A Comprehensive Assessment of the Safety of Blautia producta DSM 2950
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Draft Genome Sequencing
2.3. Profile of Safety-Related Genes
2.4. Hemolytic Activity
2.5. Antibiotic Susceptibility Test
2.6. Animals
2.7. Acute Toxicity Experimental Design
2.8. Hematological Measurements and Biochemical Parameter Analysis
2.9. Organ Index
2.10. Histology Study
2.11. Statistical Analysis
3. Results
3.1. Virulence Genes and Genomic Islands of DSM 2950
3.2. Genomic Islands of DSM 2950
3.3. Antibiotic Resistance of DSM 2950
3.4. Hemolytic Activity
3.5. Effects of DSM 2950 on Body Weight and Food and Water Intake
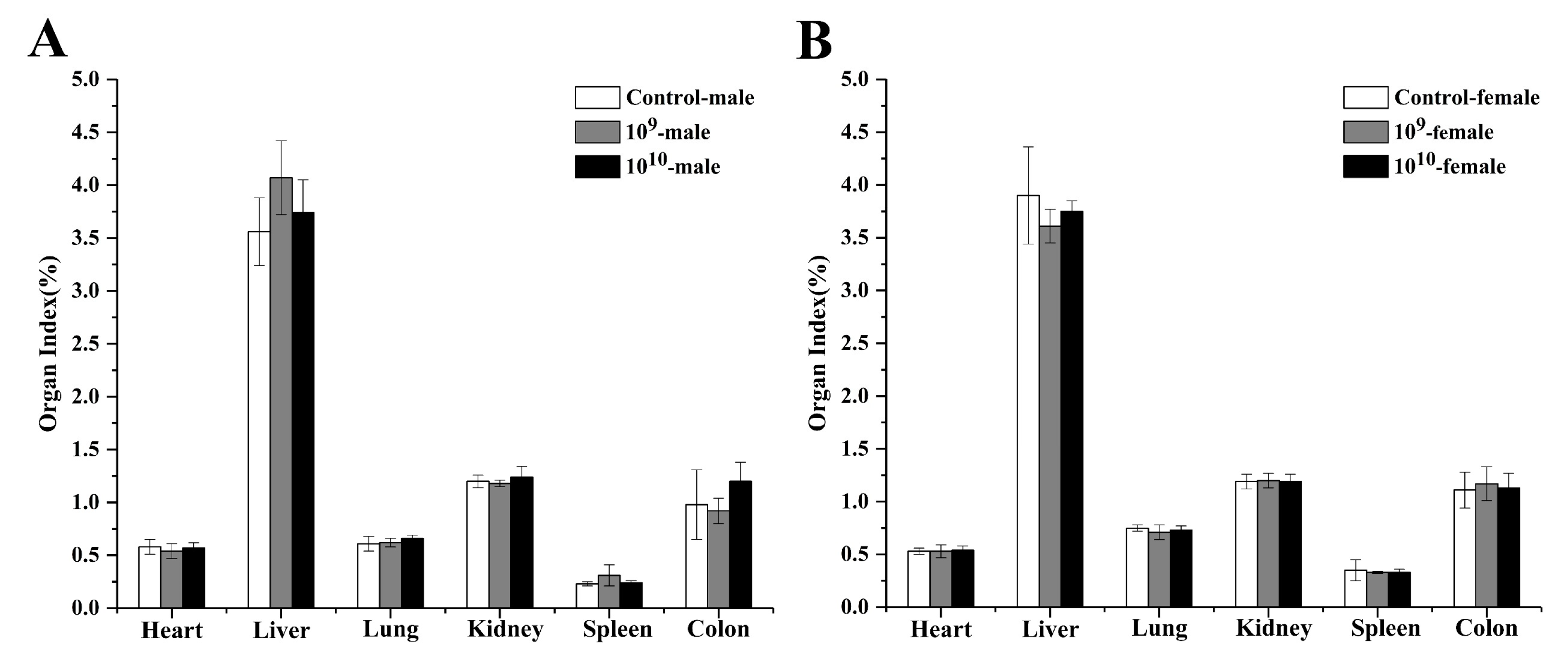
3.6. Effects of DSM 2950 on the Organ Index
3.7. Effects of DSM 2950 on Biochemical Parameters
3.8. Effects of DSM 2950 on Tissue Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Silva, M.S.; Ramos, C.L.; Gonzalez-Avila, M.; Gschaedler, A.; Arrizon, J.; Schwan, R.F.; Dias, D.R. Probiotic properties of Weissella cibaria and Leuconostoc citreum isolated from tejuino—A typical Mexican beverage. LWT Food Sci. Technol. 2017, 86, 227–232. [Google Scholar] [CrossRef]
- Borthakur, A.; Anbazhagan, A.N.; Kumar, A.; Raheja, G.; Singh, V.; Ramaswamy, K.; Dudeja, P.K. The probiotic Lactobacillus plantarum counteracts TNF-α-induced downregulation of SMCT1 expression and function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G928–G934. [Google Scholar] [CrossRef]
- Silva, D.R.; Orlandi Sardi, J.d.C.; Pitangui, N.d.S.; Roque, S.M.; da Silva, B.A.C.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, J.; Liu, B.; Lu, J.; Chen, M.; Liu, B.; Bai, W.; Rao, P.; Ni, L.; Lv, X. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020, 11, 6818–6833. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-L.; Shu, C.-C.; Lai, W.-F.; Tzeng, C.-M.; Lai, H.-C.; Lu, C.-C. Investiture of next generation probiotics on amelioration of diseases—Strains do matter. Med. Microecol. 2019, 1–2, 100002. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, S.K.; Jayasudha, R.; Prashanthi, G.S.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.S.; El Tawab, A.M.A.; Fouad, M.T. Isolation and Characterization of Anaerobic Bacteria from Frozen Rumen Liquid and its Potential Characterizations. Int. J. Dairy Sci. 2016, 12, 47–51. [Google Scholar] [CrossRef][Green Version]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhou, R.; Ng, S.C.; Li, J.; Huang, M.; Zhou, F.; Wang, X.; Shen, B.; Kamm, M.A.; et al. Characteristics of Fecal and Mucosa-Associated Microbiota in Chinese Patients With Inflammatory Bowel Disease. Medicine 2014, 93, e51. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. Npj Biofilms Microbiomes 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pathmanathan, S.G.; Lawley, B.; McConnell, M.; Baird, M.A.; Tannock, G.W. Gut bacteria characteristic of the infant microbiota down-regulate inflammatory transcriptional responses in HT-29 cells. Anaerobe 2020, 61, 102112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Becattini, S.; Moody, T.U.; Shliaha, P.V.; Littmann, E.R.; Seok, R.; Gjonbalaj, M.; Eaton, V.; Fontana, E.; Amoretti, L.; et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nat. Cell Biol. 2019, 572, 665–669. [Google Scholar] [CrossRef]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control. 2020, 108, 106872. [Google Scholar] [CrossRef]
