Minerals Determined a Special Ecological Niche and Selectively Enriched Microbial Species from Bulk Water Communities in Hot Springs
Abstract
1. Introduction
2. Materials and Methods
2.1. The Hot Spring Datasets Used for Co-Occurrence Network Construction
2.2. Co-Occurrence Network Construction and Statistical Analysis
2.3. Hot Spring Selection and Mineral Microcosms Design
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Data Processing and Statistical Analysis
3. Results
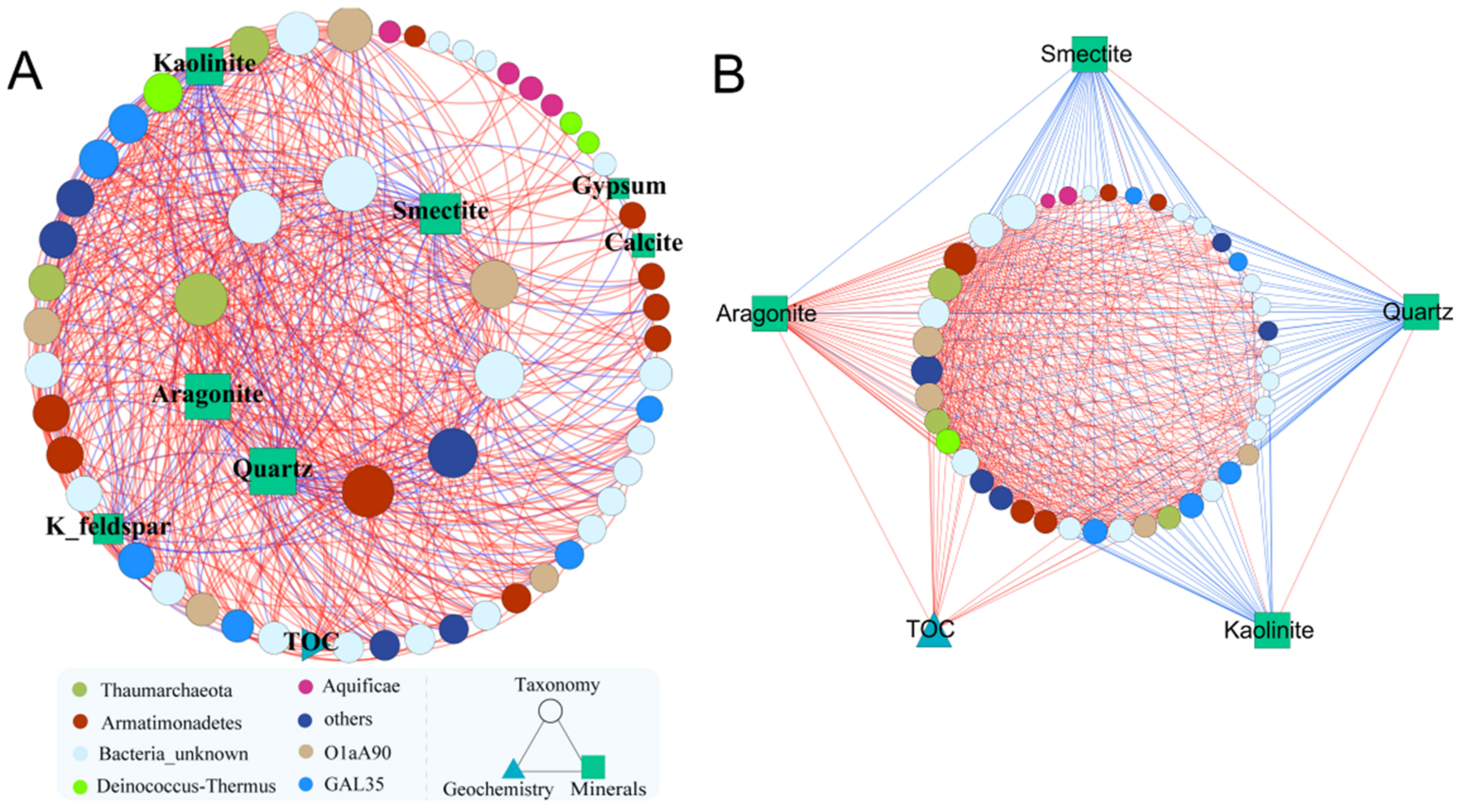
3.1. Highly Modular Structure Revealed by Microbial Network Analysis
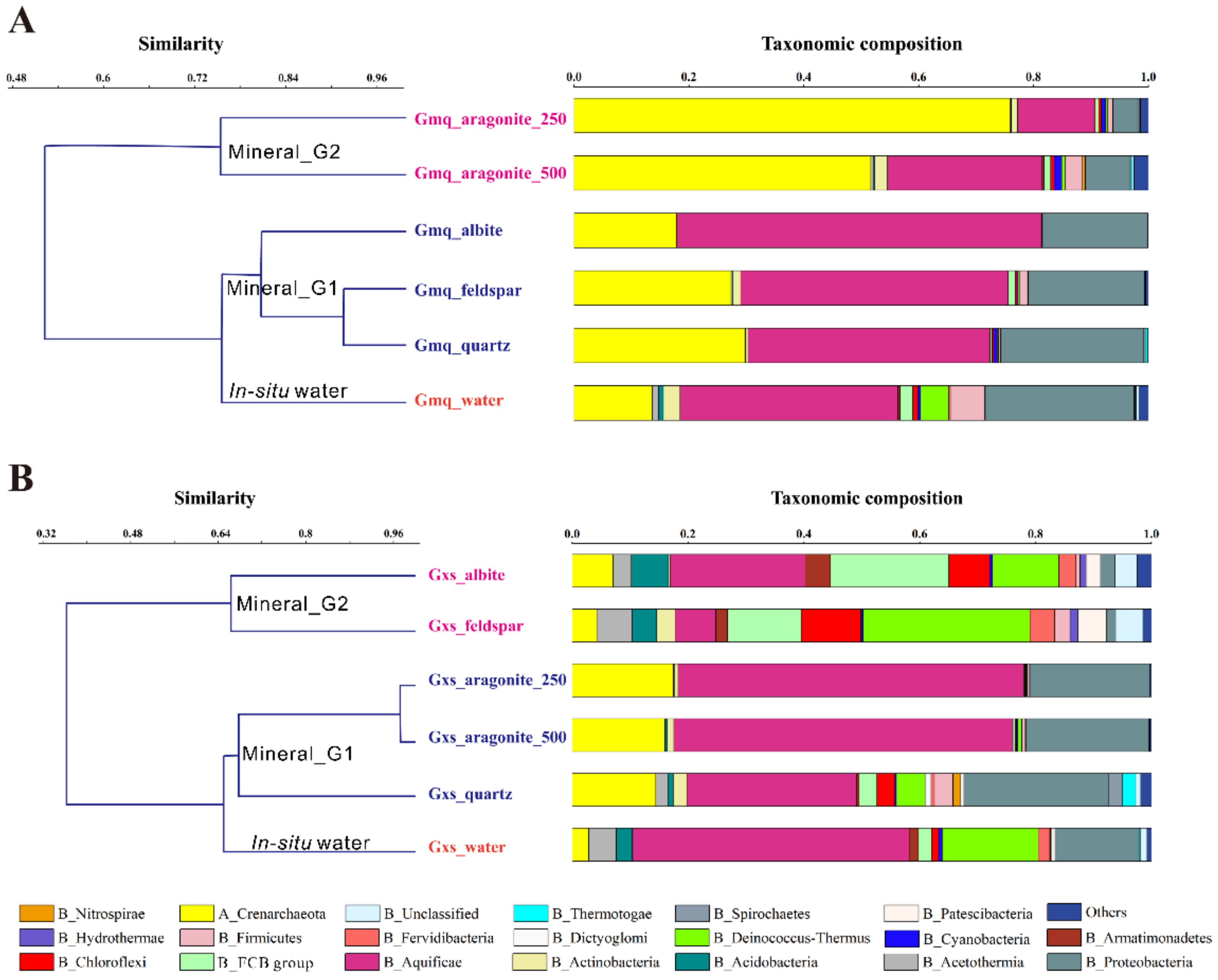
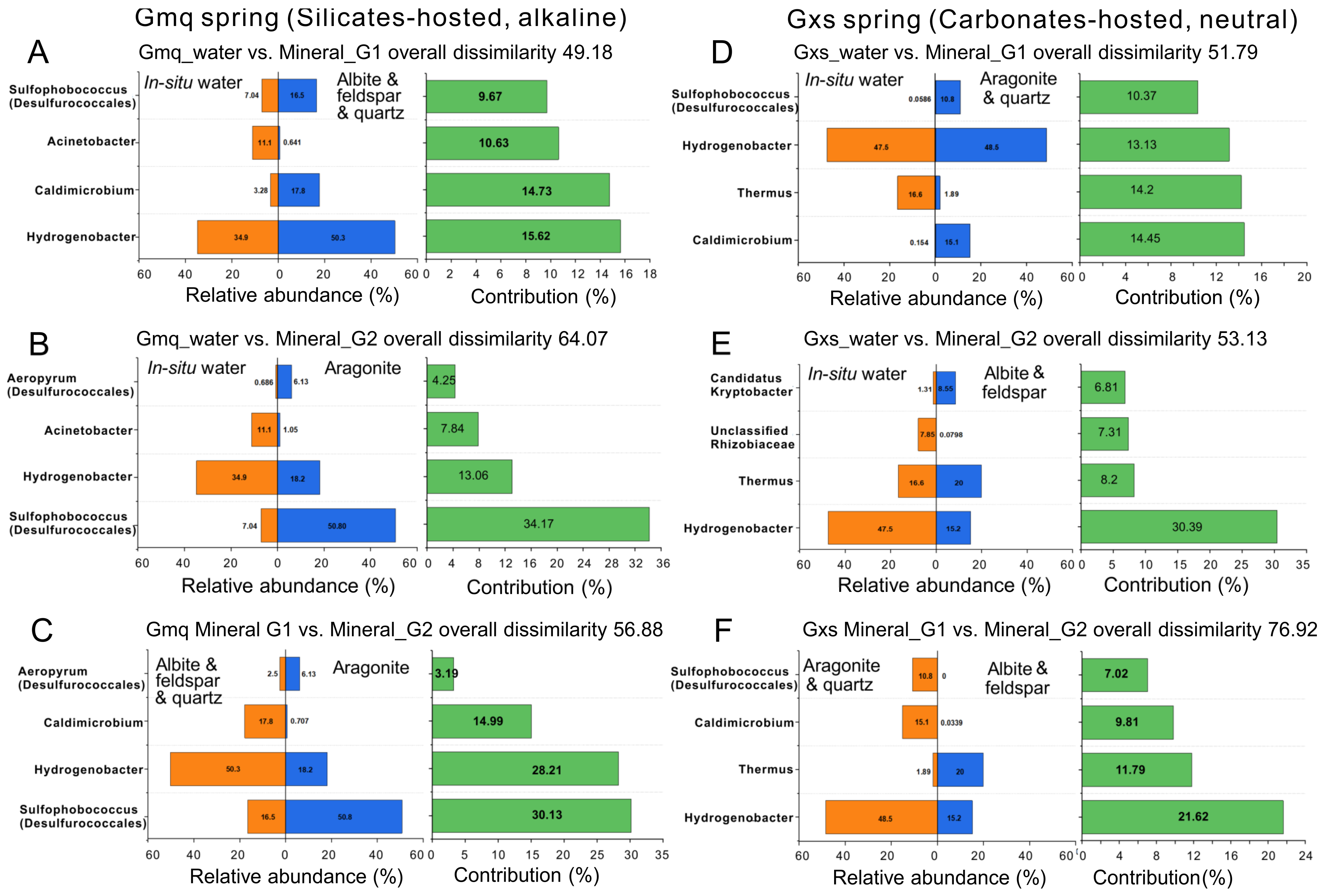
3.2. Microbial Community Composition in Gmq In-Situ Water and Mineral Microcosms
3.3. Microbial Community Composition in Gxs In-Situ Water and Mineral Microcosms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morrison, S.M.; Buongiorno, J.; Downs, R.T.; Eleish, A.; Fox, P.; Giovannelli, D.; Golden, J.J.; Hummer, D.R.; Hystad, G.; Kellogg, L.H.; et al. Exploring Carbon Mineral Systems: Recent Advances in C Mineral Evolution, Mineral Ecology, and Network Analysis. Front. Earth Sci. 2020, 8, 208. [Google Scholar] [CrossRef]
- Hazen, R. Evolution of Minerals. Sci. Am. 2010, 302, 58–65. [Google Scholar] [CrossRef]
- Delgado-Serrano, L.; Lopez, G.; Bohorquez, L.C.; Bustos, J.R.; Rubiano, C.; Osorio-Forero, C.; Junca, H.; Baena, S.; Zambrano, M.M. Neotropical Andes hot springs harbor diverse and distinct planktonic microbial communities. FEMS Microbiol. Ecol. 2014, 89, 56–66. [Google Scholar] [CrossRef]
- Hedlund, B.P.; Murugapiran, S.K.; Alba, T.W.; Levy, A.; Dodsworth, J.A.; Goertz, G.B.; Ivanova, N.; Woyke, T. Uncultivated thermophiles: Current status and spotlight on ‘Aigarchaeota’. Curr. Opin. Microbiol. 2015, 25, 136–145. [Google Scholar] [CrossRef]
- Hua, Z.S.; Qu, Y.N.; Zhu, Q.; Zhou, E.M.; Qi, Y.L.; Yin, Y.R.; Rao, Y.Z.; Tian, Y.; Li, Y.X.; Liu, L.; et al. Genomic inference of the metabolism and evolution of the archaeal phylum Aigarchaeota. Nat. Commun. 2018, 9, 2832. [Google Scholar] [CrossRef] [PubMed]
