bean, rice, barley), and small (pearl millet) sized seeds were treated with conidia concentrations ranging from 2000–5000, 500–1500, and 50 conidia/seed, respectively. Specifics of the trials (year, locations, plot sizes, replications, treatment, cooperators) are listed in the figure legends. Trials and plots sizes varied from 10 ft × 50 ft to 50 acres with 1–8 replications/trial. All treated seeds regardless of their label designation in the field studies presented was treated with the ThSM3a formulation.
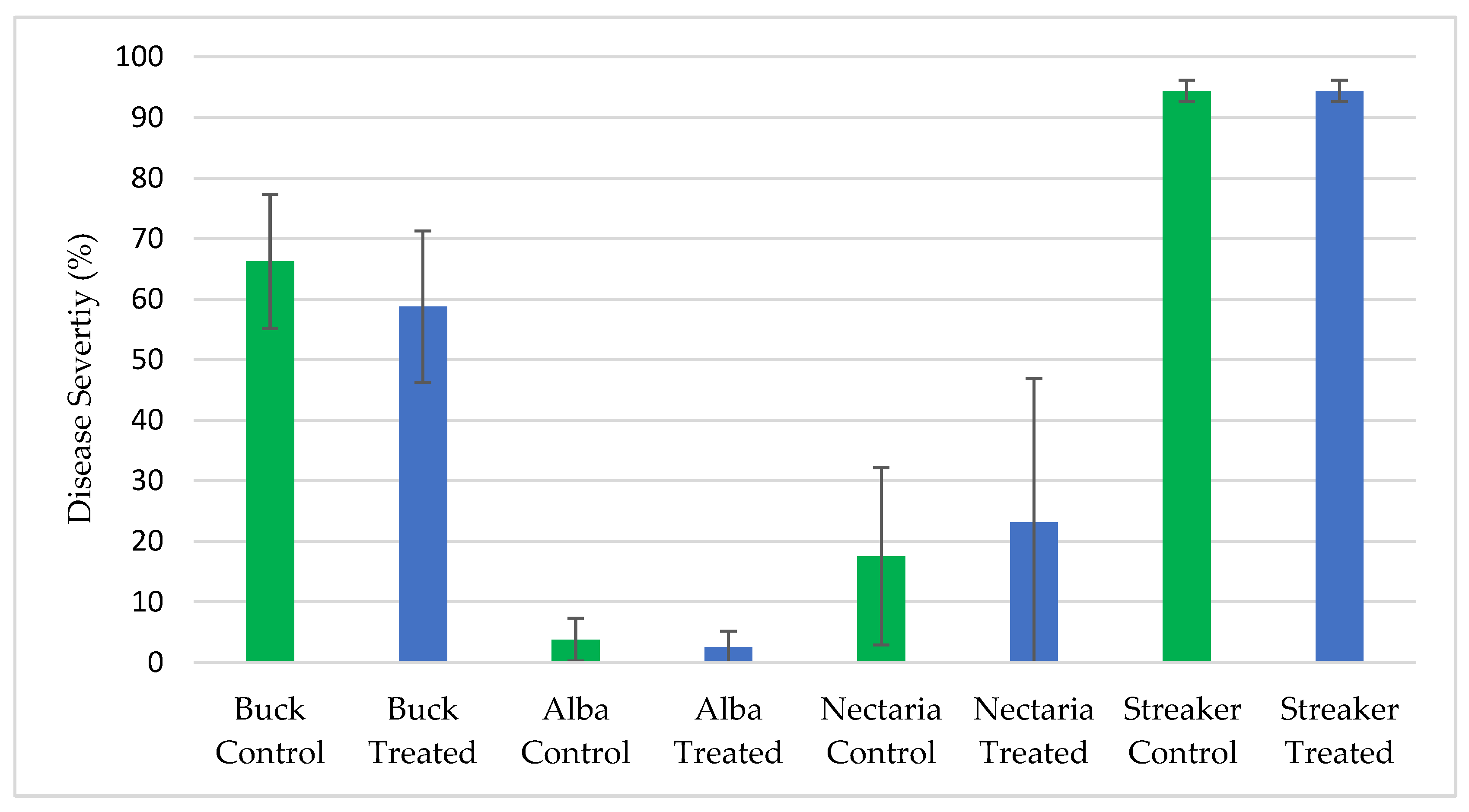
2.11. Screening ThSM3a for Biopesticidal Activity in Field Plots
All of the field testing for biopesticidal activity was performed by faculty at Michigan State University, Texas A&M AgriLife Research University and Oregon State University from 2013–2018 in locations with historical disease incidence. Testing was performed on soybean, rice, and barley. The field tests were comparative between ThTMS3a treated vs untreated control plants and the diseases screened were sudden death syndrome of soybean (Fusarium virguliforme), barley scald (Rhyncosporium secalis), and stripe rust (Puccinia striiformis) of barley, and brown spot of rice (Cochliobolus miyabeanus). In all of these field tests there was sufficient disease pressure to detect significant biopesticidal activity if present.
