Invasive Pneumococcal Disease in Adults in Portugal: The Importance of Serotypes 8 and 3 (2015–2018)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Isolate Collection
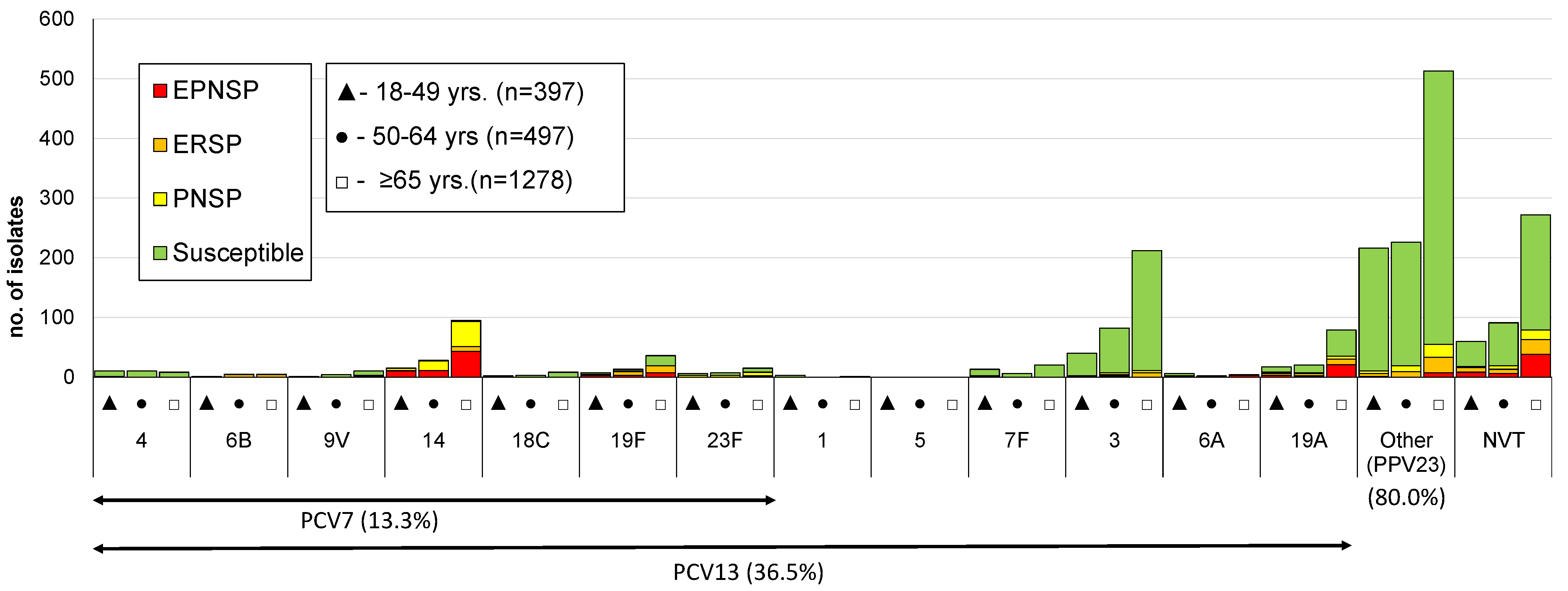
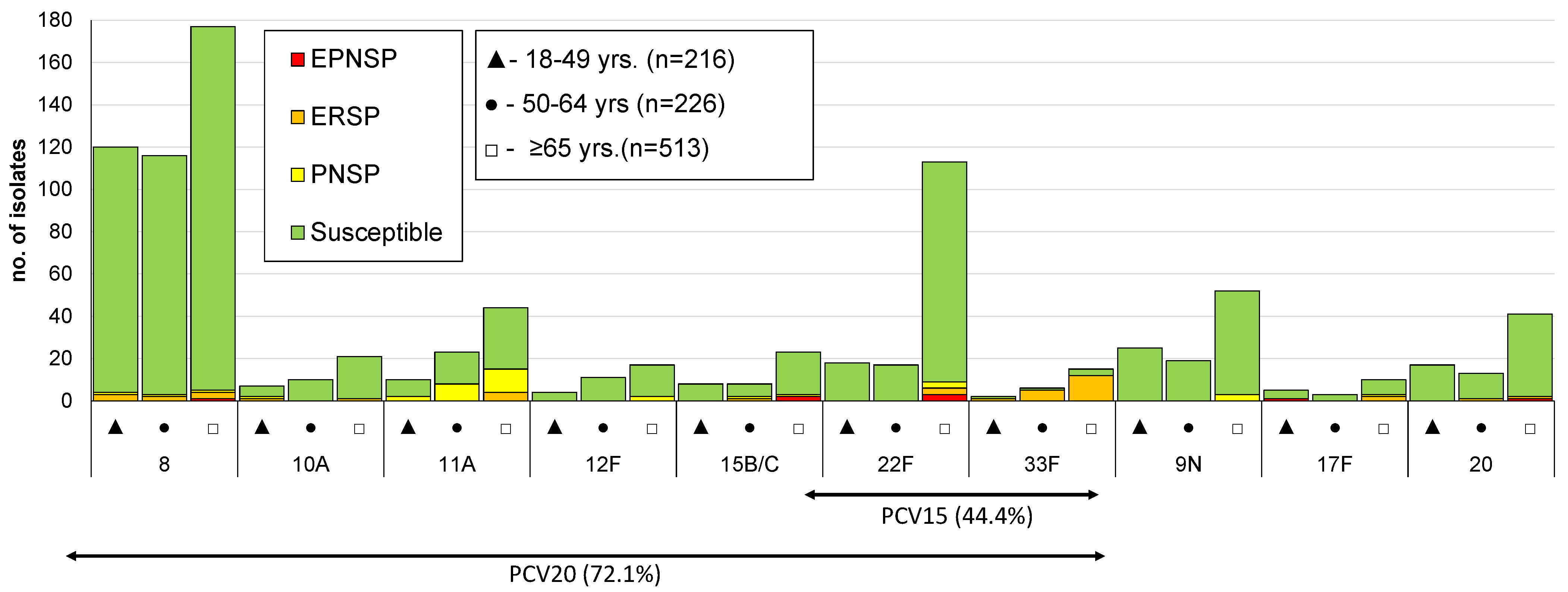
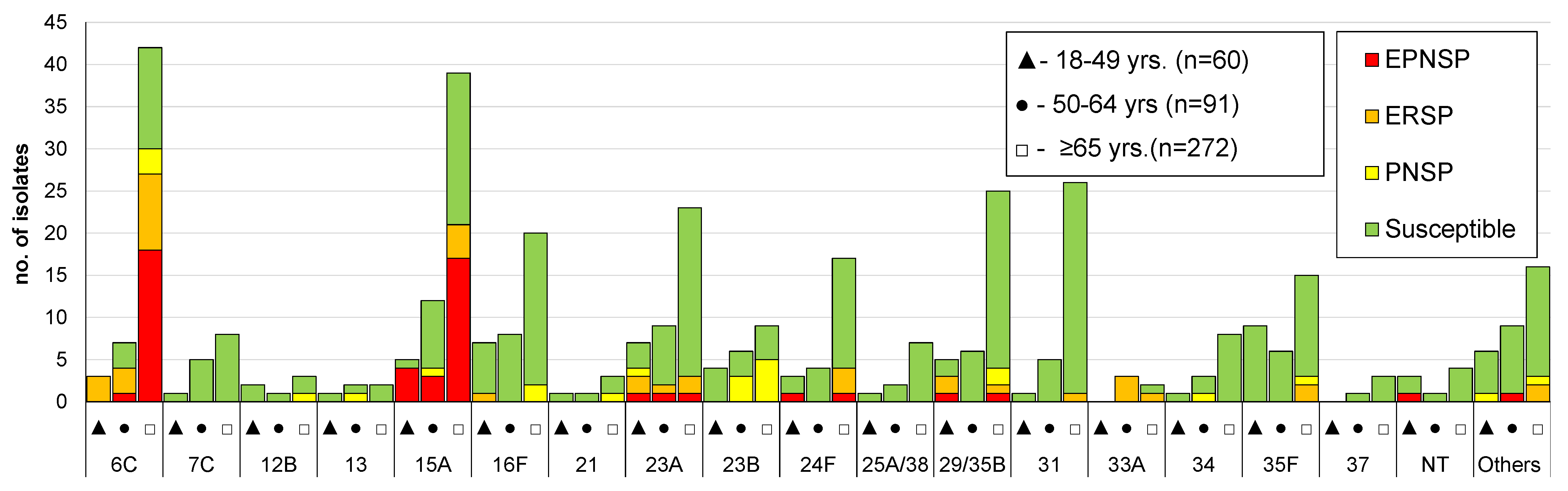
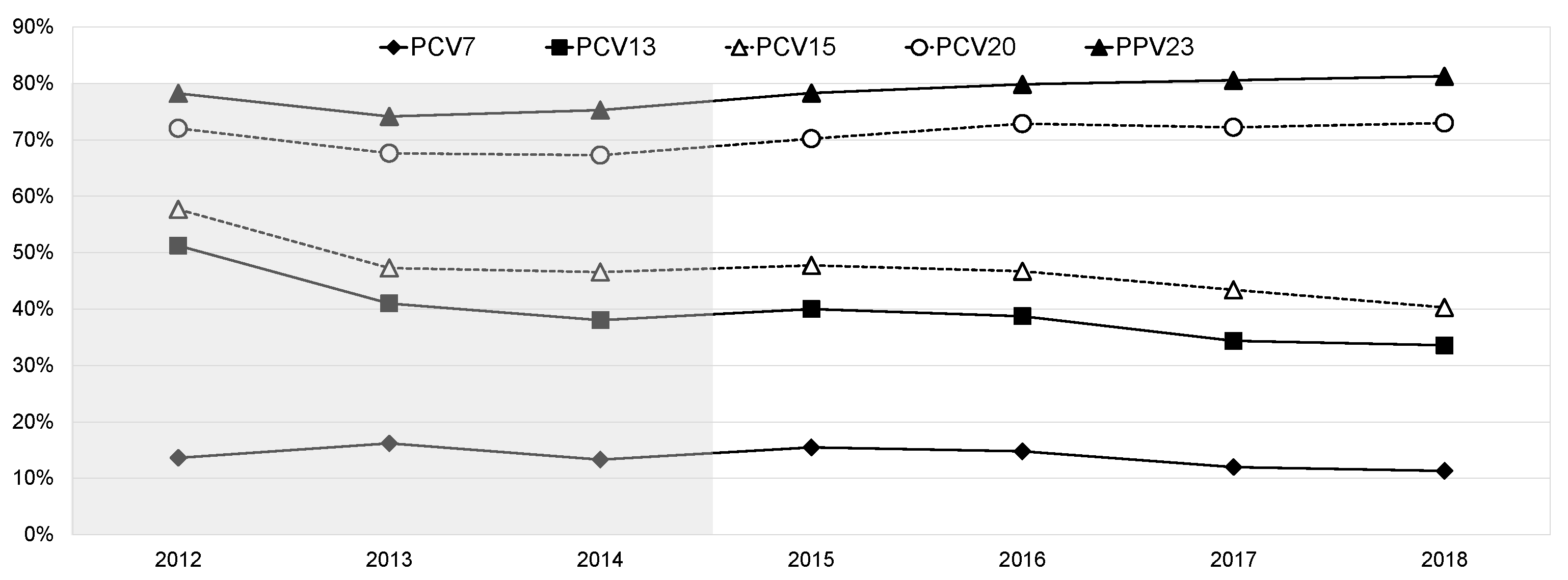
3.2. Serotype Distribution
3.3. Antimicrobial Susceptibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horácio, A.N.; Silva-Costa, C.; Lopes, J.P.; Ramirez, M.; Melo-Cristino, J.; Portuguese Group for the Study of Streptococcal Infections. Serotype 3 Remains the Leading Cause of Invasive Pneumococcal Disease in Adults in Portugal (2012–2014) despite Continued Reductions in Other 13-Valent Conjugate Vaccine Serotypes. Front. Microbiol. 2016, 7, 1616. [Google Scholar] [CrossRef]
- Horácio, A.N.; Diamantino-Miranda, J.; Aguiar, S.I.; Ramirez, M.; Melo-Cristino, J.; The Portuguese Group for the Study of Streptococcal Infections. The Majority of Adult Pneumococcal Invasive Infections in Portugal Are Still Potentially Vaccine Preventable in Spite of Significant Declines of Serotypes 1 and 5. PLoS ONE 2013, 8, e73704. [Google Scholar] [CrossRef]
