Detection of Quebec Polyomavirus DNA in Samples from Different Patient Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Samples
2.2. DNA Purification and PCR
2.3. Sequencing and Analysis of VP1
2.4. Statistical Analysis
3. Results
3.1. Detection of QPyV DNA in Specimens from Different Patient Groups
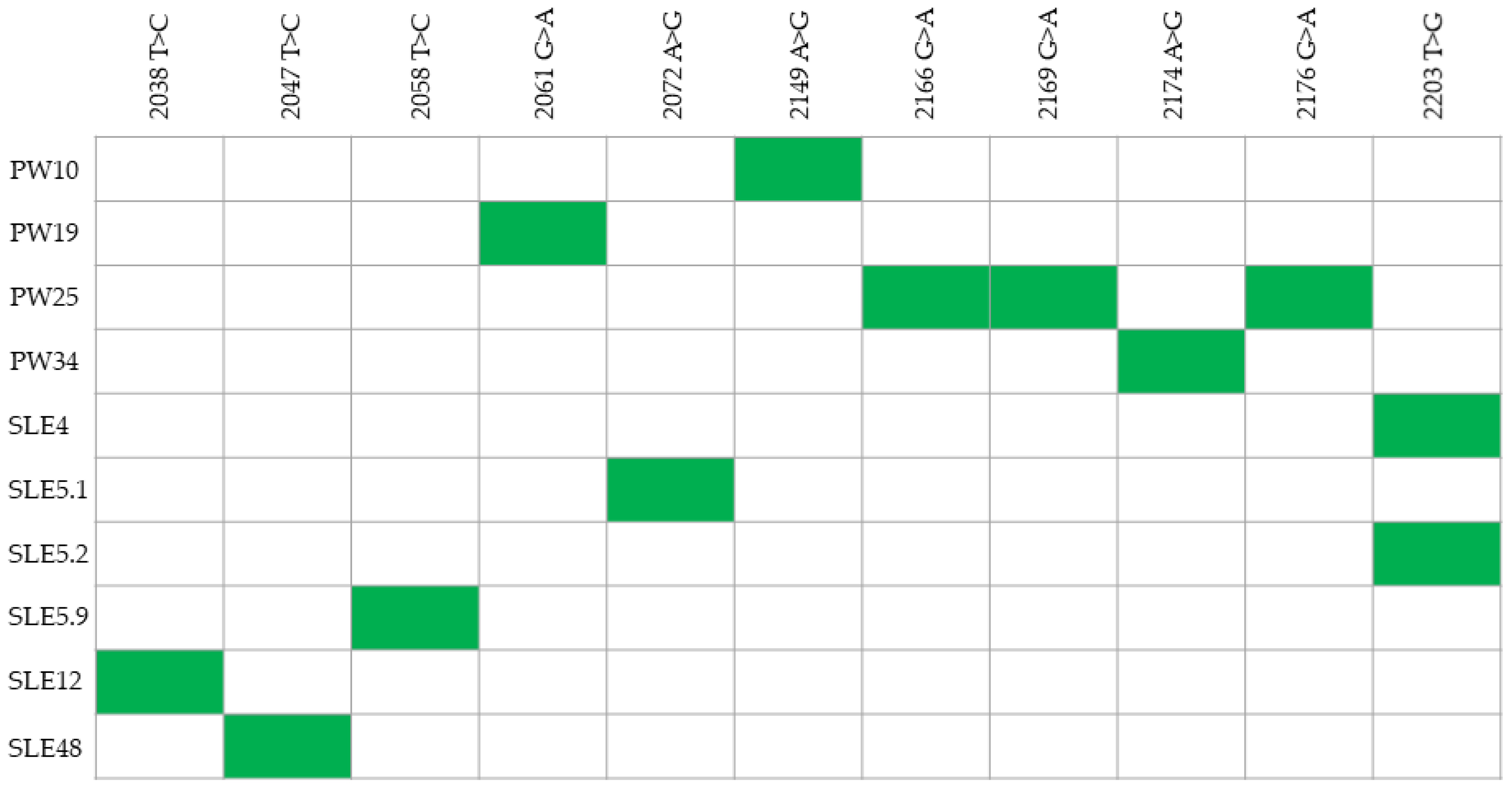
3.2. Nucleotide Sequence Analysis
3.3. Amino Acid Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moens, U.; Krumbholz, A.; Ehlers, B.; Zell, R.; Johne, R.; Calvignac-Spencer, S.; Lauber, C. Biology, evolution, and medical importance of polyomaviruses: An update. Infect. Genet. Evol. 2017, 54, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.S.; Enderlein, D.; Nelson, C.D.; Carter, W.S.; Kawano, M.; Xing, L.; Swenson, R.D.; Olson, N.H.; Baker, T.S.; Cheng, R.H.; et al. The structure of avian polyomavirus reveals variably sized capsids, non-conserved inter-capsomere interactions, and a possible location of the minor capsid protein VP4. Virology 2011, 411, 142–152. [Google Scholar] [CrossRef]
- Buck, C.B.; Van Doorslaer, K.; Peretti, A.; Geoghegan, E.M.; Tisza, M.J.; An, P.; Katz, J.P.; Pipas, J.M.; McBride, A.A.; Camus, A.C.; et al. The Ancient Evolutionary History of Polyomaviruses. PLoS Pathog. 2016, 12, e1005574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, L. A filterable agent, recovered from Ak leukemic extracts, causing salivary gland carcinomas in C3H mice. Proc. Soc. Exp. Biol. Med. 1953, 83, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Calvignac-Spencer, S.; Feltkamp, M.C.; Daugherty, M.D.; Moens, U.; Ramqvist, T.; Johne, R.; Ehlers, B. A taxonomy update for the family Polyomaviridae. Arch. Virol. 2016, 161, 1739–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rott, O.; Kröger, M.; Müller, H.; Hobom, G. The genome of budgerigar fledgling disease virus, an avian polyomavirus. Virology 1988, 165, 74–86. [Google Scholar] [CrossRef]
- Peretti, A.; FitzGerald, P.C.; Bliskovsky, V.; Pastrana, D.V.; Buck, C.B. Genome Sequence of a Fish-Associated Polyomavirus, Black Sea Bass (Centropristis striata) Polyomavirus 1. Genome Announc. 2015, 3, e01476-14. [Google Scholar] [CrossRef]
- Gardner, S.D.; Field, A.M.; Coleman, D.V.; Hulme, B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1971, 1, 1253–1257. [Google Scholar] [CrossRef]
- Padgett, B.L.; Walker, D.L.; ZuRhein, G.M.; Eckroade, R.J.; Dessel, B.H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1971, 1, 1257–1260. [Google Scholar] [CrossRef]