- Gotoh, A.; Nara, M.; Sugiyama, Y.; Sakanaka, M.; Yachi, H.; Kitakata, A.; Nakagawa, A.; Minami, H.; Okuda, S.; Katoh, T.; et al. Use of Gifu Anaerobic Medium for culturing 32 dominant species of human gut microbes and its evaluation based on short-chain fatty acids fermentation profiles. Biosci. Biotechnol. Biochem. 2017, 81, 2009–2017. [Google Scholar] [CrossRef]
- Brown, J.W.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef]
- Saroj, D.B.; Gupta, A.K. Genome based safety assessment for Bacillus coagulans strain LBSC (DSM 17654) for probiotic application. Int. J. Food Microbiol. 2020, 318, 108523. [Google Scholar] [CrossRef]
- Salvetti, E.; Orrù, L.; Capozzi, V.; Martina, A.; Lamontanara, A.; Keller, D.; Cash, H.; Felis, G.E.; Cattivelli, L.; Torriani, S.; et al. Integrate genome-based assessment of safety for probiotic strains: Bacillus coagulans GBI-30, 6086 as a case study. Appl. Microbiol. Biotechnol. 2016, 100, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A.; Duarte, V.D.S.; Pakroo, S.; Corich, V.; Giacomini, A. Genomic and phenotypic assessments of safety and probiotic properties of Streptococcus macedonicus strains of dairy origin. Food Res. Int. 2020, 130, 108931. [Google Scholar] [CrossRef] [PubMed]
- Rahmdel, S.; Hosseinzadeh, S.; Shekarforoush, S.S.; Torriani, S.; Gatto, V.; Pashangeh, S. Safety hazards in bacteriocinogenic Staphylococcus strains isolated from goat and sheep milk. Microb. Pathog. 2018, 116, 100–108. [Google Scholar] [CrossRef] [PubMed]
- ISO 10932/IDF 223. Milk and Milk Products-Determination of the minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB); International Dairy Federation: Geneva, Switzerland, 2010. [Google Scholar]
- Jeong, D.-W.; Lee, J.-H. Antibiotic resistance, hemolysis and biogenic amine production assessments of Leuconostoc and Weissella isolates for kimchi starter development. LWT 2015, 64, 1078–1084. [Google Scholar] [CrossRef]
- D’Aimmo, M.R.; Modesto, M.; Biavati, B. Antibiotic resistance of lactic acid bacteria and Bifidobacterium spp. isolated from dairy and pharmaceutical products. Int. J. Food Microbiol. 2007, 115, 35–42. [Google Scholar] [CrossRef] [PubMed]
- FEEDAP. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Delgado, S.; Flórez, A.B.; Mayo, B. Antibiotic Susceptibility of Lactobacillus and Bifidobacterium Species from the Human Gastrointestinal Tract. Curr. Microbiol. 2005, 50, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, Z.-S.; Cao, S.-J.; Chang, Y.-F.; Cao, Y.-Q.; Li, J.-B.; Yao, Z.-X.; Wen, Y.-P.; Huang, X.-B.; Wu, R.; et al. Acute oral toxicity test and assessment of combined toxicity of cadmium and aflatoxin B1 in kunming mice. Food Chem. Toxicol. 2019, 131, 110577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shu, Q.; Rutherfurd, K.; Prasad, J.; Birtles, M.; Gopal, P.; Gill, H. Safety assessment of potential probiotic lactic acid bacterial strains Lactobacillus rhamnosus HN001, Lb. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int. J. Food Microbiol. 2000, 56, 87–96. [Google Scholar] [CrossRef]
