1. Introduction
Salmonellosis is the third leading cause of death among food transmitted diseases [
1] with an estimated global
Salmonella enterocolitis incidence of 95.1 million cases [
2], accounting for 50,771 deaths in 2017 [
3]. In the Caribbean,
Salmonella is the most common laboratory-confirmed cause of foodborne diseases since 2005 [
4]. Poultry has been reported to be the main carrier of
Salmonella infections to humans [
5], more common than any other animal species [
6]. Broiler meat is an economical source of protein and estimated to be the most widely consumed meat, globally.
The human population of the twin-island Republic of Trinidad and Tobago is 1,366,725 [
7] with a reported 58.3 kg per capita poultry consumption rate; 800,000 broilers are produced weekly, of which 20% are imported [
8]. Consumers purchase chicken from cottage poultry processors, where they are freshly slaughtered and from supermarkets, which offer both chilled and frozen locally processed or imported frozen chicken. Broiler processing plants are responsible for 50% of local broiler processing [
9] where supermarkets and the franchised foodservice sector are supplied with chilled chickens as well as further-processed products [
9].
Several studies have reported the high frequency of contamination with
Salmonella of chicken meat sold at the informal and formal outlets in developed and developing countries [
10,
11]. It has also been reported that the processing of chicken at commercial processing plants contributes significantly to the contamination of dressed chicken carcasses with
Salmonella before they reach the retail outlets [
12,
13]. Unhygienic carcass handling, soiled slaughter equipment [
14,
15], contaminated water (scalding and immersion chiller water), and waste generated from evisceration and the de-feathering processes have been implicated as major sources of
Salmonella contamination during broiler processing [
16,
17,
18].
Salmonella-free broilers leaving farms may potentially become contaminated by the pathogen during processing through contact with immersion chiller water contaminated with
Salmonella originating from the positive broilers [
19,
20]. This can occur, should there be improper pH and chemical agents’ concentrations, as well as a failure to maintain good sanitary practices throughout processing [
21].
With the increase in production and consumption of broiler meat over the years, the use, misuse, and overuse of veterinary drugs for prophylaxis, therapeutic, and growth promotion purposes [
22,
23] are common in countries such as Trinidad and Tobago. In the country, although regulations on the use of veterinary drugs in livestock exist, they are not routinely enforced. The increase in the isolation of
Salmonella in humans, and the resistance of
Salmonella strains to antimicrobial agents commonly used in food-producing animals is a major health concern [
24,
25]. Worldwide, of a greater concern is the emergence of multidrug-resistant (MDR)
Salmonella [
26], which has been implicated in foodborne outbreaks due to contaminated meat [
27,
28].
To isolate
Salmonella from poultry processing plants, different approaches have been reported and recommended. In the European Union, the use of neck skin (NS) maceration [
29] is most frequent whereas, in the United States, the U.S. Department of Agriculture Food Safety and Inspection Service (USDA-FSIS) [
30] recommends the use of whole carcass rinse (WCR) method. Whilst the WCR is the most commonly used method for isolation of
Salmonella in broiler carcasses [
31,
32,
33], the whole carcass enrichment (WCE) and neck skin (NS) methods have been shown to be just as effective [
34] or even more than the WCR [
35]. However, the large space required for incubating whole carcasses makes the WCE method impractical for routine testing, but it is valuable for research purposes [
36].
In Trinidad and Tobago and the Caribbean, there is a dearth of comprehensive up-to-date data on the role played by the commercial broiler processing plants in the contamination of processed chicken carcasses with
Salmonella. The only available recent published data were from studies conducted at the outlets of cottage poultry processors (‘wet market’) where the slaughtering and retailing of dressed chicken were practiced [
37] and at supermarkets where retailing of chicken from the commercial processing plants occurs [
38] and the antimicrobial resistance profiles of
Salmonella isolates from both sources were determined [
39].
Considering the limited current information on the status and dynamics of Salmonella contamination of chicken carcasses at the commercial broiler processing plants, the present study with the following objectives was conducted: (i) to determine the frequency of isolation of Salmonella longitudinally from the different stages of processing, from pre-slaughter broilers to chilled carcasses, (ii) to evaluate the efficacy of three isolation methods for Salmonella, (iii) to identify the risk factors associated with Salmonella contamination of chicken carcasses at the plants and finally, (iv) to determine the serotypes and antimicrobial resistance profiles of the isolates of the pathogen recovered from the four plants operating in Trinidad.