- Allander, T.; Andreasson, K.; Gupta, S.; Bjerkner, A.; Bogdanovic, G.; Persson, M.A.; Dalianis, T.; Ramqvist, T.; Andersson, B. Identification of a third human polyomavirus. J. Virol. 2007, 81, 4130–4136. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, A.M.; Nissen, M.D.; Whiley, D.M.; Mackay, I.M.; Lambert, S.B.; Wu, G.; Brennan, D.C.; Storch, G.A.; Sloots, T.P.; Wang, D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007, 3, e64. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Schowalter, R.M.; Pastrana, D.V.; Pumphrey, K.A.; Moyer, A.L.; Buck, C.B. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 2010, 7, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meijden, E.; Janssens, R.W.; Lauber, C.; Bouwes Bavinck, J.N.; Gorbalenya, A.E.; Feltkamp, M.C. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010, 6, e1001024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scuda, N.; Hofmann, J.; Calvignac-Spencer, S.; Ruprecht, K.; Liman, P.; Kühn, J.; Hengel, H.; Ehlers, B. A novel human polyomavirus closely related to the african green monkey-derived lymphotropic polyomavirus. J. Virol. 2011, 85, 4586–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, C.B.; Phan, G.Q.; Raiji, M.T.; Murphy, P.M.; McDermott, D.H.; McBride, A.A. Complete genome sequence of a tenth human polyomavirus. J. Virol. 2012, 86, 10887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, E.S.; Reyes, A.; Antonio, M.; Saha, D.; Ikumapayi, U.N.; Adeyemi, M.; Stine, O.C.; Skelton, R.; Brennan, D.C.; Mkakosya, R.S.; et al. Discovery of STL polyomavirus, a polyomavirus of ancestral recombinant origin that encodes a unique T antigen by alternative splicing. Virology 2013, 436, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korup, S.; Rietscher, J.; Calvignac-Spencer, S.; Trusch, F.; Hofmann, J.; Moens, U.; Sauer, I.; Voigt, S.; Schmuck, R.; Ehlers, B. Identification of a novel human polyomavirus in organs of the gastrointestinal tract. PLoS ONE 2013, 8, e58021. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.; Pereira, M.; Rhodes, R.H.; An, P.; Pipas, J.M.; Jain, K.; Kapoor, A.; Briese, T.; Faust, P.L.; Lipkin, W.I. Identification of a novel polyomavirus in a pancreatic transplant recipient with retinal blindness and vasculitic myopathy. J. Infect. Dis. 2014, 210, 1595–1599. [Google Scholar] [CrossRef] [Green Version]
- Gheit, T.; Dutta, S.; Oliver, J.; Robitaille, A.; Hampras, S.; Combes, J.D.; McKay-Chopin, S.; Le Calvez-Kelm, F.; Fenske, N.; Cherpelis, B.; et al. Isolation and characterization of a novel putative human polyomavirus. Virology 2017, 506, 45–54. [Google Scholar] [CrossRef]
- Moens, U.; Calvignac-Spencer, S.; Lauber, C.; Ramqvist, T.; Feltkamp, M.C.W.; Daugherty, M.D.; Verschoor, E.J.; Ehlers, B. ICTV Virus Taxonomy Profile: Polyomaviridae. J. Gen. Virol. 2017, 98, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Kamminga, S.; van der Meijden, E.; Feltkamp, M.C.W.; Zaaijer, H.L. Seroprevalence of fourteen human polyomaviruses determined in blood donors. PLoS ONE 2018, 13, e0206273. [Google Scholar] [CrossRef] [Green Version]