- Horácio, A.N.; Diamantino-Miranda, J.; Aguiar, S.I.; Ramirez, M.; Melo-Cristino, J.; The Portuguese Group for the Study of Streptococcal Infections. Serotype Changes in Adult Invasive Pneumococcal Infections in Portugal Did Not Reduce the High Fraction of Potentially Vaccine Preventable Infections. Vaccine 2012, 30, 218–224. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, S.; Domenech, M.; González-Camacho, F.; Sempere, J.; Vicioso, D.; Sanz, J.C.; García Comas, L.; Ardanuy, C.; Fenoll, A.; Yuste, J. Nationwide Trends of Invasive Pneumococcal Disease in Spain (2009-2019) in Children and Adults during the Pneumococcal Conjugate Vaccine Era. Clin. Infect. Dis. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Paran, Y.; Bishara, J.; Oren, I.; Chowers, M.; Tziba, Y.; Istomin, V.; Weinberger, M.; Miron, D.; Temper, V.; et al. Early Impact of PCV7/PCV13 Sequential Introduction to the National Pediatric Immunization Plan, on Adult Invasive Pneumococcal Disease: A Nationwide Surveillance Study. Vaccine 2015, 33, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Waight, P.A.; Andrews, N.J.; Ladhani, S.N.; Sheppard, C.L.; Slack, M.P.E.; Miller, E. Effect of the 13-Valent Pneumococcal Conjugate Vaccine on Invasive Pneumococcal Disease in England and Wales 4 Years after Its Introduction: An Observational Cohort Study. Lancet Infect. Dis. 2015, 15, 535–543. [Google Scholar] [CrossRef]
- Costa, R.P.; Gonçalves, C.; Sousa, J.C. de A doença pneumocócica e recomendações GRESP para a vacinação antipneumocócica na população adulta (≥18 anos). Rev. Port. Med. Geral E Fam. 2016, 32, 70–74. [Google Scholar]
- Direcção Geral de Saúde Norma 11/2015—Vacinação Contra Infeções Por Streptococcus Pneumoniae de Grupos Com Risco Acrescido Para Doença Invasiva Pneumocócica (DIP). Adultos (≥18 Anos de Idade). 2015. Available online: http://nocs.pt/wp-content/uploads/2017/11/i021902.pdf (accessed on 11 May 2016).
