eDNA-Mediated Cutaneous Protection Against UVB Damage Conferred by Staphylococcal Epidermal Colonization
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial, Ex Vivo Human Skin, and Human Keratinocytes Cultures
2.1.1. Bacterial Strains and Culture Conditions
2.1.2. Ex Vivo Human Skin Cultures
2.1.3. Human Keratinocytes Cell Cultures
2.2. High-Resolution Scanning Electron Microscopy (HR-SEM) of the Staphylococci-Infected Human Skin
2.3. Confocal Scanning Laser Microscopy (CSLM) Imaging of the Whole-Mount Staphylococci-Infected Skin
2.4. S. aureus Biofilm eDNA Isolation
2.5. Human Caspase 3 Activity Evaluation Following UVB Irradiation in Ex Vivo Skin
2.6. Nrf2 Nuclear Translocation Assessment
2.7. Statistical Analysis
3. Results
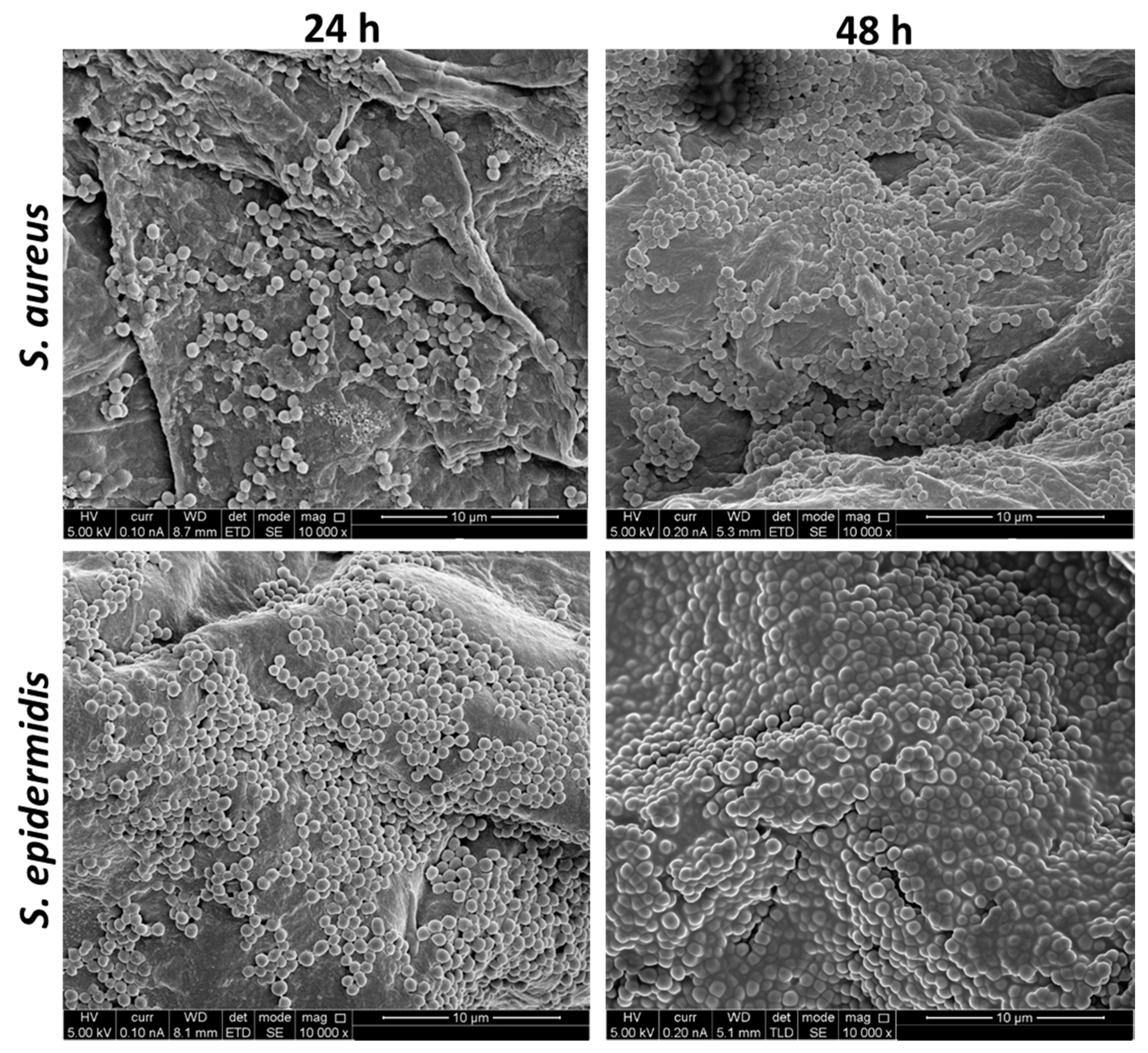
3.1. S. aureus and S. epidermidis Adhered to Ex Vivo Human Skin
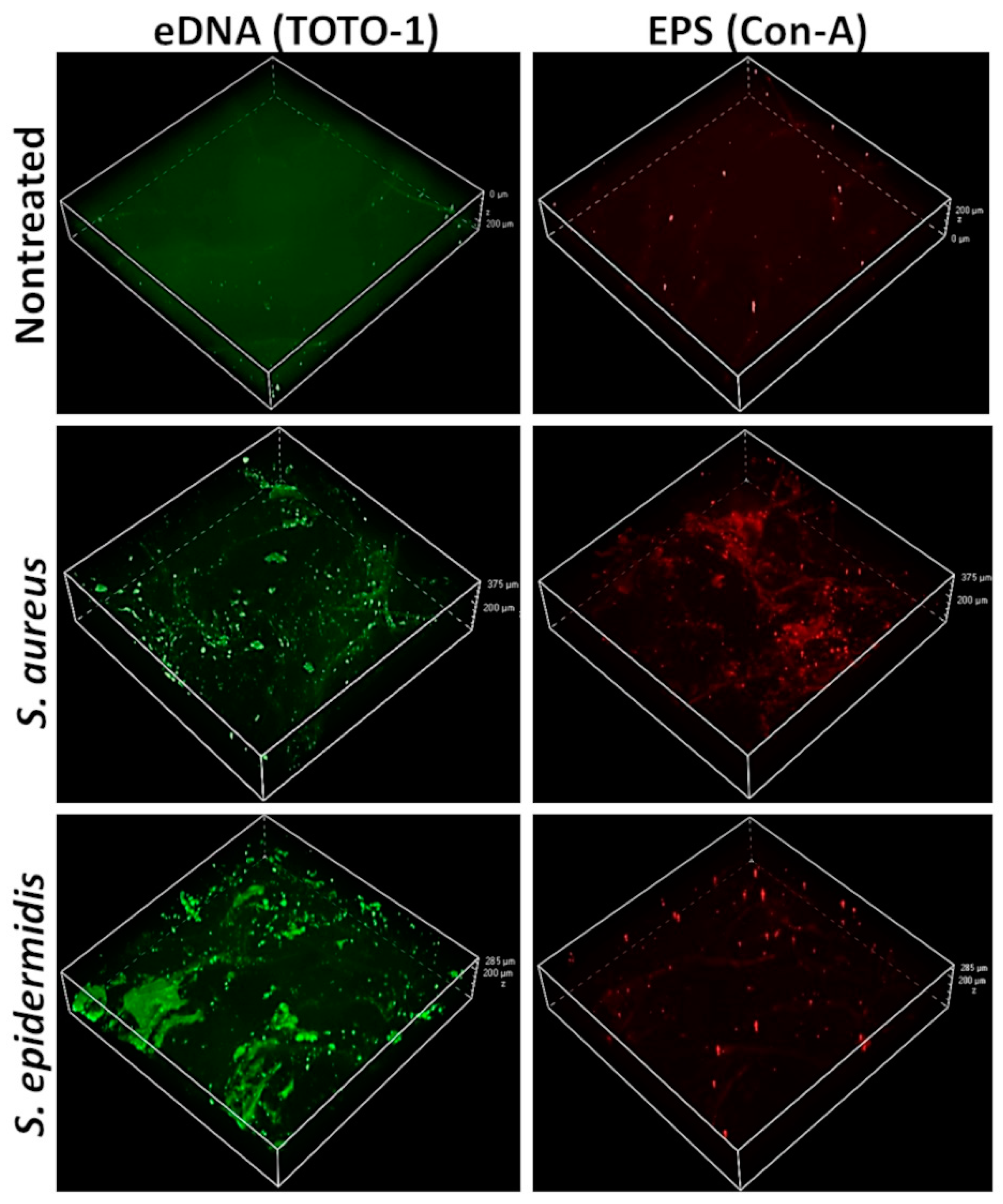
3.2. Positive Stains for eDNA and EPS in Skin-Adherent S. aureus and S. epidermidis Cultures
3.3. S. aureus and S. epidermidis Pre-Treatment of Human Skin Fragments Provided Protection against UVB-Induced Epidermal Apoptosis
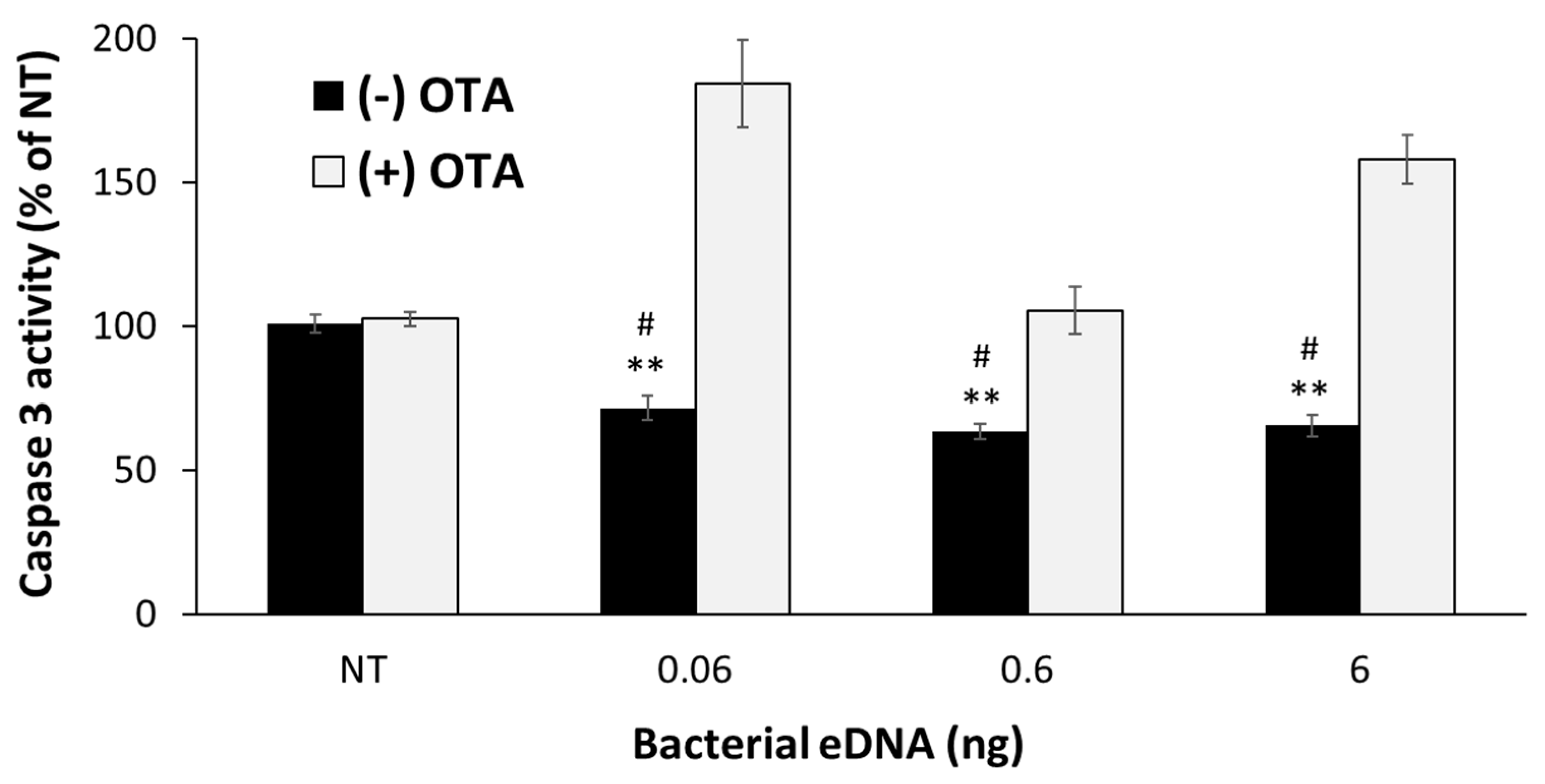
3.4. Isolated eDNA from the S. aureus Biofilm Provided Protection against UVB-Induced Epidermal Apoptosis to Ex Vivo Human Skin
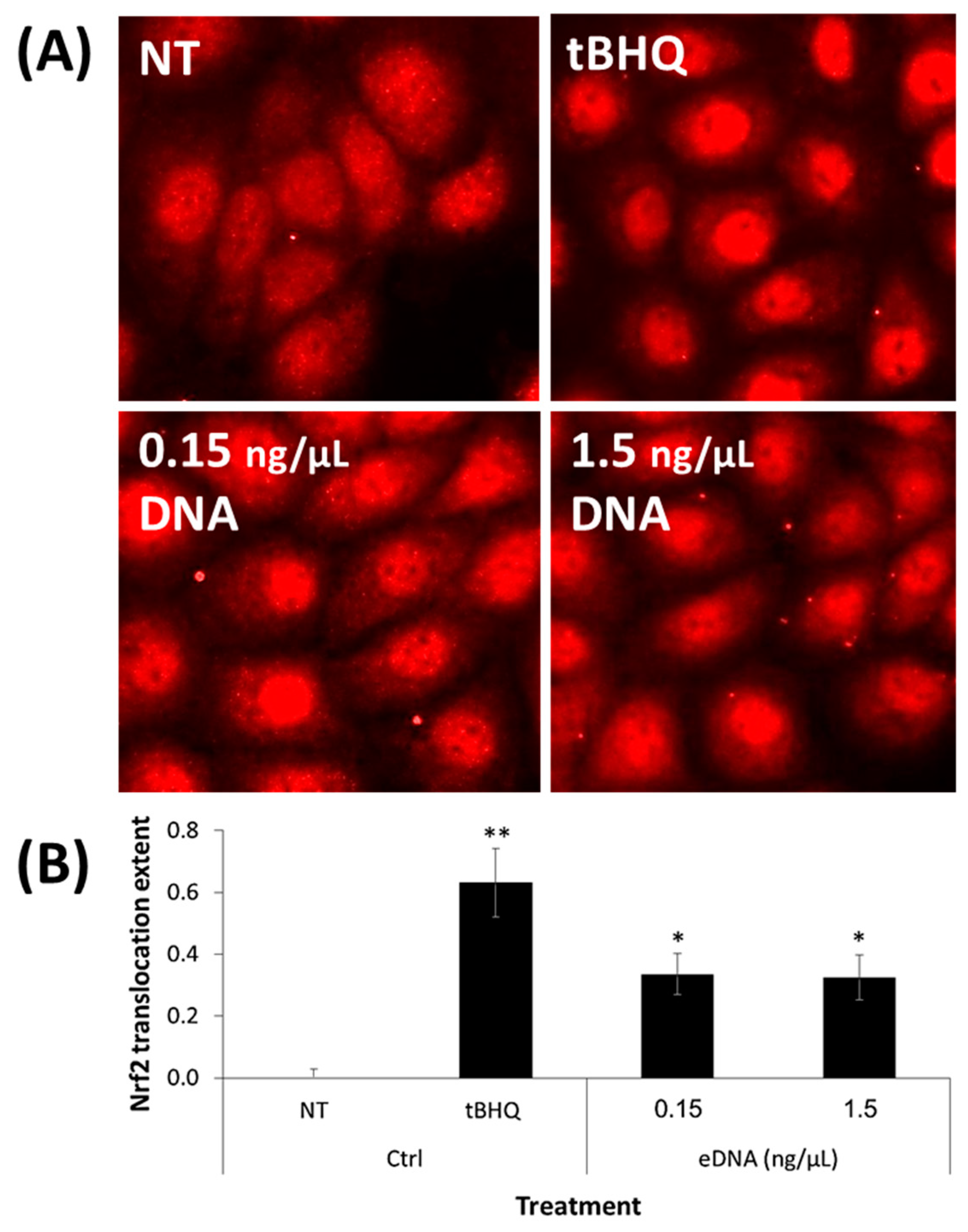
3.5. eDNA Extracted from an S. aureus Biofilm Activated the Nrf2–Keap1 Cellular Pathway in Human Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–401. [Google Scholar]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev. Dermatol. 2010, 5, 183–195. [Google Scholar] [CrossRef]