- Mall, A.; Sobotta, J.; Huber, C.; Tschirner, C.; Kowarschik, S.; Bacnik, K.; Mergelsberg, M.; Boll, M.; Hugler, M.; Eisenreich, W.; et al. Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium. Science 2018, 359, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.S.; Wang, Y.L.; Evans, P.N.; Qu, Y.N.; Goh, K.M.; Rao, Y.Z.; Qi, Y.L.; Li, Y.X.; Huang, M.J.; Jiao, J.Y.; et al. Insights into the ecological roles and evolution of methyl-coenzyme M reductase-containing hot spring Archaea. Nat. Commun. 2019, 10, 4574. [Google Scholar] [CrossRef] [PubMed]
- Nunoura, T.; Chikaraishi, Y.; Izaki, R.; Suwa, T.; Sato, T.; Harada, T.; Mori, K.; Kato, Y.; Miyazaki, M.; Shimamura, S.; et al. A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science 2018, 359, 559–562. [Google Scholar] [CrossRef]
- Lacap, D.C.; Barraquio, W.; Pointing, S.B. Thermophilic microbial mats in a tropical geothermal location display pronounced seasonal changes but appear resilient to stochastic disturbance. Environ. Microbiol. 2007, 9, 3065–3076. [Google Scholar] [CrossRef]
- Briggs, B.R.; Brodie, E.L.; Tom, L.M.; Dong, H.; Jiang, H.; Huang, Q.; Wang, S.; Hou, W.; Wu, G.; Huang, L.; et al. Seasonal patterns in microbial communities inhabiting the hot springs of Tengchong, Yunnan Province, China. Environ. Microbiol. 2014, 16, 1579–1591. [Google Scholar] [CrossRef]
- Wang, S.; Dong, H.; Hou, W.; Jiang, H.; Huang, Q.; Briggs, B.R.; Huang, L. Greater temporal changes of sediment microbial community than its waterborne counterpart in Tengchong hot springs, Yunnan Province, China. Sci. Rep. 2014, 4, 7479. [Google Scholar] [CrossRef]
- Merkel, A.Y.; Pimenov, N.V.; Rusanov, I.I.; Slobodkin, A.I.; Slobodkina, G.B.; Tarnovetckii, I.Y.; Frolov, E.N.; Dubin, A.V.; Perevalova, A.A.; Bonch-Osmolovskaya, E.A. Microbial diversity and autotrophic activity in Kamchatka hot springs. Extremophiles 2017, 21, 307–317. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Power, J.F.; Washburne, A.; Cary, S.C.; Stott, M.B.; Fierer, N. The ecology and diversity of microbial eukaryotes in geothermal springs. ISME J. 2018, 12, 1918–1928. [Google Scholar] [CrossRef]