4. Discussion
This is considered the first cross-sectional study conducted in the broiler processing plants in Trinidad and Tobago that documented the frequency of isolation of Salmonella along the processing lines. The study also characterized the isolates regarding their serotypes and antimicrobial resistance to currently used antimicrobial agents in the poultry industry. The food safety importance of the study cannot be underestimated because the four processing plants operational in the country supply the majority of local chickens and chicken products sold at supermarkets.
Of food safety concern, is the high level of contamination found in pre-packaged chilled whole carcasses (44.4%) and chilled chicken parts (40.0%) across the four processing plants. Salmonellosis has been reported in humans who consume inadequately cooked
Salmonella-contaminated chicken meat [
52,
53]. Our findings agree with the prevalence of
Salmonella found in chilled chicken carcasses in abattoirs elsewhere, where 48.0% [
43], 45.2% [
54], and 50.0% [
55] were reported in the United States, China, and Brazil, respectively. These findings were higher than the 8.3% [
56] and 3.75% [
57] reported in Iran and the Czech Republic, respectively. It is interesting to note that the most recent study on the prevalence of
Salmonella in chickens that originated from commercial processing plants in Trinidad was 8.3% [
38]. The differences in the prevalence have been reported to be affected by the carriage of
Salmonella during de-feathering [
57], evisceration, and spray washing steps [
58] as well as by contaminated chiller water [
59].
The strategy used in our study which included the collection of samples from the time of reception of live chickens to the finished chilled chickens longitudinally, from the pre-evisceration samples to chilled carcasses during each visit provided evidence of statistically significant (
p = 0.023) increased levels of contamination along the stages of processing. The differences in the frequencies of isolation of
Salmonella in the samples between and within the four processing plants, could be due in part, to the different management, production, and risk factors at these plants. These findings were not surprising because other studies have reported progressive increases in the frequency of contamination with
Salmonella during processing [
60,
61].
It is significant that the frequency of isolation of
Salmonella from the cloacal swabs pre-slaughter across the four plants was 2.2% ranging from 0.0% to 10.0%. This is an indication that the prevalence of
Salmonella was relatively low on the poultry farms from where the slaughtered birds originated. Our findings agree with the prevalence of
Salmonella in cloacal swabs of broilers pre-slaughter reported in Trinidad and Tobago, 3.95% (3/76) [
62]; Brazil, 7.0% (7/100) [
20]; and Colombia, 12.5% (8/64) [
63].
It was of epidemiological relevance to have detected that 71.4% of the 14 risk factors investigated demonstrated statistically significant association with the contamination of chicken carcasses during processing at the plants. Significantly higher frequencies of isolation of
Salmonella were detected among the following factors including medium-sized plants, use of more than 100 contract farmers, employment of less than 150 workers directly involved in processing, the average mortality rate of over 0.5% in broilers on arrival at the plant, i.e., dead on arrival, the use of pre-chillers, and the use of sanitizers in chiller water, used sanitizers for general cleaning of plants, among other factors. Many of these risk factors have been documented to be associated with the isolation of
Salmonella in processing plants by others [
15,
64,
65,
66,
67]. Standardized sanitation protocols with surveillance to monitor the efficacy and the development of resistance is suggested. In addition, frequent training programs for processing plant workers and farmers to educate them on the current best-practices will be beneficial in reducing cross-contamination along the continuum. Interestingly, further regression analyses and the odds ratio (OR) revealed that
Salmonella was 4.4 more likely (95% CI: 2.68–7.34) to be isolated from chickens in plants that received birds from more than 100 farmers. This risk could be attributed to the increased possibility of slaughtering broilers from
Salmonella-infected farms. Similarly, it was detected that plants that allowed the slaughter of chickens from batches with mortality rates of over 0.5% on arrival at the plants were 2.3 times more likely (95% CI: 1.45–3.74) to lead to the isolation of
Salmonella from chickens at those plants. Although the specific pathogens responsible for deaths experienced during transportation to the plant were not known, the possibility exists that
Salmonella may be involved. The contamination of feathers of chickens from direct contact with feces of infected broilers shedding
Salmonella and exposure to the pathogen in the transport vehicle on its way to the plant has been documented [
68,
69]. Similarly, the risk of contamination of chickens increased considerably by 4.4 times (95% CI: 2.68–7.34) in plants that permitted the slaughter of sick birds, albeit being processed last instead of being rejected at farms. The possibility of seeding the plant environment with pathogens, including
Salmonella, is pertinent, if the cleaning of the plant is inadequate.
Salmonella was isolated at a significantly higher frequency in plants that used chlorine (29.0%) than those that used hot water (11.4%). This is because
Salmonella has been reported to develop resistance to sanitizers [
70,
71,
72]. Additionally, our study noted that plants that used pre-chillers but did not add chemical agents were found to be 1.7 times more likely (95% CI: 0.94–3.02) to result in the recovery of
Salmonella. The proper use of chillers and sanitizers in processing plants can therefore not be ignored [
73,
74].