- Sousa, M.; Cavadas, L.F.; Santos, R.B.; Macedo, A. Avaliação Da Qualidade Da Prescrição Da Vacina Anti-Pneumocócica Aos Idosos. Rev. Port. Clín. Geral 2009, 25, 531–536. [Google Scholar] [CrossRef]
- Hurley, D.; Griffin, C.; Young, M.; Scott, D.A.; Pride, M.W.; Scully, I.L.; Ginis, J.; Severs, J.; Jansen, K.U.; Gruber, W.C.; et al. Safety, Tolerability, and Immunogenicity of a 20-Valent Pneumococcal Conjugate Vaccine (Pcv20) in Adults 60 to 64 Years of Age. Clin. Infect. Dis. 2020, in press. [Google Scholar] [CrossRef]
- Peterson, J.T.; Stacey, H.L.; MacNair, J.E.; Li, J.; Hartzel, J.S.; Sterling, T.M.; Benner, P.; Tamms, G.M.; Musey, L.K. Safety and Immunogenicity of 15-Valent Pneumococcal Conjugate Vaccine Compared to 13-Valent Pneumococcal Conjugate Vaccine in Adults ≥65 Years of Age Previously Vaccinated with 23-Valent Pneumococcal Polysaccharide Vaccine. Hum. Vaccines Immunother. 2019, 15, 540–548. [Google Scholar] [CrossRef]
- Serrano, I.; Ramirez, M.; Melo-Cristino, J.; Portuguese Surveillance Group for the Study of Respiratory Pathogens. Invasive Streptococcus Pneumoniae from Portugal: Implications for Vaccination and Antimicrobial Therapy. Clin. Microbiol. Infect. 2004, 10, 652–656. [Google Scholar] [CrossRef]
- Sørensen, U.B. Typing of Pneumococci by Using 12 Pooled Antisera. J. Clin. Microbiol. 1993, 31, 2097–2100. [Google Scholar] [CrossRef]
- Spencer, B.L.; Shenoy, A.T.; Orihuela, C.J.; Nahm, M.H. The Pneumococcal Serotype 15C Capsule Is Partially O-Acetylated and Allows for Limited Evasion of PPV23-Elicited Anti-Serotype 15B Antibodies. Clin. Vaccine Immunol. CVI 2017, 24, e00099-17. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Melo-Cristino, J.; Ramirez, M.; Serrano, N.; Hänscheid, T.; The Portuguese Surveillance Group for the Study of Respiratory Pathogens. Macrolide Resistance in Streptococcus Pneumoniae Isolated from Patients with Community-Acquired Lower Respiratory Tract Infections in Portugal: Results of a 3-Year (1999–2001) Multicenter Surveillance Study. Microb. Drug Resist. 2003, 9, 73–80. [Google Scholar] [CrossRef]
- Carriço, J.A.; Silva-Costa, C.; Melo-Cristino, J.; Pinto, F.R.; de Lencastre, H.; Almeida, J.S.; Ramirez, M. Illustration of a Common Framework for Relating Multiple Typing Methods by Application to Macrolide-Resistant Streptococcus Pyogenes. J. Clin. Microbiol. 2006, 44, 2524–2532. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Shiri, T.; Datta, S.; Madan, J.; Tsertsvadze, A.; Royle, P.; Keeling, M.J.; McCarthy, N.D.; Petrou, S. Indirect Effects of Childhood Pneumococcal Conjugate Vaccination on Invasive Pneumococcal Disease: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e51–e59. [Google Scholar] [CrossRef]