- Brandwein, M.; Steinberg, D.; Meshner, S. Microbial biofilms and the human skin microbiome. NPJ Biofilms Microbiomes 2016, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.; Biagini Myers, J.M.; Herr, A.B.; Khurana Hershey, G.K. Staphylococcal Biofilms in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2017, 17, 81. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.E.; Bubeck Wardenburg, J. Staphylococcus aureus and the skin: A longstanding and complex interaction. Skinmed 2015, 13, 111–119, quiz 120. [Google Scholar]
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F.; Corvec, S.; Dreno, B. Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology 2019, 235, 287–294. [Google Scholar] [CrossRef]
- Sabate Bresco, M.; Harris, L.G.; Thompson, K.; Stanic, B.; Morgenstern, M.; O’Mahony, L.; Richards, R.G.; Moriarty, T.F. Pathogenic Mechanisms and Host Interactions in Staphylococcus epidermidis Device-Related Infection. Front. Microbiol. 2017, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Speer, C.P.; Glaser, K. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence 2018, 9, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4, 529–566. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Buttner, H.; Mack, D.; Rohde, H. Structural basis of Staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front. Cell. Infect. Microbiol. 2015, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Ben-Yehuda Greenwald, M.; Ben-Sasson, S.; Bianco-Peled, H.; Kohen, R. Skin Redox Balance Maintenance: The Need for an Nrf2-Activator Delivery System. Cosmetics 2016, 3, 1. [Google Scholar] [CrossRef]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- McCormick, T.S.; Weinberg, A. Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontol 2000 2010, 54, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Ron-Doitch, S.; Kohen, R. The Cutaneous Physiological Redox: Essential to Maintain but Difficult to Define. Antioxidants 2020, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Nrf2—A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ron-Doitch, S.; Soroka, Y.; Frusic-Zlotkin, M.; Barasch, D.; Steinberg, D.; Kohen, R. Saturated and aromatic aldehydes originating from skin and cutaneous bacteria activate the Nrf2-keap1 pathway in human keratinocytes. Exp. Dermatol. 2020, 1–7. [Google Scholar] [CrossRef]
- Giudice, A.; Arra, C.; Turco, M.C. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol. Biol. 2010, 647, 37–74. [Google Scholar] [CrossRef]
- Dominguez, M.C.; de La Rosa, M.; Borobio, M.V. Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. J. Antimicrob. Chemother. 2001, 47, 391–398. [Google Scholar] [CrossRef]
- Farkash, Y.; Feldman, M.; Ginsburg, I.; Steinberg, D.; Shalish, M. Green Tea Polyphenols and Padma Hepaten Inhibit Candida albicans Biofilm Formation. Evid. Based Complement. Altern. Med. 2018, 2018, 1690747. [Google Scholar] [CrossRef]
- Okshevsky, M.; Meyer, R.L. Evaluation of fluorescent stains for visualizing extracellular DNA in biofilms. J. Microbiol. Methods 2014, 105, 102–104. [Google Scholar] [CrossRef]