- Power, J.F.; Carere, C.R.; Lee, C.K.; Wakerley, G.L.J.; Evans, D.W.; Button, M.; White, D.; Climo, M.D.; Hinze, A.M.; Morgan, X.C.; et al. Microbial biogeography of 925 geothermal springs in New Zealand. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Colman, D.R.; Lindsay, M.R.; Boyd, E.S. Mixing of meteoric and geothermal fluids supports hyperdiverse chemosynthetic hydrothermal communities. Nat. Commun. 2019, 10, 681. [Google Scholar] [CrossRef]
- Li, L.W.; Li, W.; Zou, Q.; Ma, Z.S. Network analysis of the hot spring microbiome sketches out possible niche differentiations among ecological guilds. Ecol. Model. 2020, 431, 109147. [Google Scholar] [CrossRef]
- Podar, P.T.; Yang, Z.M.; Bjornsdottir, S.H.; Podar, M. Comparative Analysis of Microbial Diversity Across Temperature Gradients in Hot Springs From Yellowstone and Iceland. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Kawano, M.; Tomita, K. Geochemical modeling of bacterially induced mineralization of schwertmannite and jarosite in sulfuric acid spring water. Am. Mineral. 2001, 86, 1156–1165. [Google Scholar] [CrossRef]
- Phillips-Lander, C.M.; Fowle, D.A.; Taunton, A.; Hernandez, W.; Mora, M.; Moore, D.; Shinogle, H.; Roberts, J.A. Silicate Dissolution in Las Pailas Thermal Field: Implications for Microbial Weathering in Acidic Volcanic Hydrothermal Spring Systems. Geomicrobiol. J. 2013, 31, 23–41. [Google Scholar] [CrossRef]
- Shock, E.L.; Holland, M.; Meyer-Dombard, D.; Amend, J.P.; Osburn, G.R.; Fischer, T.P. Quantifying inorganic sources of geochemical energy in hydrothermal ecosystems, Yellowstone National Park, USA. Geochim. Cosmochim. Acta 2010, 74, 4005–4043. [Google Scholar] [CrossRef]
- Amenabar, M.J.; Shock, E.L.; Roden, E.E.; Peters, J.W.; Boyd, E.S. Microbial substrate preference dictated by energy demand rather than supply. Nat. Geosci. 2017, 10, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Amenabar, M.J.; Boyd, E.S. A review of the mechanisms of mineral-based metabolism in early Earth analog rock-hosted hydrothermal ecosystems. World J. Microbiol. Biotechnol. 