Carbon Monoxide Induced Metabolic Shift in the Carboxydotrophic Parageobacillus thermoglucosidasius DSM 6285
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Media and Growth Condition
2.2. Sampling and Analytics
3. Results
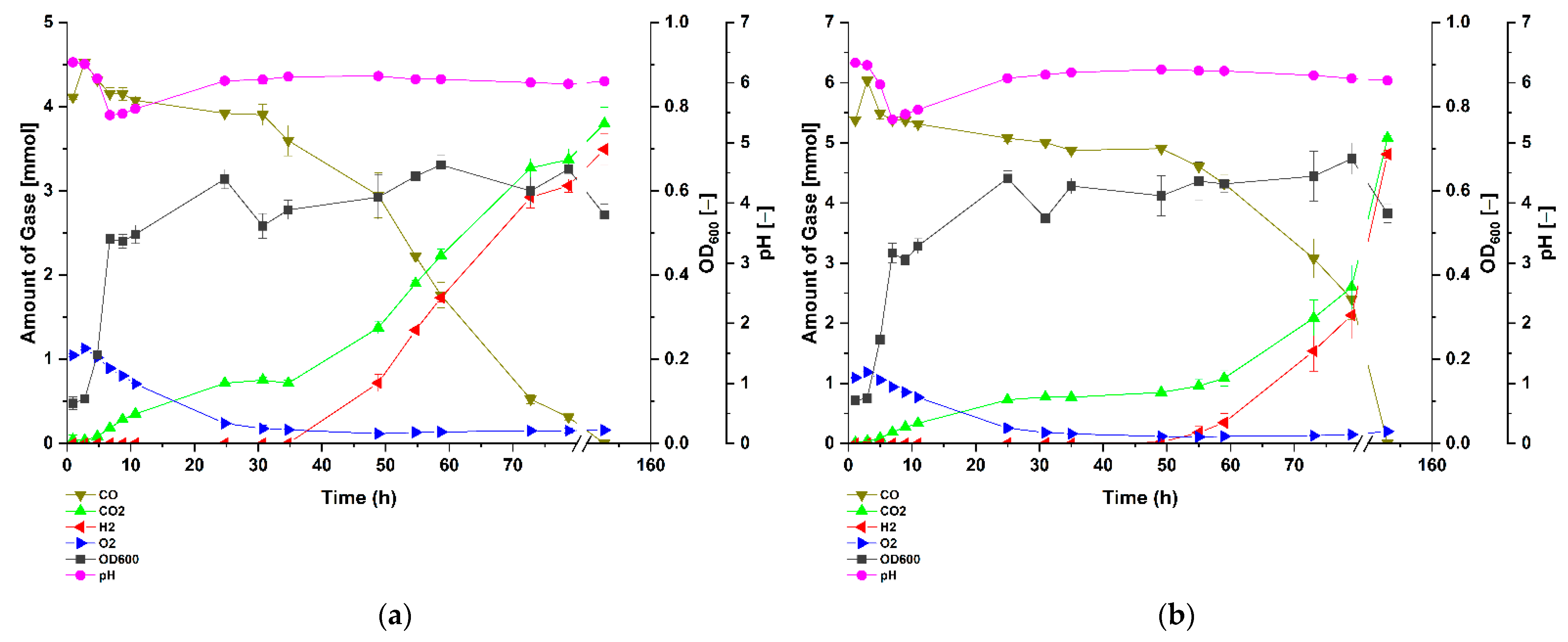
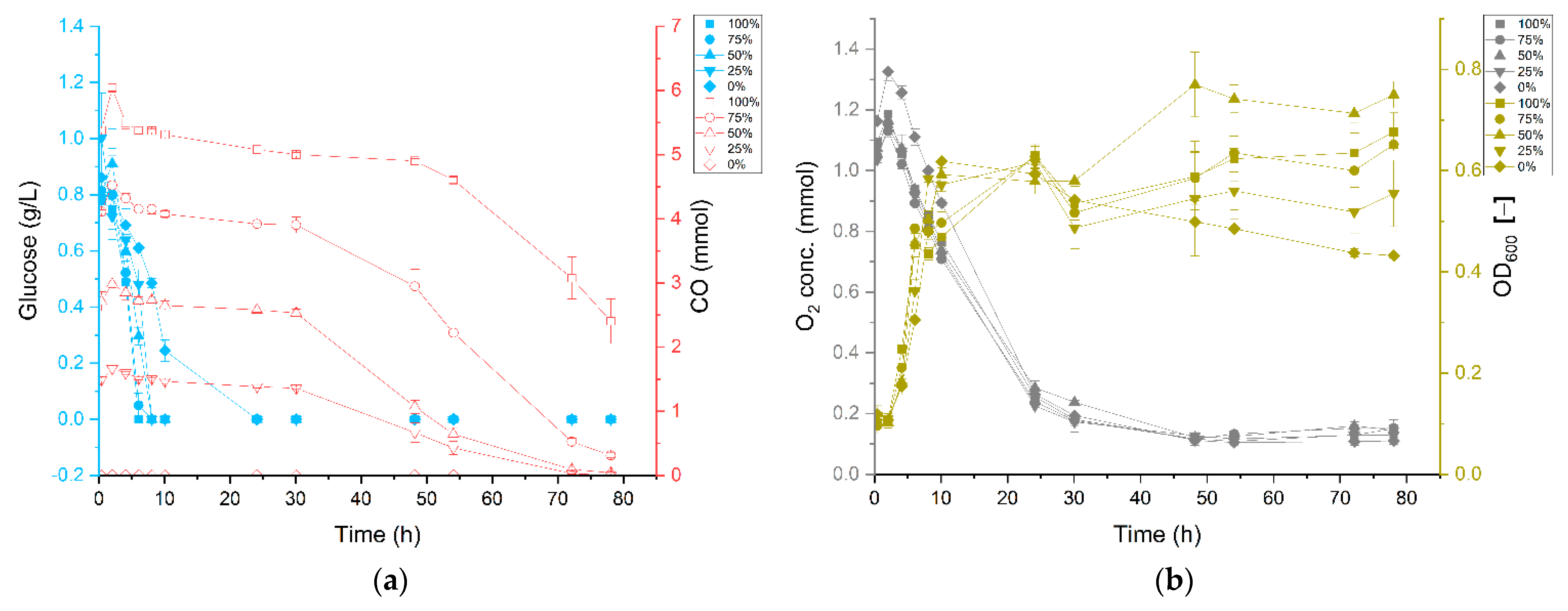
3.1. Carbon Monoxide Enhances Growth of P. thermoglucosidasius DSM 6285
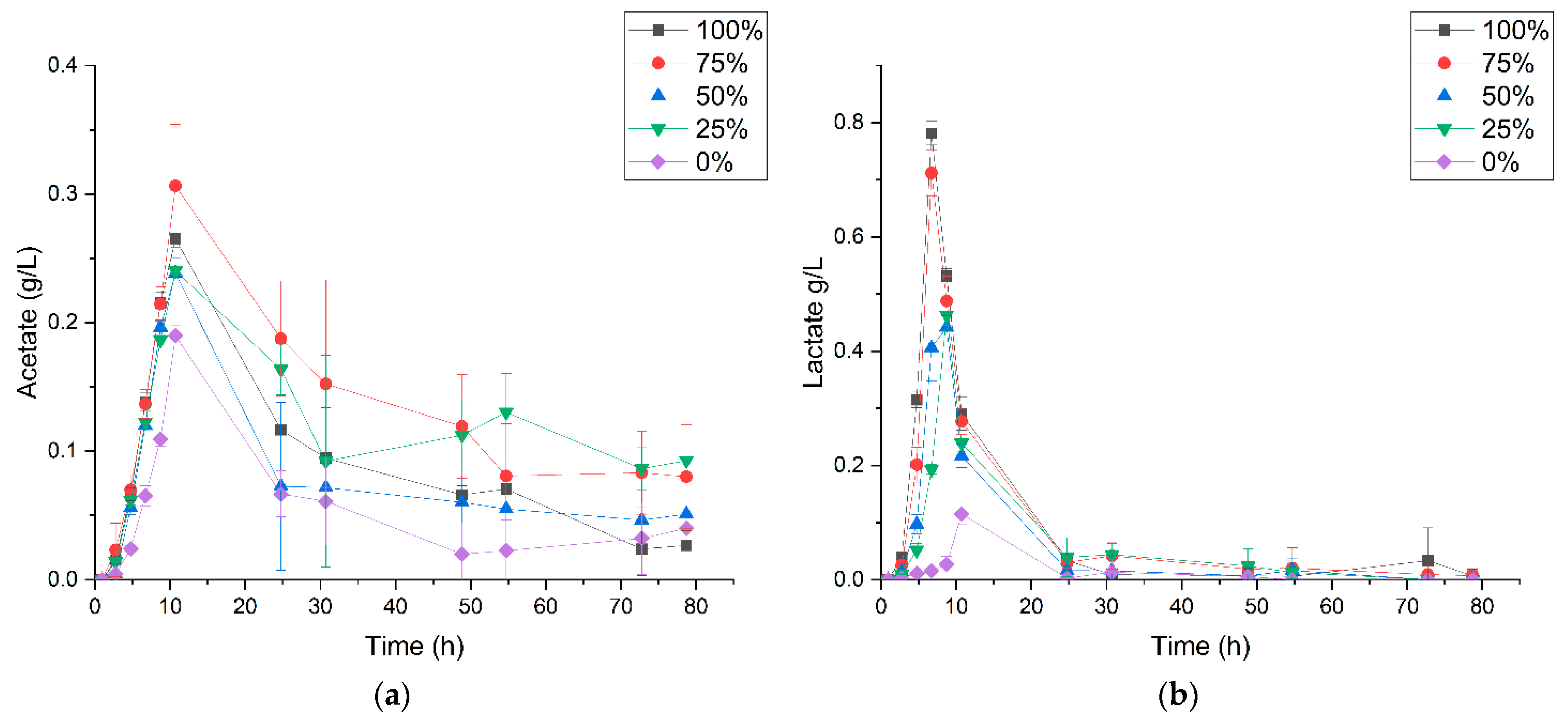
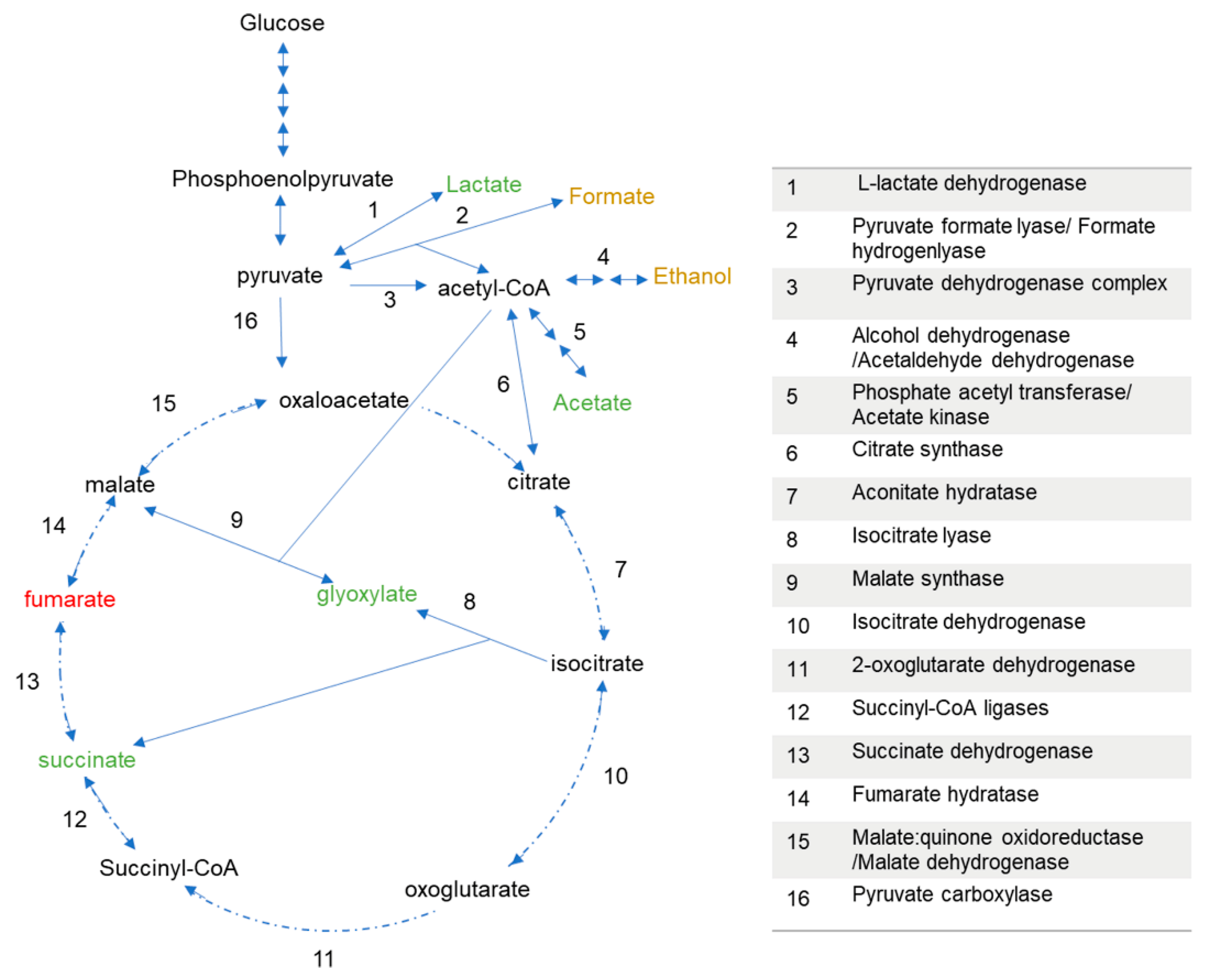
3.2. P. thermoglucosidasius DSM 6285 Cope with Carbon Monoxide by Rerouting Metabolic Intermediates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Phylogenomic re-assessment of the thermophilic genus Geobacillus. Syst. Appl. Microbiol. 2016, 39, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Corrigendum to “Phylogenomic re-assessment of the thermophilic genus Geobacillus”. Syst. Appl. Microbiol. 2018, 41, 529. [Google Scholar] [CrossRef]
- Zeigler, D.R. The Geobacillus paradox: Why is a thermophilic bacterial genus so prevalent on a mesophilic planet? Microbiology 2014, 160, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maayer, P.; Brumm, P.J.; Mead, D.A.; Cowan, D.A. Comparative analysis of the Geobacillus hemicellulose utilization locus reveals a highly variable target for improved hemicellulolysis. BMC Genom. 2014, 15, 836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumm, P.; De Maayer, P.; Cowan, D.A.; MEAD, D.A. Genomic analysis of six new Geobacillus strains reveals highly conserved carbohydrate degradation architectures and strategies. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.H.; Lisowska, B.K.; Leak, D.J. The genus Geobacillus and their biotechnological potential. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 92, pp. 1–48. [Google Scholar]