- Hanquet, G.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.N.; Nuorti, J.P.; Lepoutre, A.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effect of Childhood Pneumococcal Conjugate Vaccination on Invasive Disease in Older Adults of 10 European Countries: Implications for Adult Vaccination. Thorax 2019, 74, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, N.; Varon, E.; Levy, C.; Angoulvant, F.; Georges, S.; Ploy, M.-C.; Kempf, M.; Cremniter, J.; Cohen, R.; Bruhl, D.L.; et al. Invasive Pneumococcal Disease Incidence in Children and Adults in France during the Pneumococcal Conjugate Vaccine Era: An Interrupted Time-Series Analysis of Data from a 17-Year National Prospective Surveillance Study. Lancet Infect. Dis. 2021, 21, 137–147. [Google Scholar] [CrossRef]
- Amin-Chowdhury, Z.; Collins, S.; Sheppard, C.; Litt, D.; Fry, N.K.; Andrews, N.; Ladhani, S.N. Characteristics of Invasive Pneumococcal Disease Caused by Emerging Serotypes after the Introduction of the 13-Valent Pneumococcal Conjugate Vaccine in England: A Prospective Observational Cohort Study, 2014–2018. Clin. Infect. Dis. 2020, 71, e235–e243. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, G.; Garcia-Garcia, S.; Lanaspa, M.; Ciruela, P.; Esteva, C.; Fernandez de Sevilla, M.; Diaz-Conradi, A.; Marti, C.; Motje, M.; Galles, C.; et al. Serotype and Clonal Distribution Dynamics of Invasive Pneumococcal Strains after PCV13 Introduction (2011–2016): Surveillance Data from 23 Sites in Catalonia, Spain. PLoS ONE 2020, 15, e0228612. [Google Scholar] [CrossRef] [PubMed]
- Wijayasri, S.; Hillier, K.; Lim, G.H.; Harris, T.M.; Wilson, S.E.; Deeks, S.L. The Shifting Epidemiology and Serotype Distribution of Invasive Pneumococcal Disease in Ontario, Canada, 2007–2017. PLoS ONE 2019, 14, e0226353. [Google Scholar] [CrossRef] [PubMed]
- Zintgraff, J.; Fossati, S.; Pereira, C.S.; Veliz, O.; Regueira, M.; Moscoloni, M.A.; Irazu, L.; Lara, C.; Napoli, D.; Argentina Spn Working Group. Distribution of PCV13 and PPSV23 Streptococcus Pneumoniae Serotypes in Argentinean Adults with Invasive Disease, 2013–2017. Rev. Argent. Microbiol. 2020, 52, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, K.; Kosai, K.; Mikamo, H.; Mukae, H.; Takesue, Y.; Abe, M.; Taniguchi, K.; Petigara, T.; Kaku, M. Serotype Distribution and Antimicrobial Susceptibility of Streptococcus Pneumoniae Associated with Invasive Pneumococcal Disease among Adults in Japan. Int. J. Infect. Dis. 2021, 102, 260–268. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hanage, W.P. Making Sense of Differences in Pneumococcal Serotype Replacement. Lancet Infect. Dis. 2019, 19, e213–e220. [Google Scholar] [CrossRef]
- Hespanhol, V.; Bárbara, C. Pneumonia Mortality, Comorbidities Matter? Pulmonology 2020, 26, 123–129. [Google Scholar] [CrossRef]
| Serotype | No of Isolates (%) | CA 1 | ||
|---|---|---|---|---|
| 18–49 Years | 50–64 Years | ≥65 Years | ||
| 8 | 120 (30.2) | 116 (23.3) | 177 (13.8) | <0.001 |