2019, 35, 29. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, Y.H.; Yang, Y.F.; He, Z.L.; Luo, F.; Zhou, J.Z. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Han, X.; Deng, Y. Higher precipitation strengthens the microbial interactions in semi-arid grassland soils. Glob. Ecol. Biogeogr. 2018, 27, 570–580. [Google Scholar] [CrossRef]
- Buongiorno, J.; Herbert, L.C.; Wehrmann, L.M.; Michaud, A.B.; Laufer, K.; Roy, H.; Jorgensen, B.B.; Szynkiewicz, A.; Faiia, A.; Yeager, K.M.; et al. Complex Microbial Communities Drive Iron and Sulfur Cycling in Arctic Fjord Sediments. Appl. Environ. Microbiol. 2019, 85, 14. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef]
- Wild, B.; Daval, D.; Beaulieu, E.; Pierret, M.-C.; Viville, D.; Imfeld, G. In-situ dissolution rates of silicate minerals and associated bacterial communities in the critical zone (Strengbach catchment, France). Geochim. Cosmochim. Acta 2019, 249, 95–120. [Google Scholar] [CrossRef]
- Ahmed, E.; Hugerth, L.W.; Logue, J.B.; Brüchert, V.; Andersson, A.F.; Holmström, S.J.M. Mineral Type Structures Soil Microbial Communities. Geomicrobiol. J. 2016, 34, 538–545. [Google Scholar] [CrossRef]
- Hutchens, E. Microbial selectivity on mineral surfaces: Possible implications for weathering processes. Fungal Biol. Rev. 2009, 23, 115–121. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Lafrenière, M.J.; Skidmore, M.L.; Boyd, E.S. Influence of bedrock mineral composition on microbial diversity in a subglacial environment. Geology 2013, 41, 855–858. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennett, P.C. Mineral stimulation of subsurface microorganisms: Release of limiting nutrients from silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Whitman, T.; Neurath, R.; Perera, A.; Chu-Jacoby, I.; Ning, D.; Zhou, J.; Nico, P.; Pett-Ridge, J.; Firestone, M. Microbial community assembly differs across minerals in a rhizosphere microcosm. Environ. Microbiol. 2018, 20, 4444–4460. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Zhou, H.; Yang, J.; Ge, H.; Jiao, N.; Luan, X.; Zhang, C.; Klotz, M.G. Thaumarchaeotal signature gene distribution in sediments of the northern South China Sea: An indicator of the metabolic intersection of the marine carbon, nitrogen, and phosphorus cycles? Appl. Environ. Microbiol. 2013, 79, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A. Inhibition and enhancement of microbial surface colonization: The role of silicate composition. Chem. Geol. 2004, 212, 313–327. [Google Scholar] [CrossRef]
- Lepleux, C.; Turpault, M.P.; Oger, P.; Frey-Klett, P.; Uroz, S. Correlation of the Abundance of Betaproteobacteria on Mineral Surfaces with Mineral Weathering in Forest Soils. Appl. Environ. Microbiol. 2012, 78, 7114–7119. [Google Scholar] [CrossRef]
- Kelly, L.C.; Colin, Y.; Turpault, M.P.; Uroz, S. Mineral Type and Solution Chemistry Affect the Structure and Composition of Actively Growing Bacterial Communities as Revealed by Bromodeoxyuridine Immunocapture and 16S rRNA Pyrosequencing. Microb. Ecol. 2016, 72, 428–442. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Hou, W.; Feng, K.; Li, F.; Hai, W.; Zhang, Y.; Sun, Y.; Deng, Y. Temperature and microbial interactions drive the deterministic assembly processes in sediments of hot springs. Sci Total Environ. 2021, 772, 145465. [Google Scholar] [CrossRef]
- Hedlund, B.P.; Cole, J.K.; Williams, A.J.; Hou, W.; Zhou, E.; Li, W.; Dong, H. A review of the microbiology of the Rehai geothermal field in Tengchong, Yunnan Province, China. Geosci. Front. 2012, 3, 273–288. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, C.Q.; Liu, H.; Jin, Z.; Han, G.; Li, L. Geochemistry of the Rehai and Ruidian geothermal waters, Yunnan Province, China. Geothermics 2008, 37, 73–83. [Google Scholar] [CrossRef]
- Hou, W.; Wang, S.; Dong, H.; Jiang, H.; Briggs, B.R.; Peacock, J.P.; Huang, Q.; Huang, L.; Wu, G.; Zhi, X.; et al. A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS ONE 2013, 8, e53350. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput. Biol. 2012, 8. [Google Scholar] [CrossRef]
