Identification of a Toxin–Antitoxin System That Contributes to Persister Formation by Reducing NAD in Pseudomonas aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Construction of Plasmids and Mutation Strains
2.3. Bacteria Killing Assay
2.4. RNA Isolation and Quantitative Real-Time PCR
2.5. Transcriptional Reporter Assay
2.6. Expression and Purification of the PA14_51020 Protein
2.7. Electrophoretic Mobility Shift Assay (EMSA)
2.8. Pull Down Assay
3. Results
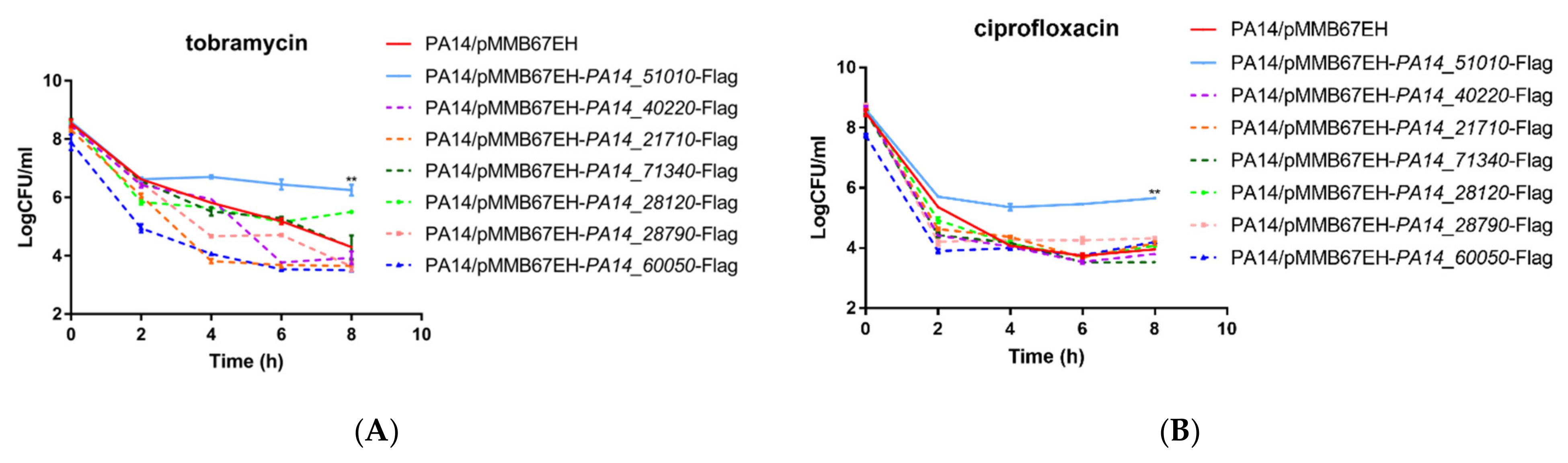
3.1. Identification of Novel Persister Formation Related Genes
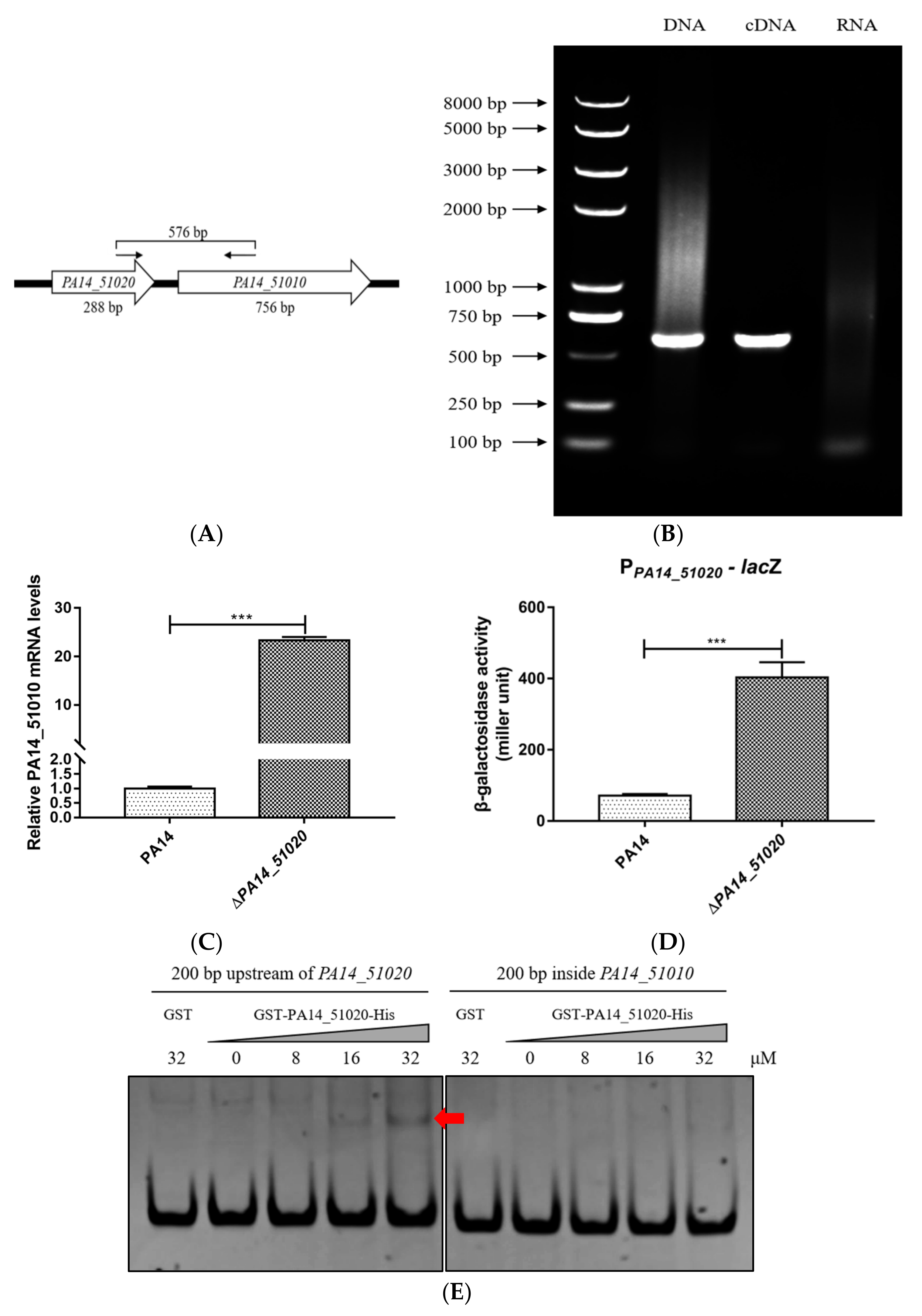
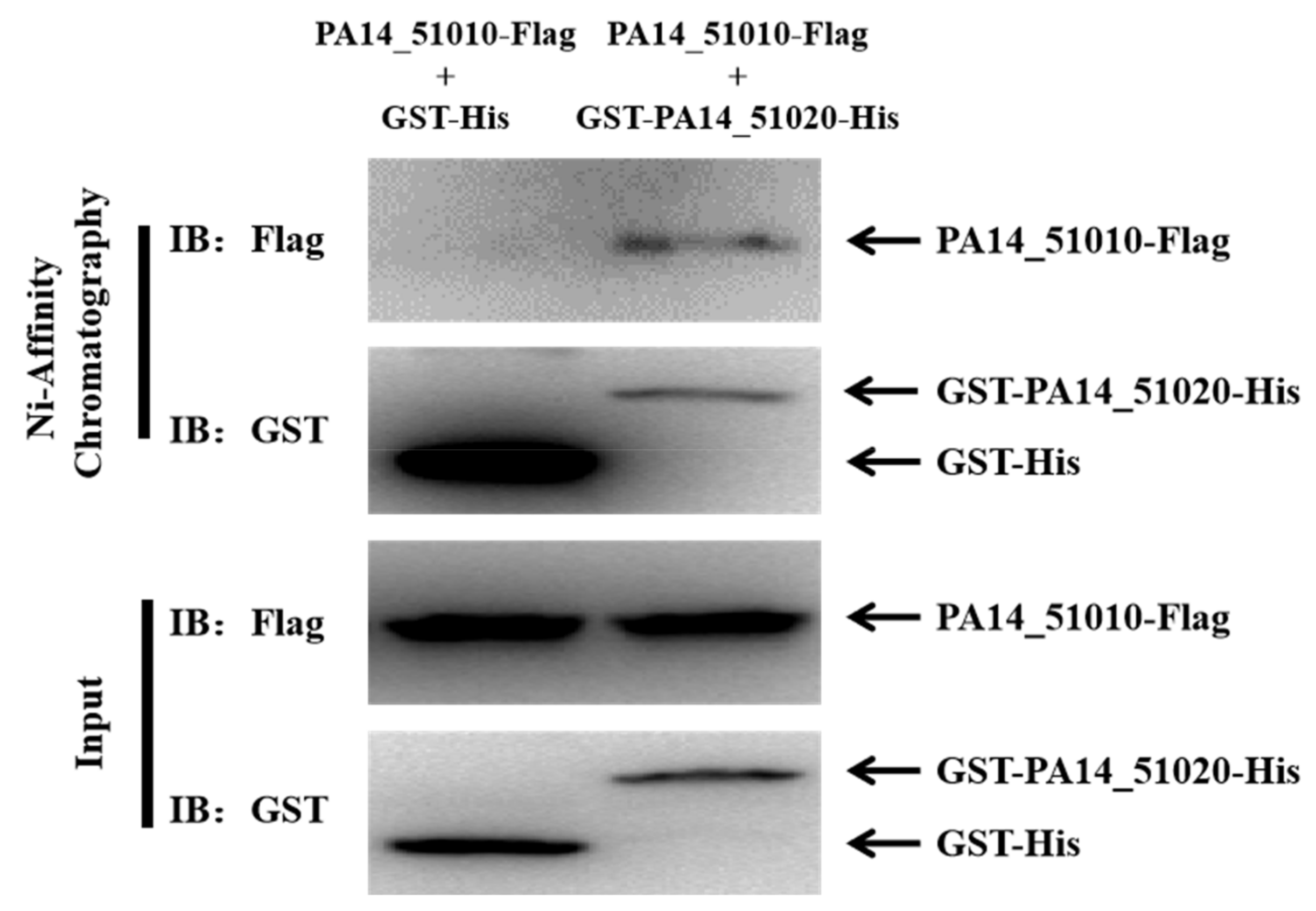
3.2. PA14_51020 Regulates the Operon of PA14_51020 and PA14_51010
3.3. PA14_51020 and PA14_51010 Regulates Persister Formation
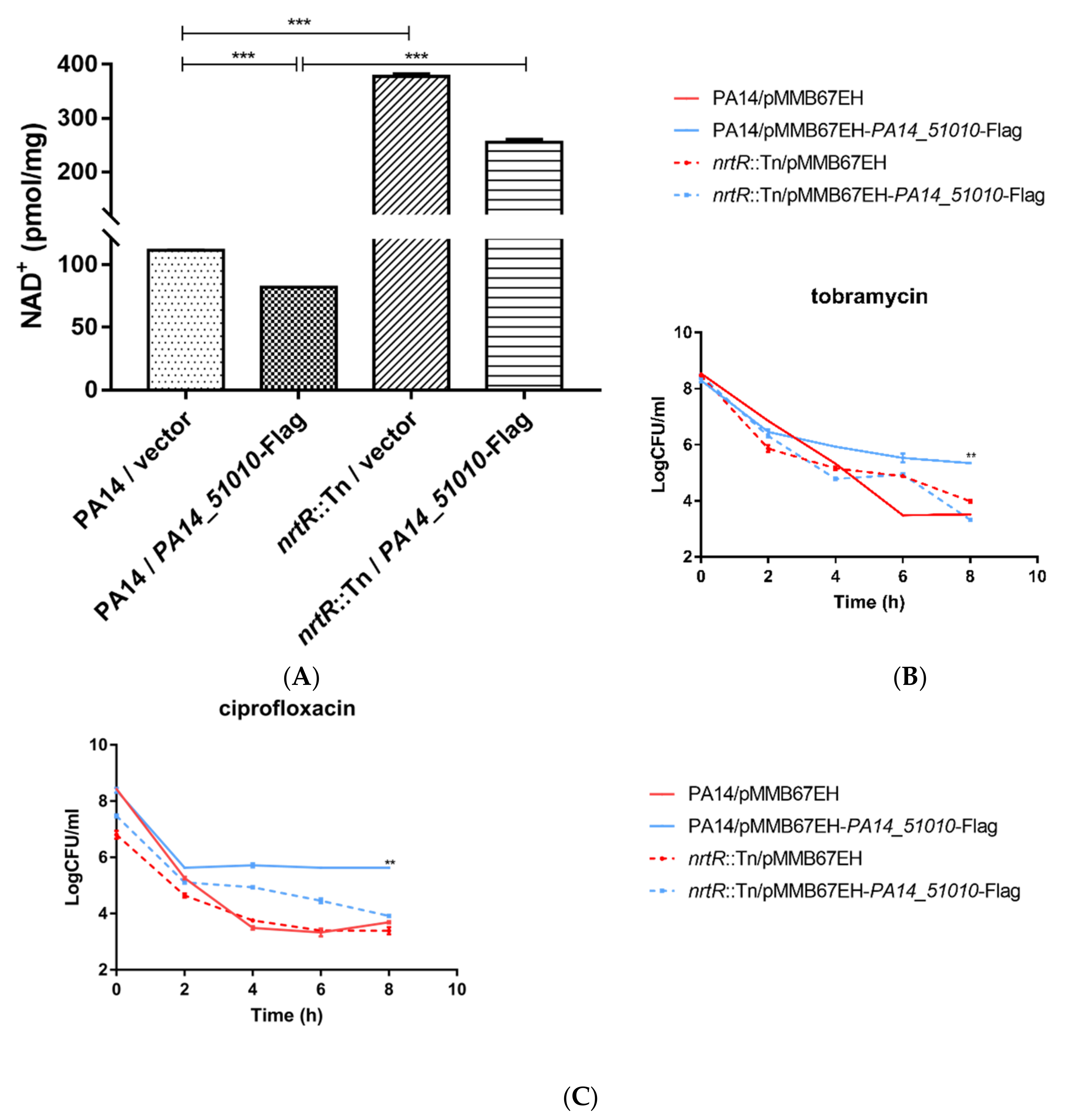
3.4. PA14_51010 Promotes Persister Formation by Reducing the Intracellular Level of NAD+
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, T.K. Combatting bacterial persister cells. Biotechnol. Bioeng. 2015, 113, 476–483. [Google Scholar] [CrossRef]
- Paranjape, S.S.; Shashidhar, R. Comparison of Starvation-Induced Persister Cells with Antibiotic-Induced Persister Cells. Curr. Microbiol. 2019, 76, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Genet. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Vallette, F.M.; Olivier, C.; Lézot, F.; Oliver, L.; Cochonneau, D.; Lalier, L.; Cartron, P.-F.; Heymann, D. Dormant, quiescent, tolerant and persister cells: Four synonyms for the same target in cancer. Biochem. Pharmacol. 2019, 162, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial persistence as a phenotypic switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial persistence and the road to drug resistance. Cell Host Microbe 2013, 13, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Swaminath, S.; Nair, R.R.; Jakkala, K.; Pradhan, A.; Ajitkumar, P. De novo emergence of genetically resistant mutants of mycobacterium tuberculosis from the persistence phase cells formed against antituberculosis drugs in vitro. Antimicrob. Agents Chemother. 2017, 61, e01343-16. [Google Scholar] [CrossRef]