- Wada, K.; Suzuki, H. Chapter 15—Biotechnological platforms of the moderate thermophiles, Geobacillus species: Notable properties and genetic tools. In Physiological and Biotechnological Aspects of Extremophiles; Salwan, R., Sharma, V., Eds.; Academic Press: London, UK, 2020; pp. 195–218. [Google Scholar] [CrossRef]
- Tang, Y.J.; Sapra, R.; Joyner, D.; Hazen, T.C.; Myers, S.; Reichmuth, D.; Blanch, H.; Keasling, J.D. Analysis of metabolic pathways and fluxes in a newly discovered thermophilic and ethanol-tolerant Geobacillus strain. Biotechnol. Bioeng. 2009, 102, 1377–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftie-Eaton, W.; Taylor, M.; Horne, K.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Balancing redox cofactor generation and ATP synthesis: Key microaerobic responses in thermophilic fermentations. Biotechnol. Bioeng. 2013, 110, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Mohr, T.; Aliyu, H.; Küchlin, R.; Polliack, S.; Zwick, M.; Neumann, A.; Cowan, D.; De Maayer, P. CO-dependent hydrogen production by the facultative anaerobe Parageobacillus thermoglucosidasius. Microb. Cell Factories 2018, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, G.; Ntaikou, I.; Stamatelatou, K.; Lyberatos, G. Biological and fermentative production of hydrogen. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2011; pp. 305–346. [Google Scholar]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J.M. Microbiology of synthesis gas fermentation for biofuel production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef]
- Schoelmerich, M.C.; Müller, V. Energy conservation by a hydrogenase-dependent chemiosmotic mechanism in an ancient metabolic pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 6329–6334. [Google Scholar] [CrossRef] [Green Version]