- Wu, J.; Xi, C. Evaluation of different methods for extracting extracellular DNA from the biofilm matrix. Appl. Environ. Microbiol. 2009, 75, 5390–5395. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Danovaro, R.; Dell’Anno, A. Simultaneous recovery of extracellular and intracellular DNA suitable for molecular studies from marine sediments. Appl. Environ. Microbiol. 2005, 71, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Frusic-Zlotkin, M.; Pergamentz, R.; Michel, B.; David, M.; Mimouni, D.; Brégégère, F.; Milner, Y. The Interaction of Pemphigus Autoimmunoglobulins with Epidermal Cells: Activation of the Fas Apoptotic Pathway and the Use of Caspase Activity for Pathogenicity Tests of Pemphigus Patients. Ann. N. Y. Acad. Sci. 2005, 1050, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Egert, M.; Simmering, R. The Microbiota of the Human Skin. Adv. Exp. Med. Biol. 2016, 902, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Renaud, G.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Wolfsberg, T.G.; Turner, M.L.; Segre, J.A. A diversity profile of the human skin microbiota. Genome Res. 2008, 18, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Shu, J.C.; Lin, L.P.; Chong, K.Y.; Cheng, Y.W.; Du, J.F.; Liu, S.T. Elucidating the crucial role of poly N-acetylglucosamine from Staphylococcus aureus in cellular adhesion and pathogenesis. PLoS ONE 2015, 10, e0124216. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Capitanio, B.; Ascenzioni, F.; Pimpinelli, F.; Morrone, A.; Ensoli, F. Staphylococcus aureus and the Cutaneous Microbiota Biofilms in the Pathogenesis of Atopic Dermatitis. Microorganisms 2019, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.B.; Vaze, N.D.; Choi, C.; Hailu, T.; Tulbert, B.H.; Cusack, C.A.; Joshi, S.G. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol. 2014, 150, 260–265. [Google Scholar] [CrossRef]
- Akiyama, H.; Hamada, T.; Huh, W.K.; Yamasaki, O.; Oono, T.; Fujimoto, W.; Iwatsuki, K. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br. J. Dermatol. 2003, 148, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Bitschar, K.; Staudenmaier, L.; Klink, L.; Focken, J.; Sauer, B.; Fehrenbacher, B.; Herster, F.; Bittner, Z.; Bleul, L.; Schaller, M.; et al. Staphylococcus aureus Skin Colonization Is Enhanced by the Interaction of Neutrophil Extracellular Traps with Keratinocytes. J. Investig. Dermatol. 2020, 140, 1054–1065. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Joshi, S.; Vig, K.; Sahu, R.; Dixit, S.; Baganizi, R.; Dennis, V.A.; Singh, S.R.; Pillai, S. A three-dimensional human skin model to evaluate the inhibition of Staphylococcus aureus by antimicrobial peptide-functionalized silver carbon nanotubes. J. Biomater. Appl. 2019, 33, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Q.; Wang, S.; Wang, G.; Li, T.; Tang, P.F.; Liu, D. Impact of negative-pressure wound therapy on bacterial behaviour and bioburden in a contaminated full-thickness wound. Int. Wound J. 2019, 16, 1214–1221. [Google Scholar] [CrossRef]