- Carkaci, D.; Højholt, K.; Nielsen, X.C.; Dargis, R.; Rasmussen, S.; Skovgaard, O.; Fuursted, K.; Andersen, P.S.; Stegger, M.; Christensen, J.J. Genomic characterization, phylogenetic analysis, and identification of virulence factors in Aerococcus sanguinicola and Aerococcus urinae strains isolated from infection episodes. Microb. Pathog. 2017, 112, 327–340. [Google Scholar] [CrossRef]
- King, T.; Schmidt, S.; Thakur, S.; Fedorka-Cray, P.; Keelara, S.; Harden, L.; Essack, S. Resistome of a carbapenemase-producing novel ST232 Klebsiella michiganensis isolate from urban hospital effluent in South Africa. J. Glob. Antimicrob. Resist. 2021, 24, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Liu, X.; Yang, S.; Bao, C.; He, P.; Dai, Q. An efficient genomic signature ranking method for genomic island prediction from a single genome. J. Theor. Biol. 2019, 467, 142–149. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, M.; Fu, J.; Zhong, C.; Zong, G.; Cao, G. Identification of a mobilizable, multidrug-resistant genomic island in Myroides odoratimimus isolated from Tibetan pasture. Sci. Total Environ. 2020, 723, 137970. [Google Scholar] [CrossRef]
- Francuski, D.; Saenger, W. Crystal Structure of the Antitoxin–Toxin Protein Complex RelB–RelE from Methanococcus jannaschii. J. Mol. Biol. 2009, 393, 898–908. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Zhang, Q.; Tang, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Preliminary safety assessment of a new Bacteroides fragilis isolate. Food Chem. Toxicol. 2020, 135, 110934. [Google Scholar] [CrossRef]
- Furuya, H.; Ide, Y.; Hamamoto, M.; Asanuma, N.; Hino, T. Isolation of a novel bacterium, Blautia glucerasei sp. nov., hydrolyzing plant glucosylceramide to ceramide. Arch. Microbiol. 2010, 192, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hatziioanou, D.; Gherghisan-Filip, C.; Saalbach, G.; Horn, N.; Wegmann, U.; Duncan, S.H.; Flint, H.J.; Mayer, M.J.; Narbad, A. Discovery of a novel lantibiotic nisin O from Blautia obeum A2-162, isolated from the human gastrointestinal tract. Microbiology 2017, 163, 1292–1305. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Del Pugar, E.M.G.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y.; Jenq, R. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5, e00857-19. [Google Scholar] [CrossRef]
- Tyler, A.D.; Knox, N.; Kabakchiev, B.; Milgrom, R.; Kirsch, R.; Cohen, Z.; McLeod, R.S.; Guttman, D.S.; Krause, D.O.; Silverberg, M.S. Characterization of the Gut-Associated Microbiome in Inflammatory Pouch Complications Following Ileal Pouch-Anal Anastomosis. PLoS ONE 2013, 8, e66934. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.L.; Liguori, G.; Lamas, B.; Brandi, G.; Da Costa, G.; Hoffmann, T.W.; Di Simone, M.P.