For soybean sudden death syndrome (SDS) screening, plots were randomly distributed throughout the field and soybean field plots were four rows at 76 cm spacing and 5.3 m long, planted on 1 May 2013 and harvested on 8 October 2013. There were 15–45 replicate plots/treatment, two treatments, control (untreated), and treated (3000 ThMS3a conidia/seed), and three soybean varieties tested. Yield data consists of percent moisture, kg/plot, Bushels/Ac, and the corrected Bushels/Ac. Some plots were damaged by the pivot irrigation. As a result, estimates of row feet lost were made and data corrected for the damaged sections. Stand counts were complete counts of one of the center two rows. Vigor rating 9 (bad) → 1 (good); an estimate of average plant vigor (score of 5) was determined and then plots were rated compared to that average.
Soybean SDS ratings were conducted on 16 August and SDS ratings done using a 0–9 scale (0 = no symptoms, 9 = dead). Disease severity (DS) ratings were done by estimating the average SDS value for affected plants and the disease incidence (DI) was estimated as the percentage of affected plants/plot. The disease index (DIX) was calculated using the equation ((DS/9)*DI) and used to compare plots. MSU staff collected and analyzed all the data.
For barley scald and stripe rust screening, barley plots were 37 m long (six plots, 6.1 m 1.5 m per plot, six rows per plot and 90 g seed per plot). Spatially, varieties were planted north/south in strips across the field. In the spring, plots are mowed and a 1.5 m alley between plots (east-west) created to facilitate access. Thus, the harvested area per plot is about 1.5 × 4.6 m. After measuring the actual plot length, the data was extrapolated to yield in kg/ha. Disease incidence was measured throughout the growing season and both resistant and susceptible varieties were treated (ThMS3a) for comparison to controls (untreated). Each year from 2015–2018, multiple barley varieties were tested with six replicate plots/treatment (untreated control and ThMS3a treated) and plots were randomly distributed in the field. Oregon State University staff collected all the data.
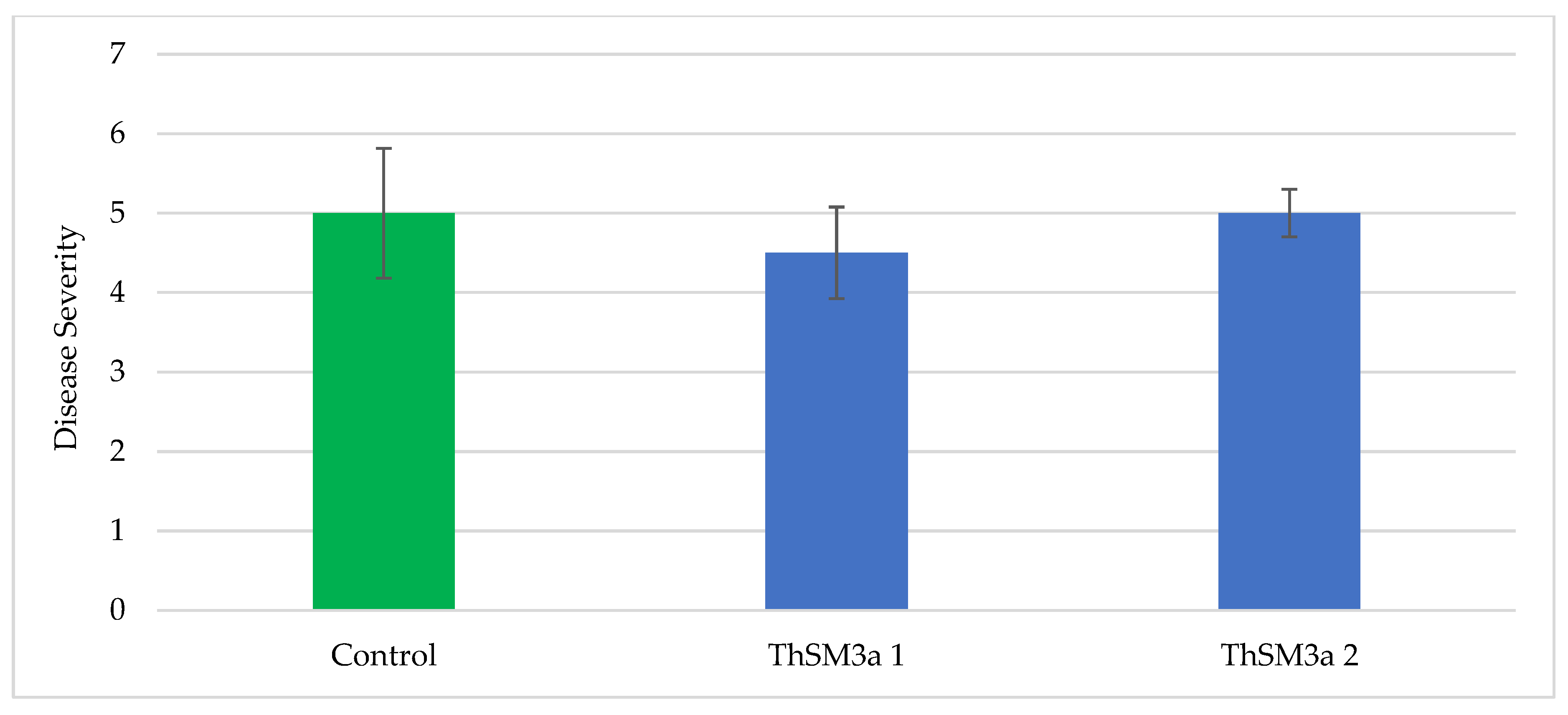
For rice brown spot screening, plots consisted of seven 4.6 m rows, and spaced 17.8 cm between rows. Three treatments [control, ThSM3a1 (500 conidia/seed) and ThMS3a2 (1500 conidia/seed)] were arranged in a randomized complete block design with four replications. Seeds of Presidio were treated with ThMS3a by AST staff prior to planting. Rice was drill seeded at 135 kg/ha on 11 May 2017. A permanent flood was established on 11 May to control weeds. Plots received 168 kg N/ha of Nature Safe (10-2-8, N-P-K) on 20 June. On 23 May, stand