- Page, R.; Peti, W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 2016, 12, 208–214. [Google Scholar] [CrossRef]
- Kim, J.-S.; Heo, P.; Yang, T.-J.; Lee, K.-S.; Cho, D.-H.; Kim, B.T.; Suh, J.-H.; Lim, H.-J.; Shin, D.; Kim, S.-K.; et al. Selective killing of bacterial persisters by a single chemical compound without affecting normal antibiotic-sensitive cells. Antimicrob. Agents Chemother. 2011, 55, 5380–5383. [Google Scholar] [CrossRef]
- Svenningsen, M.S.; Veress, A.; Harms, A.; Mitarai, N.; Semsey, S. Birth and Resuscitation of (p)ppGpp Induced Antibiotic Tolerant Persister Cells. Science Rep. 2019, 9, 6056. [Google Scholar] [CrossRef]
- Wen, Y.; Behiels, E.; Devreese, B. Toxin-Antitoxin systems: Their role in persistence, biofilm formation, and pathogenicity. Pathog. Dis. 2014, 70, 240–249. [Google Scholar] [CrossRef]
- Schuster, C.F.; Bertram, R. Toxin-antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiol. Lett. 2013, 340, 73–85. [Google Scholar] [CrossRef]
- García-Contreras, R.; Zhang, X.-S.; Kim, Y.; Wood, T.K. Protein translation and cell death: The role of rare tRNAs in biofilm formation and in activating dormant phage killer genes. PLoS ONE 2008, 3, e2394. [Google Scholar] [CrossRef]
- Marimon, O.; Teixeira, J.M.C.; Cordeiro, T.N.; Soo, V.W.C.; Wood, T.L.; Mayzel, M.; Amata, I.; García, J.; Morera, A.; Gay, M.; et al. An oxygen-sensitive toxin–antitoxin system. Nat. Commun. 2016, 7, 13634. [Google Scholar] [CrossRef]
- Yu, X.; Gao, X.; Zhu, K.; Yin, H.; Mao, X.; Wojdyla, J.A.; Qin, B.; Huang, H.; Wang, M.; Sun, Y.-C.; et al. Characterization of a toxin-antitoxin system in Mycobacterium tuberculosis suggests neutralization by phosphorylation as the antitoxicity mechanism. Commun. Biol. 2020, 3, 216. [Google Scholar] [CrossRef]
- Yao, J.; Zhen, X.; Tang, K.; Liu, T.; Xu, X.; Chen, Z.; Guo, Y.; Liu, X.; Wood, T.K.; Ouyang, S.; et al. Novel polyadenylylation-dependent neutralization mechanism of the HEPN/MNT toxin/antitoxin system. Nucleic Acids Res. 2020, 48, 11054–11067. [Google Scholar] [CrossRef]