- Arthur, R.R.; Shah, K.V.; Baust, S.J.; Santos, G.W.; Saral, R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N. Engl. J. Med. 1986, 315, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Starrett, G.J.; Buck, C.B. The case for BK polyomavirus as a cause of bladder cancer. Curr. Opin. Virol. 2019, 39, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Saade, A.; Styczynski, J.; Cesaro, S. BK virus infection in allogeneic hematopoietic cell transplantation: An update on pathogenesis, immune responses, diagnosis and treatments. J. Infect. 2020, 81, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2021, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Moore, P.S. Merkel cell carcinoma: A virus-induced human cancer. Annu. Rev. Pathol. 2012, 7, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Arora, R.; Chang, Y.; Moore, P.S. MCV and Merkel cell carcinoma: A molecular success story. Curr. Opin. Virol. 2012, 2, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.; Jedrych, J.J.; Feng, H.; Natalie, A.A.; Grandinetti, L.; Mirvish, E.; Crespo, M.M.; Yadav, D.; Fasanella, K.E.; Proksell, S.; et al. Human polyomavirus 7-associated pruritic rash and viremia in transplant recipients. J. Infect. Dis 2015, 211, 1560–1565. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.D.; Lee, E.E.; Yue, Y.; Stork, J.; Pock, L.; North, J.P.; Vandergriff, T.; Cockerell, C.; Hosler, G.A.; Pastrana, D.V.; et al. Human polyomavirus 6 and 7 are associated with pruritic and dyskeratotic dermatoses. J. Am. Acad. Dermatol. 2017, 76, 932–940.e933. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.D.B.; Erdag, G.; Cuda, J.D.; Rangwala, S.; Girardi, N.; Bibee, K.; Orens, J.B.; Prono, M.D.; Toptan, T.; Loss, M.J. Treatment of human polyomavirus-7-associated rash and pruritus with topical cidofovir in a lung transplant patient: Case report and literature review. Transpl. Infect. Dis. 2018, 20, e12793. [Google Scholar] [CrossRef]
- Canavan, T.N.; Baddley, J.W.; Pavlidakey, P.; Tallaj, J.A.; Elewski, B.E. Human polyomavirus-7-associated eruption successfully treated with acitretin. Am. J. Transplant. 2018, 18, 1278–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenstein, R.K.; Pastrana, D.V.; Starrett, G.J.; Sapio, M.R.; Hill, N.T.; Jo, J.H.; Lee, C.R.; Iadarola, M.J.; Buck, C.B.; Kong, H.H.; et al. Host-Pathogen Interactions in Human Polyomavirus 7 (HPyV7)-associated Pruritic Skin Eruption. J. Investig. Dermatol. 2021, 141, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Rascovan, N.; Monteil Bouchard, S.; Grob, J.J.; Collet-Villette, A.M.; Gaudy-Marqueste, C.; Penicaud, M.; Lepidi, H.; Raoult, D.; Desnues, C. Human Polyomavirus-6 Infecting Lymph Nodes of a Patient with an Angiolymphoid Hyperplasia With Eosinophilia or Kimura Disease. Clin. Infect. Dis. 2016, 62, 1419–1421. [Google Scholar] [CrossRef]