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 2018, 9, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, Z.; Sun, L.; Yang, J.; Jin, Q. VFDB 2012 update: Toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Res. 2011, 40, D641–D645. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Hirata, H.; Tsuyumu, S. SlyA regulates motA and motB, virulence and stress-related genes under conditions induced by the PhoP-PhoQ system in Dickeya dadantii 3937. Res. Microbiol. 2015, 166, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Avraham, R.; Haseley, N.; Brown, D.; Penaranda, C.; Jijon, H.B.; Trombetta, J.J.; Satija, R.; Shalek, A.K.; Xavier, R.J.; Regev, A.; et al. Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses. Cell 2015, 162, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Liu, M.; Gu, J.; Liu, Q.; Zhao, L.; Ma, Y.; Wei, D. Metagenomic sequencing reveals microbial gene catalogue of phosphinothricin-utilized soils in South China. Gene 2019, 711, 143942. [Google Scholar] [CrossRef]
- Pinheiro-Hubinger, L.; Brito, C.I.; De Oliveira, A.; Martins, P.Y.F.; Pereira, V.C.; Cunha, M.D.L.R.D.S.D. Staphylococcus epidermidis and Staphylococcus haemolyticus: Molecular Detection of Cytotoxin and Enterotoxin Genes. Toxins 2015, 7, 3688–3699. [Google Scholar] [CrossRef] [PubMed]
- Seilie, E.S.; Wardenburg, J.B. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin. Cell Dev. Biol. 2017, 72, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Berube, B.J.; Wardenburg, J.B. Staphylococcus aureus α-Toxin: Nearly a Century of Intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.M.; Gerstmeier, J.; Pace, S.; Bilancia, R.; Rao, Z.; Börner, F.; Miek, L.; Gutiérrez-Gutiérrez, Ó.; Arakandy, V.; Rossi, A.; et al. Staphylococcus aureus-Derived α-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution. Cell Rep. 2020, 33, 108247. [Google Scholar] [CrossRef] [PubMed]
- Tabata, A.; Ohkuni, H.; Hino, H.; Saigo, T.; Kodama, C.; Tang, Q.; Tomoyasu, T.; Fukunaga, Y.; Itoh, Y.; Nagamune, H. Cytotoxic property of Streptococcus mitis strain producing two different types of cholesterol-dependent cytolysins. Infect. Genet. Evol. 2020, 85, 104483. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.-R.; Kim, H.-N.; Park, K.-H.; Ro, I.-W.; Kim, M.-A.; Kim, N.-Y.; Par, J.-W.; Kim, H.-R. Species-specific hemolysis by Vibrio vulnificus cytolysin. Exp. Mol. Med. 1996, 28, 95–99. [Google Scholar] [CrossRef][Green Version]
- Shankar, N.; Coburn, P.; Pillar, C.; Haas, W.; Gilmore, M. Enterococcal cytolysin: Activities and association with other virulence traits in a pathogenicity island. Int. J. Med. Microbiol. 2004, 293, 609–618. [Google Scholar] [CrossRef]
- Haas, W.; Shepard, B.D.; Gilmore, M.S. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 2002, 415, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Labra, Á.; Arredondo-Zelada, O.; Flores-Herrera, P.; Marshall, S.H.; Gómez, F.A. In sílico identification and characterization of putative Dot/Icm secreted virulence effectors in the fish pathogen Piscirickettsia salmonis. Microb. Pathog. 2016, 92, 11–18. [Google Scholar] [CrossRef]