- Wang, X.; Yao, J.; Sun, Y.-C.; Wood, T.K. Type VII toxin/antitoxin classification system for antitoxins that enzymatically neutralize toxins. Trends Microbiol. 2020. [Google Scholar] [CrossRef]
- Syed, M.A.; Lévesque, C.M. Chromosomal bacterial type II toxin–antitoxin systems. Can. J. Microbiol. 2012, 58, 553–562. [Google Scholar] [CrossRef]
- Sala, A.; Bordes, P.; Genevaux, P. Multiple toxin-antitoxin systems in Mycobacterium tuberculosis. Toxins 2014, 6, 1002–1020. [Google Scholar] [CrossRef]
- Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Type II Toxin-Antitoxin Systems: Evolution and revolutions. J. Bacteriol. 2020, 202, e00763-19. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Viducic, D.; Ono, T.; Murakami, K.; Susilowati, H.; Kayama, S.; Hirota, K.; Miyake, Y. Functional Analysis ofspoT, relA and dksA Genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol. Immunol. 2006, 50, 349–357. [Google Scholar] [CrossRef]
- Soares, A.; Alexandre, K.; Etienne, M. Tolerance and persistence of Pseudomonas aeruginosa in biofilms exposed to antibiotics: Molecular mechanisms, antibiotic strategies and therapeutic perspectives. Front. Microbiol. 2020, 11, 2057. [Google Scholar] [CrossRef]
- Muthuramalingam, M.; White, J.C.; Murphy, T.; Ames, J.R.; Bourne, C.R. The toxin from a ParDE toxin-antitoxin system found in Pseudomonas aeruginosa offers protection to cells challenged with anti-gyrase antibiotics. Mol. Microbiol. 2018, 111, 441–454. [Google Scholar] [CrossRef]
- Li, G.; Shen, M.; Lu, S.; Le, S.; Tan, Y.; Wang, J.; Zhao, X.; Shen, W.; Guo, K.; Yang, Y.; et al. Identification and characterization of the HicAB Toxin-Antitoxin System in the opportunistic pathogen Pseudomonas aeruginosa. Toxins 2016, 8, 113. [Google Scholar] [CrossRef]
- Coskun, U.S.S. Effect of mazEF, higBA and relBE Toxin-Antitoxin Systems on antibiotic resistance in Pseudomonas aeruginosa and Staphylococcus isolates. Malawi Med. J. 2018, 30, 67–72. [Google Scholar] [CrossRef]