- Carr, A.; Diener, C.; Baliga, N.S.; Gibbons, S.M. Use and abuse of correlation analyses in microbial ecology. ISME J. 2019, 13, 2647–2655. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Aronesty, E. Comparison of Sequencing Utility Programs. Open Bioinforma. J. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.L.; McGarrell, D.M.; Sun, Y.N.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Sokal, R.; Rohlf, F. Biometry: The Principles and Practice of Statistics in Biological Research; WH Freeman & Co.: New York, NY, USA, 1995. [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Jiao, S.; Liu, Z.; Lin, Y.; Yang, J.; Chen, W.; Wei, G. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577. [Google Scholar] [CrossRef]
- Hensel, R. Sulfophobococcus zilligii gen. nov., spec. nov. a Novel Hyperthermophilic Archaeum Isolated from Hot Alkaline Springs of Iceland. Syst. Appl. Microbiol. 1997, 20, 102–110. [Google Scholar] [CrossRef]
- Miroshnichenko, M.L.; Lebedinsky, A.V.; Chernyh, N.A.; Tourova, T.P.; Kolganova, T.V.; Spring, S.; Bonch-Osmolovskaya, E.A. Caldimicrobium rimae gen. nov., sp nov., an extremely thermophilic, facultatively lithoautotrophic, anaerobic bacterium from the Uzon Caldera, Kamchatka. Int. J. Syst. Evol. Microbiol. 2009, 59, 1040–1044. [Google Scholar] [CrossRef][Green Version]
- Sako, Y.; Nomura, N.; Uchida, A.; Ishida, Y.; Morii, H.; Koga, Y.; Hoaki, T.; Maruyama, T. Aeropyrum pernix gen. nov., sp. nov., a Novel Hyperthermophilic Archaeon Growing Temperatures up to 100 °C. Int. J. Syst. Evol. Microbiol. 1996, 46, 1070–1077. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Paez-Espino, D.; Jarett, J.; Dunfield, P.F.; Hedlund, B.P.; Dekas, A.E.; Grasby, S.E.; Brady, A.L.; Dong, H.; Briggs, B.R.; et al. Global metagenomic survey reveals a new bacterial candidate phylum in geothermal springs. Nat. Commun. 2016, 7, 10476. [Google Scholar] [CrossRef]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling Interactions in the Microbiome: A Network Perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Calcagno, V.; Jarne, P.; Loreau, M.; Mouquet, N.; David, P. Diversity spurs diversification in ecological communities. Nat. Commun. 