- Osanai, A.; Li, S.-J.; Asano, K.; Sashinami, H.; Hu, D.-L.; Nakane, A. Fibronectin-binding protein, FbpA, is the adhesin responsible for pathogenesis ofListeria monocytogenesinfection. Microbiol. Immunol. 2013, 57, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, K.M.; Bhunia, A.K. Listeria monocytogenes Uses Listeria Adhesion Protein (LAP) To Promote Bacterial Transepithelial Translocation and Induces Expression of LAP Receptor Hsp60. Infect. Immun. 2010, 78, 5062–5073. [Google Scholar] [CrossRef] [PubMed]
- Reading, D.S.; Hallberg, R.L.; Myers, A.M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature 1989, 337, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zheng, S.-W.; Shen, Y.-J.; Zheng, G.-D.; Liu, H.-T.; Wu, Z.-Y. Complete genome sequence provides insights into the biodrying-related microbial function of Bacillus thermoamylovorans isolated from sewage sludge biodrying material. Bioresour. Technol. 2018, 260, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.G.; Hurtado, R.; Gomes, L.G.R.; Profeta, R.; Rifici, C.; Attili, A.R.; Spier, S.J.; Mazzullo, G.; Morais-Rodrigues, F.; Gomide, A.C.P.; et al. Complete genome analysis of Glutamicibacter creatinolyticus from mare abscess and comparative genomics provide insight of diversity and adaptation for Glutamicibacter. Gene 2020, 741, 144566. [Google Scholar] [CrossRef]
- Karlin, S. Detecting anomalous gene clusters and pathogenicity islands in diverse bacterial genomes. Trends Microbiol. 2001, 9, 335–343. [Google Scholar] [CrossRef]
- Averina, O.; Alekseeva, M.; Shkoporov, A.; Danilenko, V. Functional analysis of the type II toxin–antitoxin systems of the MazEF and RelBE families in Bifidobacterium longum subsp. infantis ATCC 15697. Anaerobe 2015, 35, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Moslem, M.S.; Kouhsari, E.; Pakzad, I.; Ghafouri, Z.; Khaghani, S.; Sadeghifard, N. Virulence-associated genes and toxin-antitoxin system genes of Shigella flexneri: Presence and expression in normal and thermal stress conditions. Meta Gene 2021, 27, 100825. [Google Scholar] [CrossRef]
- Das, D.J.; Shankar, A.; Johnson, J.B.; Thomas, S. Critical insights into antibiotic resistance transferability in probiotic Lactobacillus. Nutrition 2020, 69, 110567. [Google Scholar] [CrossRef]
- Terai, T.; Kato, K.; Ishikawa, E.; Nakao, M.; Ito, M.; Miyazaki, K.; Kushiro, A.; Imai, S.; Nomura, Y.; Hanada, N.; et al. Safety assessment of the candidate oral probiotic Lactobacillus crispatus YIT 12319: Analysis of antibiotic resistance and virulence-associated genes. Food Chem. Toxicol. 2020, 140, 111278. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, F.; Wang, B.; Gao, J.; Wang, Y.; Shao, Y. Evaluation of kanamycin and neomycin resistance in Lactobacillus plantarum using experimental evolution and whole-genome sequencing. Food Control. 2019, 98, 262–267. [Google Scholar] [CrossRef]
- Cauwerts, K.; Pasmans, F.; Devriese, L.A.; Martel, A.; Haesebrouck, F.; Decostere, A. CloacalLactobacillusisolates from broilers show high prevalence of resistance towards macrolide and lincosamide antibiotics. Avian Pathol. 2006, 35, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef] [PubMed]