In our study, the WCE method yielded a statistically significant higher (53.9%) frequency of isolation of
Salmonella than either the WCR (35.0%) or the NS (42.2.%) methods, making it the most sensitive method for
Salmonella detection as reported by others [
43,
75]. Berrang et al. [
36] attributed this increased sensitivity to the ability of the WCE method to facilitate the proliferation of
Salmonella in low quantities or those firmly attached to the skin of the chicken. However, the challenges associated with WCE method, particularly the considerably larger incubator space requirement compared with the use of WCR and NS methods, cannot be disregarded thereby making it an impractical method for routine surveillance testing but applicable as a research tool. It has been reported that the types of samples and the methods of enrichment affect their sensitivities to detect
Salmonella in chickens [
76,
77].
The predominant serotypes of
Salmonella isolated were Enteritidis, Javiana, and Infantis. These serotypes have similarly been isolated from chicken-associated samples in the country, such as chickens sampled from supermarkets that originated from broiler processing plants and outlets of cottage poultry processors [
38] and chicken layers [
78]. In the current study, it was found that the serotypes were detected at different frequencies from the types of samples tested in the processing plants, a finding that agrees with published reports [
79,
80]. Of food safety and public health, the significance is the fact that some of these predominant serotypes were determined in the Caribbean Public Health Agency (CARPHA) State of Public Health report [
81], to be amongst the top 15 human
Salmonella serotypes detected in the region. Similarly, the predominant serotypes in our study were also reported to be the most commonly
Salmonella serotypes associated with human salmonellosis in Trinidad and Tobago between 2005–2012 [
81]. It cannot be underestimated that serotype Enteritidis has globally been associated with poultry meat and eggs, and responsible for human cases and epidemics of salmonellosis [
82,
83].
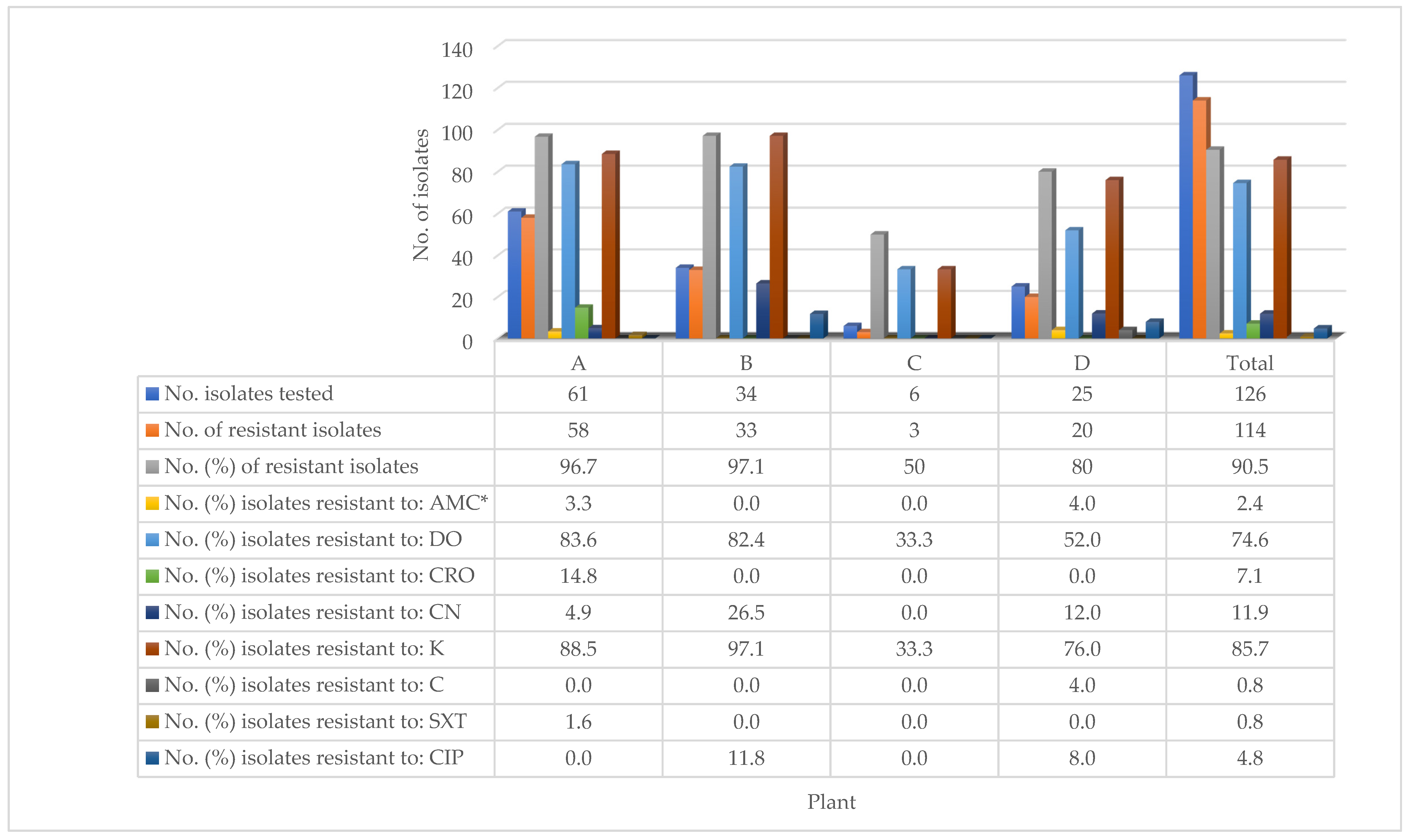
The high prevalence of resistance (90.5%) to antimicrobial agents by the 126 isolates of
Salmonella recovered from the four processing plants, has both zoonotic and therapeutic implications. It is important to have detected that the high prevalence of resistance was exhibited to antimicrobial agents routinely used in the poultry industry in the country. It has been reported that zoonotic spread of
Salmonella to workers at the commercial processing plants may occur [
84,
85] and as well as therapeutic failure in consumers of improperly cooked chickens contaminated by antimicrobial resistant-
Salmonella [
80]. Similarly, a high prevalence of resistance to antimicrobial agents (100.0%) has been reported in chilled chickens from supermarkets and cottage poultry processors [
39]. Although the current study was not farm-based, the prevalence of resistant
Salmonella in chickens processed at the plants may be indicative of the level of resistance of