| 3 | 40 (10.1) | 82 (16.5) | 212 (16.6) | 0.005 |
| 22F | 18 (4.5) | 17 (3.4) | 113 (8.8) | <0.001 |
| 14 | 15 (3.8) | 28 (5.6) | 95 (7.4) | 0.007 |
| 19A | 17 (4.3) | 20 (4.0) | 79 (6.2) | 0.068 |
| 9N | 25 (6.3) | 19 (3.8) | 52 (4.1) | 0.109 |
| 11A | 10 (2.5) | 23 (4.6) | 44 (3.4) | 0.682 |
| 20 | 17 (4.3) | 13 (2.6) | 41 (3.2) | 0.455 |
| 15A | 5 (1.3) | 12 (2.4) | 39 (3.0) | 0.050 |
| 19F | 7 (1.8) | 13 (2.6) | 36 (2.8) | 0.276 |
| 6C | 3 (0.8) | 7 (1.4) | 42 (3.3) | 0.001 |
| 7F | 13 (3.3) | 6 (1.2) | 20 (1.6) | 0.067 |
| 15B/C | 8 (2.0) | 8 (1.6) | 23 (1.8) | 0.863 |
| 23A | 7 (1.8) | 9 (1.8) | 23 (1.8) | 0.972 |
| 10A | 7 (1.8) | 10 (2.0) | 21 (1.6) | 0.765 |
| 16F | 7 (1.8) | 8 (1.6) | 20 (1.6) | 0.792 |
| 29/35B | 3 (0.8) | 5 (1.0) | 25 (2.0) | 0.053 |
| 31 | 1 (0.3) | 5 (1.0) | 26 (2.0) | 0.006 |
| 12F | 4 (1.0) | 11 (2.2) | 17 (1.3) | 0.998 |
| 35F | 9 (2.3) | 6 (1.2) | 15 (1.2) | 0.145 |
| 4 | 10 (2.5) | 10 (2.0) | 8 (0.6) | 0.001 |
| 23F | 6 (1.5) | 7 (1.4) | 15 (1.2) | 0.564 |
| 24F | 3 (0.8) | 4 (0.8) | 17 (1.3) | 0.262 |
| 33F | 2 (0.5) | 6 (1.2) | 15 (1.2) | 0.324 |
| Serotype 1 | No. of Isolates (%) | CA 2 | CA 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Current Study Period | |||||||||
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2015–2018 | 2012–2018 | |
| PCV13 | |||||||||
| 1 | 12 (3.0) | 7 (1.8) | 7 (1.9) | 2 (0.4) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0.063 | <0.001 |
| 3 | 66 (16.3) | 45 (11.7) | 50 (13.3) | 72 (13.6) | 79 (15.8) | 84 (14.6) | 99 (17.5) | 0.125 | 0.154 |
| 4 | 6 (1.5) | 8 (2.1) | 9 (2.4) | 7 (1.3) | 8 (1.6) | 8 (1.4) | 5 (0.9) | 0.478 | 0.171 |
| 6A | 2 (0.5) | 1 (0.3) | 4 (1.1) | 4 (0.8) | 3 (0.6) | 1 (0.2) | 4 (0.7) | 0.698 | 0.937 |
| 6B | 5 (1.2) | 5 (1.3) | 5 (1.3) | 3 (0.6) | 1 (0.2) | 6 (1.0) | 1 (0.2) | 0.795 | 0.031 |
| 7F | 33 (8.2) | 18 (4.7) | 10 (2.7) | 16 (3.0) | 9 (1.8) | 7 (1.2) | 7 (1.2) | 0.020 | <0.001 |
| 9V | 4 (1.0) | 4 (1.0) | 1 (0.3) | 7 (1.3) | 1 (0.2) | 3 (0.5) | 4 (0.7) | 0.340 | 0.344 |
| 14 | 29 (7.2) | 26 (6.8) | 18 (4.8) | 39 (7.4) | 41 (8.2) | 31 (5.4) | 27 (4.8) | 0.024 | 0.196 |
| 18C | 1 (0.2) | 4 (1.0) | 2 (0.5) | 3 (0.6) | 3 (0.6) | 5 (0.9) | 2 (0.4) | 0.795 | 0.998 |
| 19A | 39 (9.7) | 24 (6.3) | 21 (5.6) | 36 (6.8) | 27 (5.4) | 37 (6.4) | 16 (2.8) | 0.011 | <0.001 |
| 19F | 9 (2.2) | 12 (3.1) | 6 (1.6) | 17 (3.2) | 12 (2.4) | 12 (2.1) | 15 (2.7) | 0.518 | 0.964 |
| 23F | 1 (0.2) | 3 (0.8) | 9 (2.4) | 6 (1.1) | 8 (1.6) | 4 (0.7) | 10 (1.8) | 0.632 | 0.213 |
| addPPV23 | |||||||||
| 8 | 34 (8.4) | 43 (11.2) | 46 (12.2) | 79 (14.9) | 92 (18.4) | 117 (20.3) | 125 (22.1) | 0.002 | <0.001 |
| 9N | 8 (2.0) | 13 (3.4) | 18 (4.8) | 24 (4.5) | 19 (3.8) | 23 (4.0) | 30 (5.3) | 0.455 | 0.039 |
| 10A | 2 (0.5) | 8 (2.1) | 8 (2.1) | 9 (1.7) | 11 (2.2) | 8 (1.4) | 10 (1.8) | 0.815 | 0.486 |