- Dec, M.; Urban-Chmiel, R.; Stępień-Pyśniak, D.; Wernicki, A. Assessment of antibiotic susceptibility in Lactobacillus isolates from chickens. Gut Pathog. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Mac, K.; Wichmann-Schauer, H.; Peters, J.; Ellerbroek, L. Species identification and detection of vancomycin resistance genes in enterococci of animal origin by multiplex PCR. Int. J. Food Microbiol. 2003, 88, 305–309. [Google Scholar] [CrossRef]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.E. Vancomycin-Resistant Enterococcal Infections. N. Engl. J. Med. 2000, 342, 710–721. [Google Scholar] [CrossRef]
- Leclercq, R.; Derlot, E.; Duval, J.; Courvalin, P. Plasmid-Mediated Resistance to Vancomycin and Teicoplanin in Enterococcus Faecium. N. Engl. J. Med. 1988, 319, 157–161. [Google Scholar] [CrossRef]
- Van Melckebeke, H.; Vreuls, C.; Gans, P.; Filee, P.; Llabres, G.; Joris, B.; Simorre, J.P. Solution structural study of BlaI: Implications for the repression of genes involved in beta-lactam antibiotic resistance. J. Mol. Biol. 2003, 333, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods 2019, 58, 56–66. [Google Scholar] [CrossRef]
- Koirala, S.; Anal, A.K. Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims. Futur. Foods 2021, 3, 100013. [Google Scholar] [CrossRef]
- Fernández-Murga, M.L.; Sanz, Y. Safety Assessment of Bacteroides uniformis CECT 7771 Isolated from Stools of Healthy Breast-Fed Infants. PLoS ONE 2016, 11, e0145503. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pan, Y.; Li, L.; Li, T.; Liu, B.; Lv, X. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. [Google Scholar] [CrossRef] [PubMed]
- Dhouib, I.E.-B.; Lasram, M.M.; Annabi, A.; Gharbi, N.; El-Fazaa, S. A comparative study on toxicity induced by carbosulfan and malathion in Wistar rat liver and spleen. Pestic. Biochem. Physiol. 2015, 124, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, Y.; Shen, Y.; Cheng, Y.; Qiao, M.; Song, L.; Huang, X. Protective effects of folic acid on oxidative damage of rat spleen induced by lead acetate. Ecotoxicol. Environ. Saf. 2021, 211, 111917. [Google Scholar] [CrossRef] [PubMed]



| Class of Virulence Genes | Virulence Gene Function | Virulence Gene Name |
|---|---|---|
| Regulation of virulence-associated genes | Regulation | PhoP, RelA |
| Nonspecific virulence gene | Iron uptake system | FbpABC, FeoAB, HitABC |
| Offensive virulence gene | Secretion system | Dot/Icm, HSI-I, |
| Adherence | PI-2, PI-2a, Lap, LPS, FbpA, Hyaluronic acid capsule, Hsp60 | |
| Defensive virulence gene | Stress protein | MsrAB, KatA, ClpC, ClpP |
| Antiphagocytosis | Capsule, Capsule I, Alginate | |
| Unclassified virulence gene | _ | Beta-hemolysin/cytolysin, BSH, Cytolysin, Enterobactin, Hemolysin, LOS, MgtBC, mycobactin, peritrichous flagella, PgdA, Phenazines biosynthesis, type IV pili, Urease |
| Antibiotics (μg/mL) | DSM 2950 | ATCC 334 | Cut-Off Values |
|---|---|---|---|
| Kanamycin | MIC < 256 | MIC < 64 | MIC ≤ 64 |
| Streptomycin | MIC < 64 | MIC < 32 | MIC ≤ 64 |
| Neomycin | MIC < 64 | MIC < 8 | MIC ≤ 32 |