was counted as the number of seedlings in the three central rows of the plot and converted to stand per row foot. On 23 May, height of six seedlings randomly selected from each plot was measured. On 24 August, the severity of brown spot was rated on a scale of 0 to 9 where 0 = no symptoms and 9 = most severe damage (100% leaf area covered with lesions and most portion of leaves dead). Plots were not harvested at maturity for the determination of rice grain yield and milling quality due to the hurricane Harvey.
2.12. Screening ThSM3a for Soil Competitiveness and Presence in Plant Tissues
Soils: Samples were collected at the base of corn plants, between plants and between the rows (N = 10/location) in field test plots in Yakima, WA before planting, during cultivation and after over-wintering. The top debris layer (4–5 cm) was removed and a soil corer used to obtain <20 cm deep samples. Samples were placed into sealable plastic bags and stored at 4 °C until analyzed. Soils were homogenized and 100 g of soil of each sample retrieved for analysis using standard microbiological dilution plating methods. To begin, a teaspoon of measured soil was placed into 30 mL of sterile water and vigorously vortexed for 30 s, large soil debris allowed to settle for 5 s, and then serially diluted in a 1:10 dilution series seven times. Then, 0.3 mL sample of each dilution was spread plated onto a 150 mm, 0.1× PDA plate supplemented with 100 μg/mL of ampicillin and chloramphenicol. Dilution samples that exhibited approximately 100 CFU/plate was chosen as the target dilution for feasible CFU counts and fungal species identification. The target dilution was then plated 10 more times onto 0.1× PDA (1.5% agar) medium supplemented with 100 μg/mL of ampicillin and chloramphenicol without (non-selection media) and with chemical fungicides (selection media) and incubated at 25–28 °C under a 12 h light regime for 7–10 days. Collectively, these 10 replicate plates equated to approximately 1000 fungal CFU per soil sample from untreated and ThMS3a treated plants (soils collected from the base of corn plants, between plants, and between the rows, one month before, during (two weeks, two- and three-months post-planting), and after the growing season (four months post-harvest)) and were assessed for the presence of Trichoderma spp. The chemical fungicides chosen to visualize the potential presence of ThMS3a in soils was thiabendazole, hymexazol and tebuconazole, applied at lethal levels to the medium such that sensitive wildtype isolates could not grow, but fungicide resistant Trichoderma isolates could. Growth of Trichoderma on selection media plates were further analyzed microscopically to verify they were T. harzianum.