- Hashida, Y.; Higuchi, T.; Nakajima, K.; Ujihara, T.; Murakami, I.; Fujieda, M.; Sano, S.; Daibata, M. Human Polyomavirus 6 with the Asian-Japanese Genotype in Cases of Kimura Disease and Angiolymphoid Hyperplasia with Eosinophilia. J. Investig. Dermatol. 2020, 140, 1650–1653.e1654. [Google Scholar] [CrossRef] [PubMed]
- Kazem, S.; van der Meijden, E.; Feltkamp, M.C. The trichodysplasia spinulosa-associated polyomavirus: Virological background and clinical implications. APMIS 2013, 121, 770–782. [Google Scholar] [CrossRef]
- Wu, J.H.; Nguyen, H.P.; Rady, P.L.; Tyring, S.K. Molecular insight into the viral biology and clinical features of trichodysplasia spinulosa. Br. J. Dermatol. 2016, 174, 490–498. [Google Scholar] [CrossRef]
- Ondov, B.D.; Starrett, G.J.; Sappington, A.; Kostic, A.; Koren, S.; Buck, C.B.; Phillippy, A.M. Mash Screen: High-throughput sequence containment estimation for genome discovery. Genome Biol. 2019, 20, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendiksen, S.; Rekvig, O.P.; Van Ghelue, M.; Moens, U. VP1 DNA sequences of JC and BK viruses detected in urine of systemic lupus erythematosus patients reveal no differences from strains expressed in normal individuals. J. Gen. Virol. 2000, 81, 2625–2633. [Google Scholar] [CrossRef]
- Song, X.; Van Ghelue, M.; Ludvigsen, M.; Nordbø, S.A.; Ehlers, B.; Moens, U. Characterization of the non-coding control region of polyomavirus KI isolated from nasopharyngeal samples from patients with respiratory symptoms or infection and from blood from healthy blood donors in Norway. J. Gen. Virol. 2016, 97, 1647–1657. [Google Scholar] [CrossRef] [Green Version]
- Prezioso, C.; Van Ghelue, M.; Moens, U.; Pietropaolo, V. HPyV6 and HPyV7 in urine from immunocompromised patients. Virol. J. 2021, 18, 24. [Google Scholar] [CrossRef]
- Tümmler, C.; Dumitriu, G.; Wickström, M.; Coopman, P.; Valkov, A.; Kogner, P.; Johnsen, J.I.; Moens, U.; Sveinbjørnsson, B. SYK Inhibition Potentiates the Effect of Chemotherapeutic Drugs on Neuroblastoma Cells in Vitro. Cancers 2019, 11, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, L.; Ostermann, H.; Fiegl, M.; Tischer, J.; Jaeger, G.; Rieger, C.T. Reactivation of polyomavirus in the genitourinary tract is significantly associated with severe GvHD and oral mucositis following allogeneic stem cell transplantation. Infection 2016, 44, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Boukoum, H.; Nahdi, I.; Sahtout, W.; Skiri, H.; Segondy, M.; Aouni, M. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microb. Pathog. 2016, 97, 204–208. [Google Scholar] [CrossRef]