| Tetracycline | MIC < 0.125 | MIC < 2 | MIC ≤ 4 |
| Erythromycin | MIC < 0.25 | MIC < 0.5 | MIC ≤ 1 |
| Clindamycin | MIC < 8 | MIC < 0.5 | MIC ≤ 1 |
| Ampicillin | MIC < 1 | MIC < 1 | MIC ≤ 4 |
| Amoxicillin | MIC < 0.5 | MIC < 1 | MIC ≤ 2 |
| Trimethoprim | MIC < 32 | Resistance | MIC ≤ 32 |
| Ciprofloxacin | MIC < 32 | MIC < 4 | MIC ≤ 4 |
| Chloramphenicol | MIC < 4 | MIC < 8 | MIC ≤ 4 |
| Rifampin | MIC < 0.125 | MIC < 0.125 | MIC ≤ 2 |
| Vancomycin | MIC < 4 | Resistance | MIC ≤ 4 |
| ARO Category | Antibiotic Resistance Gene | Resistance Mechanism |
|---|---|---|
| efflux pump | bcrA, drrA, lmrD, novA, oleC, sav1866 | - |
| macrolide antibiotic | mtrA, macB | antibiotic efflux |
| fluoroquinolone antibiotic | arlR, CdeA, efrA, patB | antibiotic efflux |
| clindamycin antibiotic | lsaB | antibiotic target protection |
| aminoglycoside antibiotic | baeR, kdpE | antibiotic efflux |
| APH (3′)-IIIa, ANT (6)-Ia | antibiotic inactivation | |
| glycopeptide antibiotic | vanHA, vanHB, vanRA, vanRG, vanRM, vanRN, vanSA, vanTG, | antibiotic target alteration |
| vanRE, vanRF, vanRI, vanU | - | |
| streptogramin antibiotic | vatF | antibiotic inactivation |
| tetracycline antibiotic | rpsJ | antibiotic target protection |
| peptide antibiotic | bacA, PmrA, PmrE | antibiotic target alteration |
| blaI, blaR1 | - |
| Parameter | Control-Female | 109-Female | 1010-Female | Control-Male | 109-Male | 1010-Male |
|---|---|---|---|---|---|---|
| WBC (109/L) | 2.40 ± 0.47 | 2.97 ± 0.25 | 2.79 ± 0.43 | 2.58 ± 0.46 | 3.66 ± 0.89 | 2.58 ± 0.62 |
| Neu (109/L) | 0.24 ± 0.13 | 0.28 ± 0.06 | 0.41 ± 0.15 | 0.25 ± 0.06 | 0.39 ± 0.16 | 0.35 ± 0.12 |
| Lym (109/L) | 2.26 ± 0.35 | 2.61 ± 0.28 | 2.47 ± 0.26 | 1.91 ± 0.23 | 2.49 ± 0.44 | 2.10 ± 0.50 |
| Mon (109/L) | 0.05 ± 0.07 | 0.05 ± 0.04 | 0.05 ± 0.08 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.04 ± 0.04 |
| Eos (109/L) | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.03 | 0.03 ± 0.01 | 0.05 ± 0.01 |
| Bas (109/L) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.02 ± 0.02 | 0.00 ± 0.00 |
| Neu (%) | 11.44 ± 3.03 | 9.14 ± 1.62 | 10.6 ± 1.70 | 11.82 ± 2.49 | 13.32 ± 1.93 | 13.48 ± 2.33 |
| Lym (%) | 83.82 ± 5.73 | 87.64 ± 3.77 | 86.28 ± 2.16 | 80.54 ± 10.09 | 69.54 ± 11.67 | 81.62 ± 2.92 |
| Mon (%) | 2.70 ± 3.19 | 2.78 ± 4.89 | 1.84 ± 1.55 | 1.70 ± 0.70 | 3.08 ± 3.11 | 2.78 ± 3.23 |
| Eos (%) | 0.20 ± 0.22 | 0.24 ± 0.11 | 0.28 ± 0.16 | 0.40 ± 0.14 | 0.40 ± 0.16 | 0.42 ± 0.22 |
| Bas (%) | 0.24 ± 0.09 | 0.20 ± 0.10 | 0.28 ± 0.18 | 0.14 ± 0.15 | 0.30 ± 0.12 | 0.20 ± 0.12 |
| RBC (1012/L) | 7.91 ± 0.59 | 7.72 ± 0.55 | 7.38 ± 0.42 | 7.84 ± 0.27 | 7.41 ± 0.23 | 7.40 ± 0.35 |
| HGB (g/L) | 104 ± 17.83 | 100 ± 4.80 | 109.20 ± 11.30 | 105.80 ± 8.38 | 110.40 ± 6.58 | 107.00 ± 6.89 |
| HCT (%) | 40.14 ± 4.44 | 35.76 ± 4.53 | 38.38 ± 2.65 | 39.19 ± 1.98 | 40.56 ± 1.22 | 41.62 ± 1.85 |
| MCV (fL) | 43.34 ± 0.77 | 43.9 ± 1.32 | 42.4 ± 1.44 | 41.90 ± 1.70 | 43.90 ± 1.59 | 42.94 ± 0.92 |
| MCH (pg) | 15.07 ± 0.29 | 15.10 ± 0.51 | 14.94 ± 0.69 | 15.30 ± 0.26 | 14.80 ± 1.09 | 15.02 ± 0.94 |