- Wood, T.L.; Wood, T.K. The HigB/HigA toxin/antitoxin system of Pseudomonas aeruginosa influences the virulence factors pyochelin, pyocyanin, and biofilm formation. Microbiologyopen 2016, 5, 499–511. [Google Scholar] [CrossRef]
- Li, M.; Long, Y.; Liu, Y.; Liu, Y.; Chen, R.; Shi, J.; Zhang, L.; Jin, Y.; Yang, L.; Bai, F.; et al. HigB of Pseudomonas aeruginosa enhances killing of phagocytes by up-regulating the Type III secretion system in ciprofloxacin induced persister cells. Front. Cell. Infect. Microbiol. 2016, 6, 125. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, B.; Li, M.; Shi, J.; Long, Y.; Jin, Y.; Bai, F.; Cheng, Z.; Jin, S.; Wu, W. HigB reciprocally controls biofilm formation and the expression of Type III Secretion System Genes through Influencing the Intracellular c-di-GMP level in Pseudomonas aeruginosa. Toxins 2018, 10, 424. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, C.; Li, Y.; Tang, K.; Ni, S.; Wang, X. Antitoxin HigA inhibits virulence gene mvfR expression in Pseudomonas aeruginosa. Environ. Microbiol. 2019, 21, 2707–2723. [Google Scholar] [CrossRef] [PubMed]
- Liberati, N.T.; Urbach, J.M.; Miyata, S.; Lee, D.G.; Drenkard, E.; Wu, G.; Villanueva, J.; Wei, T.; Ausubel, F.M. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. USA 2006, 103, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Schweizer, H.P. mini-Tn7 insertion in bacteria with single attTn7 sites: Example Pseudomonas aeruginosa. Nat. Protoc. 2006, 1, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Fürste, J.P.; Pansegrau, W.; Frank, R.; Blöcker, H.; Scholz, P.; Bagdasarian, M.; Lanka, E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 1986, 48, 119–131. [Google Scholar] [CrossRef]
- Chen, R.; Wei, X.; Li, Z.; Weng, Y.; Xia, Y.; Ren, W.; Wang, X.; Jin, Y.; Bai, F.; Cheng, Z.; et al. Identification of a small RNA that directly controls the translation of the quorum sensing signal synthase gene rhlI in Pseudomonas aeruginosa. Environ. Microbiol. 2019, 21, 2933–2947. [Google Scholar] [CrossRef]