- Adachi, Y.; Inoue, M.; Yoshida, T.; Sako, Y. Genetic Engineering of Carbon Monoxide-dependent Hydrogen-producing Machinery in Parageobacillus thermoglucosidasius. Microbes Environ. 2020, 35, ME20101. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, D.; Pedersen, C.N.S.; Greening, C. HydDB: A web tool for hydrogenase classification and analysis. Sci. Rep. UK 2016, 6, 34212. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.-C.; Huang, J.; Smolinski, S.; Tek, V.; Vanzin, G. Energy generation from the CO oxidation-hydrogen production pathway in Rubrivivax gelatinosus. Appl. Environ. Microbiol. 2005, 71, 2870–2874. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.K.; Mayer, F.; Kang, S.G.; Müller, V. Energy conservation by oxidation of formate to carbon dioxide and hydrogen via a sodium ion current in a hyperthermophilic archaeon. Proc. Natl. Acad. Sci. USA 2014, 111, 11497. [Google Scholar] [CrossRef] [Green Version]
- Sapra, R.; Bagramyan, K.; Adams, M.W.W. A simple energy-conserving system: Proton reduction coupled to proton translocation. Proc. Natl. Acad. Sci. USA 2003, 100, 7545. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, Y.; Inoue, M.; Omae, K.; Yoshida, T.; Sako, Y. Chapter Three—Anaerobic and hydrogenogenic carbon monoxide-oxidizing prokaryotes: Versatile microbial conversion of a toxic gas into an available energy. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 110, pp. 99–148. [Google Scholar]
- Robb, F.T.; Techtmann, S.M. Life on the fringe: Microbial adaptation to growth on carbon monoxide. F1000Research 2018, 7, F1000 Faculty Rev-1981. [Google Scholar] [CrossRef]
- Nakagawa, T.; Iino, T.; Suzuki, K.-i.; Harayama, S. Ferrimonas futtsuensis sp. nov. and Ferrimonas kyonanensis sp. nov., selenate-reducing bacteria belonging to the Gammaproteobacteria isolated from Tokyo Bay. Int. J. Syst. Evol. Microbiol. 2006, 56, 2639–2645. [Google Scholar] [CrossRef]
- Srinivas, T.N.R.; Vijaya Bhaskar, Y.; Bhumika, V.; Anil Kumar, P. Photobacterium marinum sp. nov., a marine bacterium isolated from a sediment sample from Palk Bay, India. Syst. Appl. Microbiol. 