| MCHC (g/L) | 333.40 ± 9.29 | 328.60 ± 12.82 | 326.20 ± 6.46 | 330.72 ± 5.71 | 330.92 ± 13.06 | 337.14 ± 6.56 |
| RDW-CV (%) | 12.40 ± 0.22 | 12.60 ± 0.29 | 12.32 ± 0.36 | 12.50 ± 0.16 | 12.26 ± 0.24 | 12.50 ± 0.23 |
| RDW-SD (fL) | 27.52 ± 0.49 | 26.85 ± 1.78 | 27.46 ± 0.29 | 27.66 ± 0.49 | 27.83 ± 0.75 | 26.79 ± 0.39 |
| PLT (109/L) | 972.22 ± 51.26 | 991.69 ± 90.82 | 1043.00 ± 117.1 | 819.51 ± 91.73 | 762.73 ± 62.48 | 839.79 ± 75.86 |
| MPV (fL) | 4.70 ± 0.14 | 4.96 ± 0.15 | 5.00 ± 0.62 | 5.04 ± 0.96 | 4.82 ± 1.11 | 4.56 ± 0.05 |
| PDW (%) | 12.66 ± 0.05 | 12.68 ± 0.08 | 12.84 ± 0.27 | 12.54 ± 0.17 | 12.78 ± 0.51 | 12.72 ± 0.11 |
| PCT (%) | 0.46 ± 0.05 | 0.43 ± 0.02 | 0.44 ± 0.03 | 0.37 ± 0.03 | 0.36 ± 0.04 | 0.34 ± 0.02 |
| Parameter | Control-Male | 109-Male | 1010-Male | Control-Female | 109-Female | 1010-Female |
|---|---|---|---|---|---|---|
| Glu (mmol/L) | 3.70 ± 0.87 | 3.07 ± 0.39 | 3.09 ± 0.55 | 3.04 ± 0.34 | 3.03 ± 0.33 | 2.99 ± 0.51 |
| TC (mmol/L) | 2.74 ± 0.23 | 3.16 ± 0.46 | 2.86 ± 0.09 | 1.86 ± 0.23 | 1.74 ± 0.26 | 1.92 ± 0.20 |
| TG (mmol/L) | 1.33 ± 0.35 | 1.29 ± 0.23 | 1.36 ± 0.12 | 0.92 ± 0.12 | 0.86 ± 0.11 | 0.89 ± 0.14 |
| ALT(U/L) | 26.62 ± 4.17 | 29.50 ± 3.49 | 27.85 ± 2.47 | 31.50 ± 3.15 | 28.56 ± 1.78 | 28.53 ± 3.87 |
| AST(U/L) | 135.60 ± 19.29 | 126.96 ± 13.00 | 138.72 ± 23.85 | 151.68 ± 68.55 | 143.46 ± 36.60 | 153.66 ± 41.73 |
| ALP(U/L) | 174.00 ± 18.85 | 163.20 ± 29.29 | 154.20 ± 9.86 | 178.80 ± 27.13 | 202.80 ± 22.21 | 186.60 ± 21.15 |
| γ-GT (U/L) | 3.06 ± 0.68 | 2.88 ± 0.40 | 3.06 ± 0.83 | 2.70 ± 0.56 | 3.30 ± 0.60 | 2.88 ± 1.11 |
| CHE(U/L) | 4714.20 ± 469.40 | 4749.00 ± 347.61 | 4956.00 ± 390.65 | 6661.80 ± 1085.12 | 6528.00 ± 393.56 | 6400.20 ± 802.77 |
| TP(g/L) | 57.42 ± 3.68 | 52.98 ± 2.44 | 56.82 ± 2.57 | 53.40 ± 2.96 | 55.74 ± 2.89 | 54.48 ± 5.53 |
| ALB(g/L) | 35.64 ± 2.04 | 32.58 ± 2.44 | 34.92 ± 2.15 | 34.50 ± 2.34 | 36.18 ± 1.96 | 34.98 ± 4.13 |
| CK(U/L) | 1012.27 ± 229.25 | 785.61 ± 236.92 | 990.99 ± 233.37 | 716.28 ± 479.39 | 814.98 ± 308.76 | 943.80 ± 306.41 |
| LDH(U/L) | 370.20 ± 61.02 | 337.32 ± 18.91 | 324.18 ± 43.42 | 390.9 ± 158.67 | 358.74 ± 98.81 | 377.88 ± 141.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Guo, W.; Cui, S.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. A Comprehensive Assessment of the Safety of Blautia producta DSM 2950. Microorganisms 2021, 9, 908. https://doi.org/10.3390/microorganisms9050908
Liu X, Guo W, Cui S, Tang X, Zhao J, Zhang H, Mao B, Chen W. A Comprehensive Assessment of the Safety of Blautia producta DSM 2950. Microorganisms. 2021; 9(5):908. https://doi.org/10.3390/microorganisms9050908
Chicago/Turabian StyleLiu, Xuemei, Weiling Guo, Shumao Cui, Xin Tang, Jianxin Zhao, Hao Zhang, Bingyong Mao, and Wei Chen. 2021. "A Comprehensive Assessment of the Safety of Blautia producta DSM 2950" Microorganisms 9, no. 5: 908. https://doi.org/10.3390/microorganisms9050908
APA StyleLiu, X., Guo, W., Cui, S., Tang, X., Zhao, J., Zhang, H., Mao, B., & Chen, W. (2021). A Comprehensive Assessment of the Safety of Blautia producta DSM 2950. Microorganisms, 9(5), 908. https://doi.org/10.3390/microorganisms9050908