- Xia, B.; Li, M.; Tian, Z.; Chen, G.; Liu, C.; Xia, Y.; Jin, Y.; Bai, F.; Cheng, Z.; Jin, S.; et al. Oligoribonuclease contributes to tolerance to aminoglycoside and β-Lactam antibiotics by regulating KatA in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e00212-19. [Google Scholar] [CrossRef]
- Hoang, T.T.; Karkhoff-Schweizer, R.R.; Kutchma, A.J.; Schweizer, H.P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 1998, 212, 77–86. [Google Scholar] [CrossRef]
- Weng, Y.; Chen, F.; Liu, Y.; Zhao, Q.; Chen, R.; Pan, X.; Liu, C.; Cheng, Z.; Jin, S.; Jin, Y.; et al. Pseudomonas aeruginosa enolase influences bacterial tolerance to oxidative stresses and virulence. Front. Microbiol. 2016, 7, 1999. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, J.; Liu, C.; Jin, Y.; Li, K.; Chen, R.; Jin, S.; Wu, W. PrtR Homeostasis Contributes to Pseudomonas aeruginosa pathogenesis and resistance against ciprofloxacin. Infect. Immun. 2014, 82, 1638–1647. [Google Scholar] [CrossRef]
- Winsor, G.L.; Griffiths, E.J.; Lo, R.; Dhillon, B.K.; Shay, J.A.; Brinkman, F.S.L. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016, 44, D646–D653. [Google Scholar] [CrossRef]
- Skjerning, R.B.; Senissar, M.; Winther, K.S.; Gerdes, K.; Brodersen, D.E. The RES domain toxins of RES-Xre toxin-antitoxin modules induce cell stasis by degrading NAD+. Mol. Microbiol. 2018, 111, 221–236. [Google Scholar] [CrossRef]
- Freire, D.M.; Gutierrez, C.; Garza-Garcia, A.; Grabowska, A.D.; Sala, A.J.; Ariyachaokun, K.; Panikova, T.; Beckham, K.S.; Colom, A.; Pogenberg, V.; et al. An NAD+ phosphorylase toxin triggers Mycobacterium tuberculosis cell death. Mol. Cell 2019, 73, 1282–1291. [Google Scholar] [CrossRef]
- Gazzaniga, F.; Stebbins, R.; Chang, S.Z.; McPeek, M.A.; Brenner, C. Microbial NAD metabolism: Lessons from comparative genomics. Microbiol. Mol. Biol. Rev. 2009, 73, 529–541. [Google Scholar] [CrossRef]