Salmonella on the contract farms from where they originated. It has been documented that the misuse or over-use of antimicrobial agents by farmers may result in the development of resistance to antimicrobial agents [
86]. This is a common practice particularly in developing countries, including Trinidad and Tobago, where although laws governing the type and use of antimicrobial agents for prophylaxis, growth promotion, and therapy exist, prevailing challenges limit or prevent their enforcement [
87,
88].
With regard to the eight antimicrobial agents tested, it was important that the overall prevalence of resistance was comparatively low (0.8–11.9%) to six (amoxicillin-clavulanic acid, ceftriaxone, gentamicin, chloramphenicol, sulphamethoxazole–trimethoprim, and ciprofloxacin) of the antimicrobial agents, while significantly higher prevalence was exhibited to doxycycline (74.6%) and kanamycin (85.7%). Furthermore, the study found that the prevalence of resistance to the antimicrobial agents varied significantly across the processing plants from where the
Salmonella isolates originated. These findings reflect the differences in the types and the frequency of use of antimicrobial agents on the contract farms that supplied live broilers to the plants. The high prevalence of resistance exhibited to doxycycline and kanamycin has been documented in chickens in the country [
39]. The detection of a high prevalence of resistance (60.0% to 88.9%) among the top three detected serotypes (Enteritidis, Javiana, and Infantis) may also be therapeutic significance to infected broilers or humans. Differences in the prevalence of resistance to antimicrobial agents by
Salmonella have been reported to vary among serotypes of
Salmonella from chickens by others [
71,
89]. Therefore, there is a need to monitor the use of the two antimicrobial agents on broiler farms in the country.
It is concluded that the high prevalence of Salmonella (27.0%) including antimicrobial-resistant strains (90.5%), along with the predominance of three serotypes (Enteritidis, Javiana, and Infantis) among the isolates has implications for human salmonellosis in the country. The relative risk of salmonellosis posed by consumption of under-cooked Salmonella-contaminated chicken meat from these plants needs to be emphasized. The fact that 10 of the 14 risk factors investigated were statistically significantly associated with the contamination of chicken in the processing lines along with the odds ratio (OR) generated provides critical control points where interventions may be successfully applied. Our study reveals that the WCE method, which is not used for routine surveillance of Salmonella in chickens, demonstrated its significantly higher sensitivity when compared with either the WCR or NS methods, a finding that may be indicative of the potential under-reporting of the prevalence of antimicrobial resistant Salmonella in chickens in the country. The high prevalence of antimicrobial resistance exhibited by Salmonella isolates in this study poses both zoonotic and therapeutic implications to humans exposed to infected chickens. It is imperative to control the use of antimicrobial agents on poultry farms to reduce the development of antimicrobial resistance among Salmonella.