| 11A | 16 (4.0) | 18 (4.7) | 15 (4.0) | 19 (3.6) | 17 (3.4) | 14 (2.4) | 27 (4.8) | 0.453 | 0.626 |
| 12F | 6 (1.5) | 8 (2.1) | 4 (1.1) | 5 (0.9) | 3 (0.6) | 9 (1.6) | 15 (2.7) | 0.008 | 0.349 |
| 15B/C | 5 (1.2) | 9 (2.3) | 8 (2.1) | 7 (1.3) | 8 (1.6) | 16 (2.8) | 8 (1.4) | 0.578 | 0.756 |
| 17F | 5 (1.2) | 2 (0.5) | 2 (0.5) | 5 (0.9) | 2 (0.4) | 3 (0.5) | 8 (1.4) | 0.371 | 0.775 |
| 20 | 14 (3.5) | 11 (2.9) | 14 (3.7) | 18 (3.4) | 17 (3.4) | 23 (4.0) | 13 (2.3) | 0.420 | 0.665 |
| 22F | 25 (6.2) | 23 (6.0) | 31 (8.2) | 40 (7.5) | 34 (6.8) | 45 (7.8) | 29 (5.1) | 0.191 | 0.759 |
| 33F | 1 (0.2) | 1 (0.3) | 1 (0.3) | 1 (0.2) | 6 (1.2) | 7 (1.2) | 9 (1.6) | 0.031 | 0.001 |
| NVT | |||||||||
| 6C | 8 (2.0) | 14 (3.7) | 6 (1.6) | 20 (3.8) | 14 (2.8) | 8 (1.4) | 10 (1.8) | 0.011 | 0.212 |
| 7C | 1 (0.2) | 4 (1.0) | 1 (0.3) | 2 (0.4) | 2 (0.4) | 5 (0.9) | 5 (0.9) | 0.196 | 0.308 |
| 15A | 3 (0.7) | 11 (2.9) | 13 (3.5) | 12 (2.3) | 14 (2.8) | 14 (2.4) | 16 (2.8) | 0.660 | 0.235 |
| 16F | 13 (3.2) | 3 (0.8) | 7 (1.9) | 11 (2.1) | 8 (1.6) | 13 (2.3) | 3 (0.5) | 0.095 | 0.079 |
| 23A | 9 (2.2) | 8 (2.1) | 9 (2.4) | 13 (2.5) | 7 (1.4) | 12 (2.1) | 7 (1.2) | 0.161 | 0.159 |
| 23B | 4 (1.0) | 5 (1.3) | 3 (0.8) | 6 (1.1) | 3 (0.6) | 4 (0.7) | 6 (1.1) | 0.951 | 0.632 |
| 24F | 5 (1.2) | 9 (2.3) | 9 (2.4) | 7 (1.3) | 7 (1.4) | 5 (0.9) | 5 (0.9) | 0.359 | 0.075 |
| 25A/38 | 3 (0.7) | 3 (0.8) | 2 (0.5) | 1 (0.2) | 3 (0.6) | 5 (0.9) | 1 (0.2) | 0.870 | 0.448 |
| 29/35B | 10 (2.5) | 6 (1.6) | 10 (2.7) | 6 (1.1) | 7 (1.4) | 14 (2.4) | 10 (1.8) | 0.241 | 0.709 |
| 31 | 5 (1.2) | 2 (0.5) | 4 (1.1) | 6 (1.1) | 10 (2.0) | 9 (1.6) | 7 (1.2) | 0.956 | 0.309 |
| 33A | 2 (0.5) | 5 (1.3) | 2 (0.5) | 4 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0.060 | 0.010 |
| 34 | 3 (0.7) | 1 (0.3) | 4 (1.1) | 5 (0.9) | 3 (0.6) | 1 (0.2) | 3 (0.5) | 0.245 | 0.431 |
| 35F | 7 (1.7) | 4 (1.0) | 2 (0.5) | 5 (0.9) | 11 (2.2) | 6 (1.0) | 8 (1.4) | 0.904 | 0.808 |
| NT | 1 (0.2) | 3 (0.8) | 6 (1.6) | 2 (0.4) | 1 (0.2) | 3 (0.5) | 2 (0.4) | 0.834 | 0.380 |
| Other | 7 (1.7) | 12 (3.1) | 9 (2.4) | 11 (2.1) | 8 (1.6) | 14 (2.4) | 18 (3.2) | ||
| Total | 404 | 383 | 376 | 530 | 501 | 576 | 566 | - | - |
| Age Group (Years) | Serotype Group 1 | No. of Isolates (%) | CA 2 | |||
|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | |||
| 18–49 | PCV7 | 8 (7.8) | 14 (16.5) | 12 (10.9) | 8 (8.1) | 0.807 |
| addPCV13 | 23 (22.3) | 19 (22.4) | 15 (13.6) | 22 (22.2) | 0.593 | |
| addPCV15 | 13 (12.6) | 3 (3.5) | 4 (3.6) | 0 (0) | <0.001 | |
| addPCV20 | 35 (34.0) | 28 (32.9) | 44 (40.0) | 42 (42.4) | 0.139 | |
| addPPV23 | 11 (10.7) | 8 (9.4) | 13 (11.8) | 15 (15.2) | 0.286 | |
| NVT | 13 (12.6) | 13 (15.3) | 22 (20.0) | 12 (12.1) | 0.802 | |
| 50–64 | PCV7 | 21 (16.9) | 16 (14.0) | 18 (14.4) | 15 (11.2) | 0.216 |