- Wang, Y.; Keinonen, A.; Koskenmies, S.; Pitkänen, S.; Fyhrquist, N.; Sadeghi, M.; Mäkisalo, H.; Söderlund-Venermo, M.; Hedman, K. Occurrence of newly discovered human polyomaviruses in skin of liver transplant recipients and their relation with squamous cell carcinoma in situ and actinic keratosis—A single-center cohort study. Transplant. Int. 2019, 32, 516–522. [Google Scholar] [CrossRef]
- Nanda, S.; Jayan, G.; Voulgaropoulou, F.; Sierra-Honigmann, A.M.; Uhlenhaut, C.; McWatters, B.J.; Patel, A.; Krause, P.R. Universal virus detection by degenerate-oligonucleotide primed polymerase chain reaction of purified viral nucleic acids. J. Virol. Methods 2008, 152, 18–24. [Google Scholar] [CrossRef]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguière, A.; Manuguerra, J.C.; et al. Human skin microbiota: High diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS ONE 2012, 7, e38499. [Google Scholar] [CrossRef] [Green Version]
- Wylie, K.M.; Mihindukulasuriya, K.A.; Zhou, Y.; Sodergren, E.; Storch, G.A.; Weinstock, G.M. Metagenomic analysis of double-stranded DNA viruses in healthy adults. BMC Biol. 2014, 12, 71. [Google Scholar] [CrossRef]
- Rani, A.; Ranjan, R.; McGee, H.S.; Metwally, A.; Hajjiri, Z.; Brennan, D.C.; Finn, P.W.; Perkins, D.L. A diverse virome in kidney transplant patients contains multiple viral subtypes with distinct polymorphisms. Sci. Rep. 2016, 6, 33327. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, C.N.; Siqueira, J.; Li, L.; Deng, X.; Mugo, P.; Graham, S.M.; Price, M.A.; Sanders, E.J.; Delwart, E. The plasma virome of febrile adult Kenyans shows frequent parvovirus B19 infections and a novel arbovirus (Kadipiro virus). J. Gen. Virol. 2016, 97, 3359–3367. [Google Scholar] [CrossRef] [Green Version]
- Legoff, J.; Resche-Rigon, M.; Bouquet, J.; Robin, M.; Naccache, S.N.; Mercier-Delarue, S.; Federman, S.; Samayoa, E.; Rousseau, C.; Piron, P.; et al. The eukaryotic gut virome in hematopoietic stem cell transplantation: New clues in enteric graft-versus-host disease. Nat. Med. 2017, 23, 1080–1085. [Google Scholar] [CrossRef]
- Rampelli, S.; Turroni, S.; Schnorr, S.L.; Soverini, M.; Quercia, S.; Barone, M.; Castagnetti, A.; Biagi, E.; Gallinella, G.; Brigidi, P.; et al. Characterization of the human DNA gut virome across populations with different subsistence strategies and geographical origin. Environ. Microbiol. 2017, 19, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Wylie, K.M.; Wylie, T.N.; Cahill, A.G.; Macones, G.A.; Tuuli, M.G.; Stout, M.J. The vaginal eukaryotic DNA virome and preterm birth. Am. J. Obstet. Gynecol. 2018, 219, e1–e189. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.L.; Cordey, S.; Simonetta, F.; Brito, F.; Docquier, M.; Turin, L.; van Delden, C.; Boely, E.; Dantin, C.; Pradier, A.; et al. Human pegivirus persistence in human blood virome after allogeneic haematopoietic stem-cell transplantation. Clin. Microbiol. Infect. 