Plant residue: One-year post harvest of the Yakima plots, ThSM3a treated and untreated corn plant residue (crowns and upper roots) were collected from the two 6 ha plots to determine if the endophytes had survived over winter. Samples (N = 10) were collected from the center of each plot, cleaned with water, either surface sterilized or not. Plant samples were surface sterilized by immersion into 0.5% (
v/
v) sodium hypochlorite for 20 min and rinsed with 5–10 volumes of sterile distilled water. The imprint technique was utilized to verify adequate surface sterilization of plant tissues was achieved [
30]. Using aseptic techniques, plant tissues were cut into small sections (5 cm) representing the roots and shoots and placed onto non-selection or selection media plates (see above). Plates were incubated at 25–28 °C under a 12 h light regime for 7–10 days and assessed for growth of culturable fungi out of plant tissues. Fungal species were identified using standard microbiological techniques.
2.13. ThSM3a Field Trials
Rice, barley, soybean, corn, pearl millet, cotton, and mung bean seeds were treated using rotary treaters commonly used in the agricultural community. Depending upon the crop, 50–5000 conidia/seed of the T. harzianum three-strain mix ThSM3a were applied one week to nine months before planting. Seeds were planted in fields using manual methods or standard commercial equipment under standard conditions. Plot sizes ranged from four rows × 3 m to 20 ha and commercial seed varieties with standard plant protection packages (PPP’s) were used for testing. All testing was comparative between ThSM3a treated and untreated control seeds and field trials were carried out by farmers, seed companies, agricultural distributors, university faculty, agricultural cooperatives and contract research organizations (CRO’s). More details about the field trials are included in the figure legends.