2017, 8, 15810. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Widder, S.; Besemer, K.; Singer, G.A.; Ceola, S.; Bertuzzo, E.; Quince, C.; Sloan, W.T.; Rinaldo, A.; Battin, T.J. Fluvial network organization imprints on microbial co-occurrence networks. Proc. Natl. Acad. Sci. USA 2014, 111, 12799–12804. [Google Scholar] [CrossRef]
- Hedlund, B.P.; Reysenbach, A.L.; Huang, L.; Ong, J.C.; Liu, Z.; Dodsworth, J.A.; Ahmed, R.; Williams, A.J.; Briggs, B.R.; Liu, Y.; et al. Isolation of diverse members of the Aquificales from geothermal springs in Tengchong, China. Front. Microbiol. 2015, 6, 157. [Google Scholar] [CrossRef]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.K.; Peacock, J.P.; Dodsworth, J.A.; Williams, A.J.; Thompson, D.B.; Dong, H.L.; Wu, G.; Hedlund, B.P. Sediment microbial communities in Great Boiling Spring are controlled by temperature and distinct from water communities. ISME J. 2013, 7, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Certini, G.; Campbell, C.D.; Edwards, A.C. Rock fragments in soil support a different microbial community from the fine earth. Soil Biol. Biochem. 2004, 36, 1119–1128. [Google Scholar] [CrossRef]
- Jones, A.A.; Bennett, P.C. Mineral Microniches Control the Diversity of Subsurface Microbial Populations. Geomicrobiol. J. 2014, 31, 246–261. [Google Scholar] [CrossRef]
- Dockrey, J.; Lindsay, M.; Mayer, K.; Beckie, R.; Norlund, K.; Warren, L.; Southam, G. Acidic Microenvironments in Waste Rock Characterized by Neutral Drainage: Bacteria–Mineral Interactions at Sulfide Surfaces. Minerals 2014, 4, 170–190. [Google Scholar] [CrossRef]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Imai, H.; Terada, T.; Miura, T.; Yamabi, S. Self-organized formation of porous aragonite with silicate. J. Cryst. Growth 2002, 244, 200–205. [Google Scholar] [CrossRef]
- Druppel, K.; Wirth, R. Metasomatic Replacement of Albite in Nature and Experiments. Minerals 2018, 8, 214. [Google Scholar] [CrossRef]


| Spring Name | Location | Spring ID | Conductivity mS/cm | pH | Temperature °C | Main Minerals |