- Gao, R.; Wei, W.; Hassan, B.H.; Li, J.; Deng, J.-Y.; Feng, Y. A single regulator NrtR controls bacterial NAD+ homeostasis via its acetylation. eLife 2019, 8, e51603. [Google Scholar] [CrossRef]
- Okon, E.; Dethlefsen, S.; Pelnikevich, A.; Van Barneveld, A.; Munder, A.; Tümmler, B. Key role of an ADP-ribose—Dependent transcriptional regulator of NAD metabolism for fitness and virulence of Pseudomonas aeruginosa. Int. J. Med Microbiol. 2017, 307, 83–94. [Google Scholar] [CrossRef]
- Gad, G.F.; El-Domany, R.A.; Ashour, H.M. Antimicrobial Susceptibility Profile of Pseudomonas aeruginosa Isolates in Egypt. J. Urol. 2008, 180, 176–181. [Google Scholar] [CrossRef]
- Elkhatib, W.F.; Khalil, M.A.; Ashour, H.M. Integrons and antiseptic resistance genes mediate resistance of Acinetobacter baumannii and Pseudomonas aeruginosa Isolates from intensive care unit patients with wound infections. Curr. Mol. Med. 2019, 19, 286–293. [Google Scholar] [CrossRef]
- Mann, D.L.; Foale, R.A.; Gillam, L.D.; Schoenfeld, D.; Newell, J.; Weyman, A.E. Early natural history of regional left ventricular dysfunction after experimental myocardial infarction. Am. Heart J. 1988, 115, 538–546. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Li, Y.; Li, J.; Wan, Q.; Chen, J.; Tay, F.R.; Niu, L. Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci. 2019, 7, 1901872. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, Y.; Shen, Y.; Li, X.; Zhou, H.; Tai, C.; Deng, Z.; Ou, H.-Y. TADB 2.0: An updated database of bacterial Type II Toxin–Antitoxin loci. Nucleic Acids Res. 2018, 46, D749–D753. [Google Scholar] [CrossRef]
- Ariyachaokun, K.; Grabowska, A.D.; Gutierrez, C.; Neyrolles, O. Multi-Stress induction of the Mycobacterium tuberculosis MbcTA bactericidal toxin-antitoxin system. Toxins 2020, 12, 329. [Google Scholar] [CrossRef]
- Long, Y.; Fu, W.; Li, S.; Ren, H.; Li, M.; Liu, C.; Zhang, B.; Xia, Y.; Fan, Z.; Xu, C.; et al. Identification of novel genes that promote persister formation by repressing transcription and cell division in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2019, 74, 2575–2587. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef]