2013, 36, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Omae, K.; Fukuyama, Y.; Yasuda, H.; Mise, K.; Yoshida, T.; Sako, Y. Diversity and distribution of thermophilic hydrogenogenic carboxydotrophs revealed by microbial community analysis in sediments from multiple hydrothermal environments in Japan. Arch. Microbiol. 2019, 201, 969–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, T.; Aliyu, H.; Küchlin, R.; Zwick, M.; Cowan, D.; Neumann, A.; de Maayer, P. Comparative genomic analysis of Parageobacillus thermoglucosidasius strains with distinct hydrogenogenic capacities. BMC Genom. 2018, 19, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, T.; Aliyu, H.; Biebinger, L.; Gödert, R.; Hornberger, A.; Cowan, D.; de Maayer, P.; Neumann, A. Effects of different operating parameters on hydrogen production by Parageobacillus thermoglucosidasius DSM 6285. AMB Express 2019, 9, 207. [Google Scholar] [CrossRef]
- Aliyu, H.; Mohr, T.; Cowan, D.; de Maayer, P.; Neumann, A. Time-course Transcriptome of Parageobacillus thermoglucosidasius DSM 6285 Grown in the Presence of Carbon Monoxide and Air. Int. J. Mol. Sci. 2020, 21, 3870. [Google Scholar] [CrossRef] [PubMed]
- Mol, V.; Bennett, M.; Sánchez, B.J.; Lisowska, B.K.; Herrgård, M.J.; Nielsen, A.T.; Leak, D.J.; Sonnenschein, N. Genome-scale metabolic modelling of P. thermoglucosidasius NCIMB 11955 reveals metabolic bottlenecks in anaerobic metabolism. bioRxiv 2021. [Google Scholar] [CrossRef]
- Karp, P.D.; Paley, S.; Romero, P. The Pathway Tools software. Bioinformatics 2002, 18, S225–S232. [Google Scholar] [CrossRef]
- Ahmad, A.; Hartman, H.B.; Krishnakumar, S.; Fell, D.A.; Poolman, M.G.; Srivastava, S. A Genome Scale Model of Geobacillus thermoglucosidasius (C56-YS93) reveals its biotechnological potential on rice straw hydrolysate. J. Biotechnol. 2017, 251, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Henstra, A.M.; Stams, A.J.M. Novel Physiological Features of Carboxydothermus hydrogenoformans and Thermoterrabacterium ferrireducens. Appl. Environ. Microbiol. 2004, 70, 7236–7240. [Google Scholar] [CrossRef] [Green Version]