2019, 25, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garretto, A.; Thomas-White, K.; Wolfe, A.J.; Putonti, C. Detecting viral genomes in the female urinary microbiome. J. Gen. Virol. 2018, 99, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.T.; Hong, N.T.T.; Nhu, L.N.T.; Thanh, T.T.; Lau, C.Y.; Limmathurotsakul, D.; Deng, X.; Rahman, M.; Chau, N.V.V.; van Doorn, H.R.; et al. Viruses in Vietnamese Patients Presenting with Community-Acquired Sepsis of Unknown Cause. J. Clin. Microbiol. 2019, 57, e00386-19. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.Z.; Zhang, H.; Yang, J.; Zeng, J.; Wang, H. Preliminary assessment of viral metagenome from cancer tissue and blood from patients with lung adenocarcinoma. J. Med. Virol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Ströh, L.J.; Neu, U.; Blaum, B.S.; Buch, M.H.; Garcea, R.L.; Stehle, T. Structure analysis of the major capsid proteins of human polyomaviruses 6 and 7 reveals an obstructed sialic acid binding site. J. Virol. 2014, 88, 10831–10839. [Google Scholar] [CrossRef] [Green Version]

| Patient Group (n) | Specimen | QPyV Positive |
|---|---|---|
| SLE * patients (5) patient 1 (5 samples) patient 2 (7 samples) patient 3 (13 samples) patient 4 (16 samples) patient 5 (32 samples) | consecutive urine | 0 (0%) 0 (0%) 0 (0%) 1 (6.3%) 3 (9.2%) |
| SLE patients (62) | urine | 9 (14.5%) |
| pregnant women (65) | urine | 10 (15.4%) |
| suspected neurological complications (63) | CSF | 0 (0%) |
| respiratory symptoms or infection (80) | NPA | 0 (0%) |
| HIV+ (66) | urine | 0 (0%) |
| HIV+ (65) | plasma | 0 (0%) |
| mutiple sclerosis (35) | urine | 5 (14.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prezioso, C.; Van Ghelue, M.; Pietropaolo, V.; Moens, U. Detection of Quebec Polyomavirus DNA in Samples from Different Patient Groups. Microorganisms 2021, 9, 1082. https://doi.org/10.3390/microorganisms9051082
Prezioso C, Van Ghelue M, Pietropaolo V, Moens U. Detection of Quebec Polyomavirus DNA in Samples from Different Patient Groups. Microorganisms. 2021; 9(5):1082. https://doi.org/10.3390/microorganisms9051082
Chicago/Turabian StylePrezioso, Carla, Marijke Van Ghelue, Valeria Pietropaolo, and Ugo Moens. 2021. "Detection of Quebec Polyomavirus DNA in Samples from Different Patient Groups" Microorganisms 9, no. 5: 1082. https://doi.org/10.3390/microorganisms9051082
APA StylePrezioso, C., Van Ghelue, M., Pietropaolo, V., & Moens, U. (2021). Detection of Quebec Polyomavirus DNA in Samples from Different Patient Groups. Microorganisms, 9(5), 1082. https://doi.org/10.3390/microorganisms9051082