- Shan, Y.; Lazinski, D.; Rowe, S.; Camilli, A.; Lewis, K. Genetic Basis of Persister Tolerance to Aminoglycosides in Escherichia coli. mBio 2015, 6, e00078-15. [Google Scholar] [CrossRef]
- Goormaghtigh, F.; Fraikin, N.; Putrinš, M.; Hallaert, T.; Hauryliuk, V.; Garcia-Pino, A.; Sjödin, A.; Kasvandik, S.; Udekwu, K.; Tenson, T.; et al. Reassessing the role of type II toxin-antitoxin systems in formation of Escherichia coli type II persister cells. mBio 2018, 9, e00640-18. [Google Scholar] [CrossRef]
- Helaine, S.; Cheverton, A.M.; Watson, K.G.; Faure, L.M.; Matthews, S.A.; Holden, D.W. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 2014, 343, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Claudi, B.; Spröte, P.; Chirkova, A.; Personnic, N.; Zankl, J.; Schürmann, N.; Schmidt, A.; Bumann, D. Phenotypic variation of Salmonella in host tissues delays eradication by antimicrobial chemotherapy. Cell 2014, 158, 722–733. [Google Scholar] [CrossRef]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Ambroa, A.; López, M.; Bou, G.; Cantón, R.; Garcia-Contreras, R.; Wood, T.K.; et al. (p)ppGpp and its role in bacterial persistence: New challenges. Antimicrob. Agents Chemother. 2020, 64, e01283-20. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J.; Williamson, K.S.; Folsom, J.P.; Boegli, L.; James, G.A. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2015, 59, 3838–3847. [Google Scholar] [CrossRef]
- Xu, X.; Yu, H.; Zhang, D.; Xiong, J.; Qiu, J.; Xin, R.; He, X.; Sheng, H.; Cai, W.; Jiang, L.; et al. Role of ppGpp in Pseudomonas aeruginosa acute pulmonary infection and virulence regulation. Microbiol. Res. 2016, 192, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.L.; Green, C.; Stevens, K.M.; Day, B.; Erickson, D.L.; Woods, D.E.; Storey, D.G. The stringent response is essential for Pseudomonas aeruginosa virulence in the rat lung agar bead and drosophila melanogaster feeding models of infection. Infect. Immun. 