2.14. Quantifying Abiotic Stress
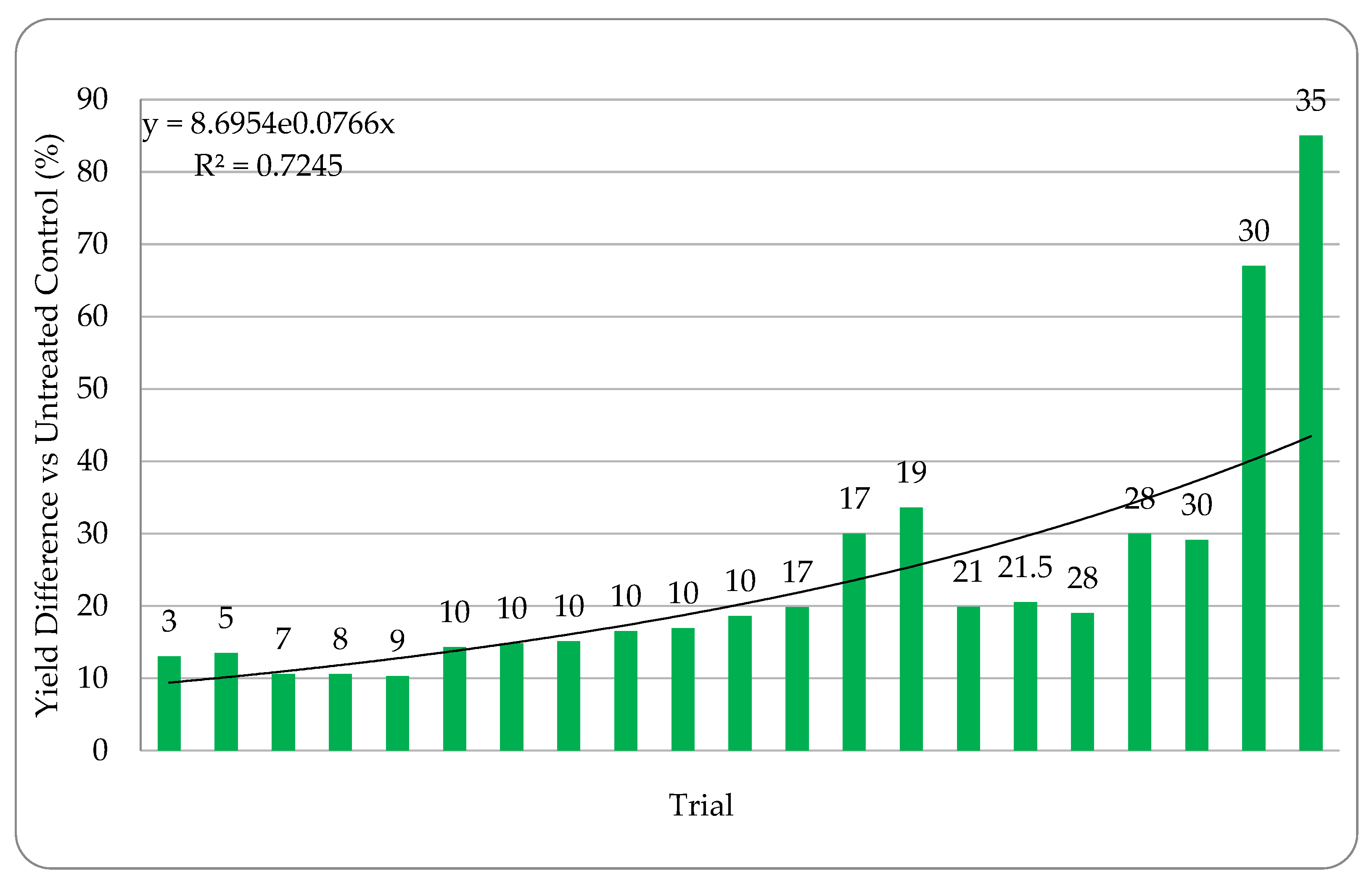
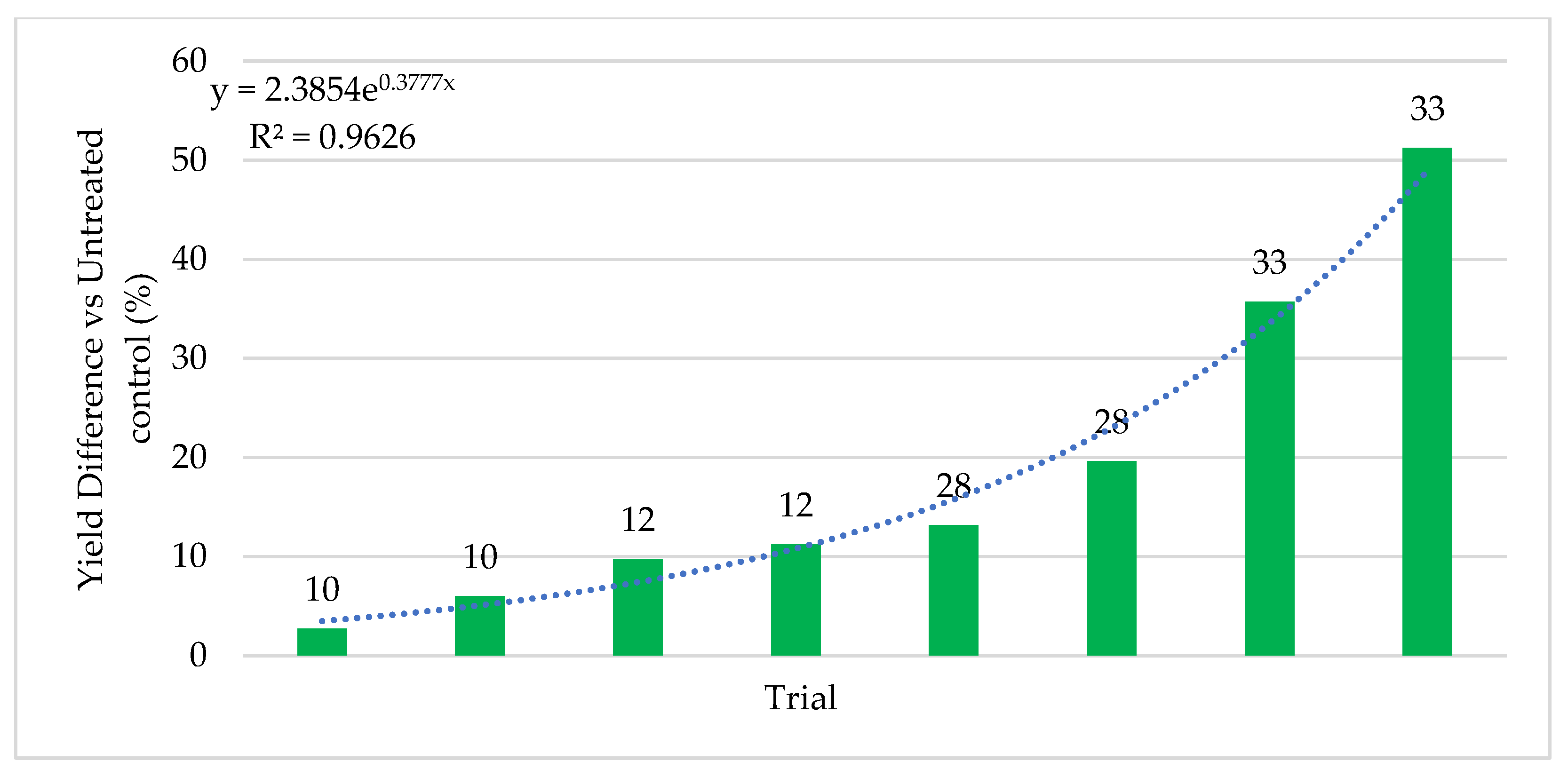
Quantifying abiotic stress relies on a myriad of factors that are nearly impossible to completely capture. In our effort to assign stress levels, we created a simple ranking system based on six common and quantifiable abiotic stressors: number of growing season days above 32 °C (heat stress), level of irrigation utilization (drought stress), soil composition, precipitation, plant growth phase of endophyte application, and plant health. Each is defined and described below. High abiotic stress at the location and year of a trial is represented with a high combined stress score. Lower abiotic stress is represented by a lower combined stress score. Stress rankings in the 24 trials ranged from 3–35. Stress definitions and ranking methodology:
- (1)
Number of growing season days above 32 °C (heat stress): Using data from the National Climatic Data Center (NCDC) and the National Oceanic and Atmospheric Administration (NOAA), totals for the number of days during the growing season (April–September for US, June–November for India) that exceeded 32 °C were recorded at each location. Ranks were then given to each location; 1 was assigned to 0–10 days, 2 to 11–20, etc. for a maximum rank of 19.
- (2)
Irrigation levels (drought stress): Ranks were determined based off the amount of irrigation provided at each location. If no irrigation was utilized a rank of 10 was given. If a trial was 100% irrigated a rank of 0 was given. Partial irrigation scores were determined on a case by case basis and scored either 5 or 2.5 depending on irrigation conditions at each site.