- Krishna, S.; Miller, L.S. Host-pathogen interactions between the skin and Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef]
- Denning, M.F.; Wang, Y.; Tibudan, S.; Alkan, S.; Nickoloff, B.J.; Qin, J.Z. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002, 9, 40–52. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Soroka, Y.; Ma’or, Z.; Oron, M.; Zioni, T.; Bregegere, F.M.; Neuman, R.; Kohen, R.; Milner, Y. Protective effects of a cream containing Dead Sea minerals against UVB-induced stress in human skin. Exp. Dermatol. 2009, 18, 781–788. [Google Scholar] [CrossRef]
- Pandey, S.; Sahukhal, G.S.; Elasri, M.O. The msaABCR Operon Regulates the Response to Oxidative Stress in Staphylococcus aureus. J. Bacteriol. 2019, 201, 21–417. [Google Scholar] [CrossRef]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal response to oxidative stress. Front. Cell. Infect. Microbiol. 2012, 2, 33. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Adi, P.; Do Thi, T.M.; Yang, J.H.; Labibah, A.S.; Huang, C.M. Skin Bacteria Mediate Glycerol Fermentation to Produce Electricity and Resist UV-B. Microorganisms 2020, 8, 1092. [Google Scholar] [CrossRef]
- Keshari, S.; Balasubramaniam, A.; Myagmardoloonjin, B.; Herr, D.R.; Negari, I.P.; Huang, C.M. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. Int. J. Mol. Sci. 2019, 20, 4477. [Google Scholar] [CrossRef]
- Nagler, M.; Insam, H.; Pietramellara, G.; Ascher-Jenull, J. Extracellular DNA in natural environments: Features, relevance and applications. Appl. Microbiol. Biotechnol. 2018, 102, 6343–6356. [Google Scholar] [CrossRef] [PubMed]