- Hopper, C.P.; De La Cruz, L.K.; Lyles, K.V.; Wareham, L.K.; Gilbert, J.A.; Eichenbaum, Z.; Magierowski, M.; Poole, R.K.; Wollborn, J.; Wang, B. Role of Carbon Monoxide in Host–Gut Microbiome Communication. Chem. Rev. 2020, 120, 13273–13311. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, D.; van der Rest, M.E.; Petrović, S. Biochemical and genetic characterization of the membrane-associated malate dehydrogenase (acceptor) from Corynebacterium glutamicum. Eur. J. Biochem. 1998, 254, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Cecchini, G. Function and structure of complex II of the respiratory chain. Annu. Rev. Biochem. 2003, 72, 77–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Rest, M.E.; Frank, C.; Molenaar, D. Functions of the Membrane-Associated and Cytoplasmic Malate Dehydrogenases in the Citric Acid Cycle of Escherichia coli. J. Bacteriol. 2000, 182, 6892–6899. [Google Scholar] [CrossRef] [Green Version]
- Borisov, V.B.; Forte, E.; Sarti, P.; Brunori, M.; Konstantinov, A.A.; Giuffrè, A. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem. Biophys. Res. Commun. 2007, 355, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.; Borisov, V.B.; Arese, M.; Sarti, P.; Forte, E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim. Et Biophys. Acta Bioenerg. 2014, 1837, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.P. The fermentation pathways of Escherichia coli. Fems Microbiol. Rev. 1989, 5, 223–234. [Google Scholar] [CrossRef]
- Chai, Y.; Kolter, R.; Losick, R. A Widely Conserved Gene Cluster Required for Lactate Utilization in Bacillus subtilis and Its Involvement in Biofilm Formation. J. Bacteriol. 2009, 191, 2423–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cypionka, H.; Meyer, O. Carbon monoxide-insensitive respiratory chain of Pseudomonas carboxydovorans. J. Bacteriol. 