- (3)
Soil composition: Using location specific data and definitions from the United States Department of Agriculture (USDA) Web Soil Survey, hydrologic soil groups were categorized based off estimates of runoff potential and overall total sand composition was determined. Soils falling into group A (high infiltration rate when thoroughly wet) were regarded as higher stress soils. Soils with high group A percentages were ranked with higher scores whereas soils with loams or clays were ranked lower. Starting with 0% sand composition with a ranking of 0, one point was added for every 5% increase in sand composition resulting in a total of 10 points for highly stressed soil.
- (4)
Precipitation (drought stress): Using historical climate data from NOAA, departures in precipitation from 30-year averages were determined at each location. If precipitation for that year and locale was above the 30-year average a 0 was given as a stress value. For every inch of precipitation below the 30-year average one point was given, rounding to the nearest whole number (range 0–12).
- (5)
Plant growth phase at time of application: Ranking ranged from 0–3 based on application onto seeds (0), seedlings (1), multi-leaved vegetative plants (2), and reproductive plants (3). The intent is that the earlier the application, the better stress protection.
- (6)
Plant health at time of application: Ranking is based on symptoms of stress impact on plants at the time of endophyte application. To decrease potential for subjective ranking bias, this was simplified to a three-ranking system: 0 for healthy plants lacking any sign of stress, 5 for significant leaf curling or browning, and 10 for severe wilting.