| addPCV13 | 33 (26.6) | 26 (22.8) | 24 (19.2) | 27 (20.1) | 0.166 | |
| addPCV15 | 2 (1.6) | 7 (6.1) | 7 (5.6) | 7 (5.2) | 0.218 | |
| addPCV20 | 30 (24.2) | 44 (38.6) | 44 (35.2) | 51 (38.1) | 0.041 | |
| addPPV23 | 13 (10.5) | 3 (2.6) | 6 (4.8) | 13 (9.7) | 0.993 | |
| NVT | 25 (20.2) | 18 (15.8) | 26 (20.8) | 21 (15.7) | 0.558 | |
| ≥65 | PCV7 | 53 (17.5) | 44 (14.6) | 39 (11.4) | 41 (12.3) | 0.033 |
| addPCV13 | 74 (24.4) | 75 (24.8) | 90 (26.4) | 77 (23.1) | 0.819 | |
| addPCV15 | 26 (8.6) | 30 (9.9) | 41 (12.0) | 31 (9.3) | 0.584 | |
| addPCV20 | 54 (17.8) | 59 (19.5) | 78 (22.9) | 92 (27.6) | 0.002 | |
| addPPV23 | 23 (7.6) | 27 (8.9) | 30 (8.8) | 23 (6.9) | 0.733 | |
| NVT | 73 (24.1) | 67 (22.2) | 63 (18.5) | 69 (20.7) | 0.183 | |
| No. Resistant Isolates (%) | |||
|---|---|---|---|
| Antimicrobial 1 | 18–49 Years (n = 397) | 50–64 Years (n = 497) | ≥65 Years (n = 1278) 2 |
| PEN | 44 (11.1) | 65 (13.8) | 217 (17.0) |
| MIC90 3 | 0.016 | 0.125 | 0.5 |
| MIC50 3 | 0.012 | 0.012 | 0.012 |
| CTX | 1 (0.3) | 2 (0.4) | 3 (0.2) |
| MIC90 3 | 0.016 | 0.125 | 0.38 |
| MIC50 3 | 0.016 | 0.016 | 0.016 |
| LEV | 2 (0.5) | 2 (0.4) | 9 (0.7) |
| ERY | 51 (12.8) | 57 (11.5) | 214 (16.7) |
| CLI | 41 (10.3) | 49 (9.9) | 185 (14.5) |
| CHL | 5 (1.3) | 12 (2.4) | 23 (1.8) |
| SXT | 47 (11.8) | 62 (12.5) | 175 (13.7) |
| TET | 53 (13.4) | 79 (15.9) | 214 (16.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Costa, C.; Gomes-Silva, J.; Teodoro, I.; Ramirez, M.; Melo-Cristino, J.; on behalf of the Portuguese Group for the Study of Streptococcal Infections. Invasive Pneumococcal Disease in Adults in Portugal: The Importance of Serotypes 8 and 3 (2015–2018). Microorganisms 2021, 9, 1016. https://doi.org/10.3390/microorganisms9051016
Silva-Costa C, Gomes-Silva J, Teodoro I, Ramirez M, Melo-Cristino J, on behalf of the Portuguese Group for the Study of Streptococcal Infections. Invasive Pneumococcal Disease in Adults in Portugal: The Importance of Serotypes 8 and 3 (2015–2018). Microorganisms. 2021; 9(5):1016. https://doi.org/10.3390/microorganisms9051016
Chicago/Turabian StyleSilva-Costa, Catarina, Joana Gomes-Silva, Inês Teodoro, Mário Ramirez, José Melo-Cristino, and on behalf of the Portuguese Group for the Study of Streptococcal Infections. 2021. "Invasive Pneumococcal Disease in Adults in Portugal: The Importance of Serotypes 8 and 3 (2015–2018)" Microorganisms 9, no. 5: 1016. https://doi.org/10.3390/microorganisms9051016
APA StyleSilva-Costa, C., Gomes-Silva, J., Teodoro, I., Ramirez, M., Melo-Cristino, J., & on behalf of the Portuguese Group for the Study of Streptococcal Infections. (2021). Invasive Pneumococcal Disease in Adults in Portugal: The Importance of Serotypes 8 and 3 (2015–2018). Microorganisms, 9(5), 1016. https://doi.org/10.3390/microorganisms9051016