1983, 156, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- Wareham, L.K.; Begg, R.; Jesse, H.E.; Van Beilen, J.W.A.; Ali, S.; Svistunenko, D.; McLean, S.; Hellingwerf, K.J.; Sanguinetti, G.; Poole, R.K. Carbon Monoxide Gas Is Not Inert, but Global, in Its Consequences for Bacterial Gene Expression, Iron Acquisition, and Antibiotic Resistance. Antioxid. Redox Signal. 2016, 24, 1013–1028. [Google Scholar] [CrossRef] [Green Version]
- Hasona, A.; Kim, Y.; Healy, F.G.; Ingram, L.O.; Shanmugam, K.T. Pyruvate Formate Lyase and Acetate Kinase Are Essential for Anaerobic Growth of Escherichia coli on Xylose. J. Bacteriol. 2004, 186, 7593–7600. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.H.; Shulla, A.; Reimann, S.A.; Keating, D.H.; Wolfe, A.J. The Intracellular Concentration of Acetyl Phosphate in Escherichia coli Is Sufficient for Direct Phosphorylation of Two-Component Response Regulators. J. Bacteriol. 2007, 189, 5574–5581. [Google Scholar] [CrossRef] [Green Version]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; de Jong, H. Acetate Metabolism and the Inhibition of Bacterial Growth by Acetate. J. Bacteriol. 2019, 201, e00147-00119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, A.J. The Acetate Switch. Microbiol. Mol. Biol. Rev. 2005, 69, 12–50. [Google Scholar] [CrossRef] [Green Version]
- Shinar, G.; Rabinowitz, J.D.; Alon, U. Robustness in Glyoxylate Bypass Regulation. PLoS Comput. Biol. 2009, 5, e1000297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.; Jung, J.; Jang, I.-A.; Madsen, E.L.; Park, W. Role of Glyoxylate Shunt in Oxidative Stress Response. J. Biol. Chem. 2017, 291, 11928–11938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, B.; Shen, T.; Zhou, H.; Liu, J.; Chen, J.; Pan, X.; Liu, H.; Wu, J.; Zheng, H.; Shi, Y. A systematic investigation of Escherichia coli central carbon metabolism in response to superoxide stress. BMC Syst. Biol. 