2.15. Statistical Analyses
Due to the variety and type of data collected, several methods of statistical analysis were performed throughout this paper as indicated in the table captions and figure legends. For all experiments unless otherwise stated, the student’s t-test was used to determine significance between two groups (i.e., untreated control vs. treated). A significance level of p < 0.05 was considered to be statistically significant unless otherwise specified.
4. Discussion
Studies with weedy rice lines add to the body of knowledge that fungal endophytes play a significant role in the ecology, biology, and adaptation of plants [
13,
32,
33,
34]. It may also explain why breeding certain traits from native plants into domesticated plants has had limited success. Both
C. brachyspora and
F. asiaticum endophytes conferred stress tolerance, increased biomass, and decreased water consumption in weedy rice and other commercial rice cultivars, did not grow into seeds or embryos and were categorized as Class 2 endophytes [
13]. Although endophytes can establish on seed coats of native plants, we have not observed endophytes establish on the seed coats of agricultural plants (not shown). Therefore, the endophytes that are responsible for abiotic stress tolerance are vertically transmitted inefficiently during the breeding process. This is supported by the fact that attempts to breed salt, heat, and drought tolerance from weedy rice into commercial varieties have been marginally successful in the field [
35,
36,
37].
There are three basic requirements of commercializing endophytes for agricultural use: (1) the ability to scale production to meet demand, (2) field efficacy, and (3) regulatory approval [
38,
39]. Unfortunately, some isolates of
C. brachyspora and
F. asiaticum are known to be pathogenic. This presented a significant impediment to obtaining regulatory approvals so the isolates were not field tested or commercialized. Instead, a series of endophytes were screened for meeting all three requirements. Most intriguing were the endophytic strains of
Trichoderma harzianum.
One impediment to field testing of fungal endophytes is the ubiquitous use of plant protection packages (PPPs) containing fungicides in commercial seed treatments of non-organic seed. Although the endophytic
T. harzianum strains were resistant to several fungicides in PPPs, they were inhibited by others such as the sterol biosynthesis inhibitors (SBIs). Generating SBI-resistant strains required successive rounds of UV light mutagenesis and selection for resistance to one or more SBIs using plate inhibition assays. A group of three isolates (ThSM3a) that collectively showed resistance to all of the commonly used fungicides were used for field testing. There are numerous reports describing the benefits of fungal endophytes on seed germination, seedling vigor, abiotic, and biotic stress tolerance, and increased biomass of crop plants [
38,
40,
41,
42,
43,
44,
45]. Although there are comparatively few reports on endophytes improving crop production under field conditions [
38], fungal endophytes have shown good field efficacy for enhancing yields, biological control, and improving yield quality [
38,
43,
44,
45]. Extensive field testing demonstrated that ThSM3a conferred tolerance to drought and temperature extremes in both a monocot (corn) and eudicot (cotton). There was a strong relationship between the level of stress and yield benefits (greater stress = greater yield). Quantifying stress levels was complicated by inaccuracies between regional versus local environmental data including precipitation, soil composition, and temperature. This could be improved by increasing the accuracy of environmental data with on-site weather stations and field soil compositional analysis. Regardless, the six-factor system used for quantifying stress levels was sufficient to visualize the relationship between stress and yield benefit.
Since
T. harzianum is commonly known to express biopesticidal activities and is a competitive soil species, ThSM3a was screened for both of these characteristics. Soil competitiveness was assessed by monitoring the presence of fungicide resistant
T. harzianum in soil before and after planting ThSM3a treated and untreated corn seeds. Although there were
Trichoderma spp. and other fungi in the soils before planting, no fungi grew on growth medium containing fungicides that ThSM3a could tolerate. Two weeks after planting seeds, fungicide resistant
T. harzianum was isolated from rhizosphere soils but not in any of the subsequent samples collected up to one year after planting. The results suggest that ThSM3a is similar to other Class 2 endophytes in regard to soil competitiveness [
13].