- Tetz, V.; Tetz, G. Bacterial DNA induces the formation of heat-resistant disease-associated proteins in human plasma. Sci. Rep. 2019, 9, 17995. [Google Scholar] [CrossRef] [PubMed]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ron-Doitch, S.; Frušić-Zlotkin, M.; Soroka, Y.; Duanis-Assaf, D.; Amar, D.; Kohen, R.; Steinberg, D. eDNA-Mediated Cutaneous Protection Against UVB Damage Conferred by Staphylococcal Epidermal Colonization. Microorganisms 2021, 9, 788. https://doi.org/10.3390/microorganisms9040788
Ron-Doitch S, Frušić-Zlotkin M, Soroka Y, Duanis-Assaf D, Amar D, Kohen R, Steinberg D. eDNA-Mediated Cutaneous Protection Against UVB Damage Conferred by Staphylococcal Epidermal Colonization. Microorganisms. 2021; 9(4):788. https://doi.org/10.3390/microorganisms9040788
Chicago/Turabian StyleRon-Doitch, Sapir, Marina Frušić-Zlotkin, Yoram Soroka, Danielle Duanis-Assaf, Dalit Amar, Ron Kohen, and Doron Steinberg. 2021. "eDNA-Mediated Cutaneous Protection Against UVB Damage Conferred by Staphylococcal Epidermal Colonization" Microorganisms 9, no. 4: 788. https://doi.org/10.3390/microorganisms9040788
APA StyleRon-Doitch, S., Frušić-Zlotkin, M., Soroka, Y., Duanis-Assaf, D., Amar, D., Kohen, R., & Steinberg, D. (2021). eDNA-Mediated Cutaneous Protection Against UVB Damage Conferred by Staphylococcal Epidermal Colonization. Microorganisms, 9(4), 788. https://doi.org/10.3390/microorganisms9040788