2010, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Pidatala, R.R.; Ramakrishna, W. Mutational, proteomic and metabolomic analysis of a plant growth promoting copper-resistant Pseudomonas spp. FEMS Microbiol. Lett. 2012, 335, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Schroeter, R.; Voigt, B.; Jürgen, B.; Methling, K.; Pöther, D.C.; Schäfer, H.; Albrecht, D.; Mostertz, J.; Mäder, U.; Evers, S. The peroxide stress response of Bacillus licheniformis. Proteomics 2011, 11, 2851–2866. [Google Scholar] [CrossRef]
- Nandakumar, M.; Nathan, C.; Rhee, K.Y. Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Durall, C.; Kukil, K.; Hawkes, J.A.; Albergati, A.; Lindblad, P.; Lindberg, P. Production of succinate by engineered strains of Synechocystis PCC 6803 overexpressing phosphoenolpyruvate carboxylase and a glyoxylate shunt. Microb. Cell. Fact. 2021, 20, 39. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliyu, H.; Kastner, R.; Maayer, P.d.; Neumann, A. Carbon Monoxide Induced Metabolic Shift in the Carboxydotrophic Parageobacillus thermoglucosidasius DSM 6285. Microorganisms 2021, 9, 1090. https://doi.org/10.3390/microorganisms9051090
Aliyu H, Kastner R, Maayer Pd, Neumann A. Carbon Monoxide Induced Metabolic Shift in the Carboxydotrophic Parageobacillus thermoglucosidasius DSM 6285. Microorganisms. 2021; 9(5):1090. https://doi.org/10.3390/microorganisms9051090
Chicago/Turabian StyleAliyu, Habibu, Ronnie Kastner, Pieter de Maayer, and Anke Neumann. 2021. "Carbon Monoxide Induced Metabolic Shift in the Carboxydotrophic Parageobacillus thermoglucosidasius DSM 6285" Microorganisms 9, no. 5: 1090. https://doi.org/10.3390/microorganisms9051090
APA StyleAliyu, H., Kastner, R., Maayer, P. d., & Neumann, A. (2021). Carbon Monoxide Induced Metabolic Shift in the Carboxydotrophic Parageobacillus thermoglucosidasius DSM 6285. Microorganisms, 9(5), 1090. https://doi.org/10.3390/microorganisms9051090