|---|---|---|---|---|---|---|
| Source | GmqS | 4.0 | 9.35 | 93 | quartz, feldspar | |
| Gumaguma | Channel | GmqC | 4.0 | 9.36 | 89 | quartz, aragonite, goethite |
| Pool | GmqP | 3.9 | 9.30 | 82.5 | quartz, feldspar | |
| Gongxiaoshe | Gxs | 7.29 | 73.8 | aragonite, calcite |
| Module No. | Node | Intra-Module Edges | Inter-Module Edges | Related Abiotic Factors | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| 1 | 88 | 663 | 53 | 61 | 81 | pH, K+, Ca2+, Na+, TN, NH4+, SO42−, NO3−, Fe2+ |
| 2 | 79 | 430 | 49 | 21 | 7 | Temperature, DOC, Biotite |
| 3 | 63 | 445 | 117 | 18 | 11 | TOC, Kaolinite, Smectite, Calcite, Aragonite, Quartz, Gypsum, K-feldspar |
| 4 | 59 | 680 | 57 | 32 | 64 | Oxygen, Mg, F− |
| 5 | 37 | 237 | 15 | 47 | 23 | Total Fe, Cl− |
| 6 | 21 | 62 | 0 | 9 | 0 | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, S.; He, Q.; Zhang, W.; Guo, D.; Zhang, Y.; Hai, W.; Sun, Y.; Dong, H.; Hou, W. Minerals Determined a Special Ecological Niche and Selectively Enriched Microbial Species from Bulk Water Communities in Hot Springs. Microorganisms 2021, 9, 1020. https://doi.org/10.3390/microorganisms9051020
Li F, Wang S, He Q, Zhang W, Guo D, Zhang Y, Hai W, Sun Y, Dong H, Hou W. Minerals Determined a Special Ecological Niche and Selectively Enriched Microbial Species from Bulk Water Communities in Hot Springs. Microorganisms. 2021; 9(5):1020. https://doi.org/10.3390/microorganisms9051020
Chicago/Turabian StyleLi, Fangru, Shang Wang, Qing He, Wenhui Zhang, Dongyi Guo, Yidi Zhang, Wanming Hai, Yuxuan Sun, Hailiang Dong, and Weiguo Hou. 2021. "Minerals Determined a Special Ecological Niche and Selectively Enriched Microbial Species from Bulk Water Communities in Hot Springs" Microorganisms 9, no. 5: 1020. https://doi.org/10.3390/microorganisms9051020
APA StyleLi, F., Wang, S., He, Q., Zhang, W., Guo, D., Zhang, Y., Hai, W., Sun, Y., Dong, H., & Hou, W. (2021). Minerals Determined a Special Ecological Niche and Selectively Enriched Microbial Species from Bulk Water Communities in Hot Springs. Microorganisms, 9(5), 1020. https://doi.org/10.3390/microorganisms9051020