The biopesticidal potential of ThSM3a was assessed under laboratory and field conditions. Interaction plating of ThSM3a against nine fungi including seven plant pathogens demonstrated a lack of biopesticidal activity against any of the fungi. Field studies carried out to assess biopesticidal activity against sudden death syndrome of soybean (
Fusarium solani), barley scald (
Rhyncosporium secalis) and brown spot on rice (
Cochliobolus miyabeanus) also revealed no biopesticidal activity by ThSM3a. The lack of biopesticidal activity and soil competitiveness is particularly interesting because mycoparasitism is considered an ancestral trait of the genus
Trichoderma which is why so many strains have been commercialized as biopesticides [
46]. Biopesticidal activity can occur via antagonism, hyperparasitism, or induced resistance depending on the species and strains [
47]. Although
Trichoderma spp. are known to colonize the outer regions of roots, a few species are known to be true endophytes living inside plant tissues. Interestingly, most of the endophytic species are thought to have recently diverged and are phylogenetically distinct from other
Trichoderma spp. with biopesticidal activities [
48].
To the best of our knowledge, this is the first assessment of the relationship between the level of abiotic stress and the resulting yield enhancement from fungal endophytes that confer stress tolerance. The strong relationship between increased stress levels and increased yields in corn (monocot) and cotton (eudicot) suggests that the symbiotic communication responsible for abiotic stress tolerance is conserved and predates the divergence of the plant lineages more than 130 million years ago [
49,
50,
51]. The fossil record indicates that the relationship between plants and fungi are ancient and may have existed when plants colonized land during the Devonian geologic period [
52]. It has been suggested that the ability of fungal endophytes to confer drought and heat stress may be been instrumental in the movement of plants onto land [
51,
52,
53].
Symbiotic yield benefits were also observed in corn, wheat, and alfalfa cultivated under low-stress conditions. However, the yield increases were lower (5%) for corn than those observed under more stressful conditions (26%). Although wheat and alfalfa were not tested under high stress conditions, average yields under low stress were 5.3% and 12.7%, respectively. Moreover, the yield benefits in alfalfa were observed with each successive cutting in a single season. This also indicates that endophyte yield benefits occur in leguminous and non-leguminous plants.
As part of a USAID/SWFF grant, Adaptive Symbiotic Technologies was asked to bring endophyte technology to small land-holding farmers in climate-vulnerable regions of India. We chose to work in the state of Rajasthan, one of the hottest and driest regions in India where the majority of its 6.3 million farmers live on less than $5/day and cultivate on less than two hectares of land. The vast majority of crops are cultivated under dry land conditions with temperatures typically exceeding 40 °C during the growing season. There are two growing seasons: the monsoon season when planting occurs in June or July depending on rainfall and a fall/winter season if there is sufficient moisture in the soil for seed germination. Farmers grow crops for sustenance, fodder for animals, carryover seed, and revenues. If they do not produce sufficient yield for sustenance a famine ensues and the government provides wheat to rural communities.
The three-strain mixture ThSM3a was the basis of the product used in India commercially branded as BioEnsure. Although the intent of field testing was to compare yields from endophyte treated seeds to untreated seeds, many farmers had other issues they wanted to address. The primary concern was that the majority of farmers use carryover seeds from previous harvests because they cannot afford to purchase fresh market seed which costs 4–8 times more than the value of carryover seed. The famers lack proper seed storage facilities so the carry over seeds have lower percent germination, seedling vigor, and final yields compared to market seeds. Compared to untreated plants, the farmers observed that treated plants had increased percent germination, seedling vigor, plant biomass, stress tolerance, and yields. More biomass translated to increased fodder for animals that produce milk, their primary source of protein. Increased yields equate to more grain for sustenance and potential revenues. Most importantly for the farmers, treated carryover seeds performed at least as well or better than market seeds (
https://vimeo.com/192003746, accessed on 20 April 2021).
As climate change has increased in severity, agricultural land around the world has become marginal for crop production, resulting in decreased food security [
54]. Food insecurity plays a critical role in human migration, poverty, famine, and political unrest [
55,
56]. Unfortunately, there is a strong relationship between poverty, food insecurity, and political instability. This has been evident in Somalia, Syria, Honduras, and Venezuela where human migrations directly correlate with food insecurity. It is just a matter of time before food insecurity begins to impact more nations. Although there are physical and chemical technologies available that aid in water delivery, retention in soil, or adsorption in plant tissues, there are very few technologies that can generate climate resilient crops. Since the vast majority of farming around the world involves dryland cultivation, food security has become dependent on generating climate resilient crops. The symbiotic technology described here allows for the generation of climate resilient crops within a single growing season that can be applied to a diversity of monocots and eudicots. These studies demonstrate that symbiotic technology may be developed as a viable commercial tool for rapidly generating climate resilient crops to enhance agricultural sustainability.