2011, 79, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, D.; Blimkie, T.M.; Wolfmeier, H.; Li, Y.; Baghela, A.; Lee, A.H.Y.; Falsafi, R.; Hancock, R.E.W. The stringent stress response controls proteases and global regulators under optimal growth conditions in Pseudomonas aeruginosa. mSystems 2020, 5, e00495-20. [Google Scholar] [CrossRef] [PubMed]



| Strain | Description | Source (Reference) |
|---|---|---|
| P. aeruginosa | ||
| PA14 | Wild-type strain of Pseudomonas aeruginosa | [32] |
| ΔPA14_51020 | PA14 deleted of PA14_51020 | This study |
| ΔPA14_51010 | PA14 deleted of PA14_51010 | This study |
| ΔPA14_51020ΔPA14_51010 | PA14 deleted of PA14_51020 and PA14_51010 | This study |
| Plasmid | ||
| pEX18Tc | Gene replacement vector; Tc r | [33] |
| pMMB67EH | Expression vector with tac promoter; Amp r | [34] |
| pUCP20-lacZ | Promoterless lacZ fusion vector; Amp r | [35] |
| pMMB67EH-PA14_51010-Flag | PA14_51010 gene with Flag-tag driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-PA14_40220-Flag | PA14_40220 gene with Flag-tag driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-PA14_21710-Flag | PA14_21710 gene with Flag-tag driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-PA14_28120-Flag | PA14_28120 gene with Flag-tag driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-PA14_28790-Flag | PA14_28790 gene with Flag-tag driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-PA14_60050-Flag | PA14_60050 gene with Flag-tag driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-PA14_71340-Flag | PA14_71340 gene with Flag-tag driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-GST-His | GST with His-tag driven by tac promoter on pMMB67EH; Amp r | [36] |
| pMMB67EH-GST-PA14_51020-His | PA14_51020 gene with His-tag fused to GST driven by tac promoter on pMMB67EH; Amp r | This study |
| pEX18Tc-ΔPA14_51020 | PA14_51020 gene of PA14 deletion on pEX18Tc; Tc r | This study |
| pEX18Tc-ΔPA14_51010 | PA14_51010 gene of PA14 deletion on pEX18Tc; Tc r | This study |
| pEX18Tc-ΔPA14_51020ΔPA14_51010 | PA14_51020 and PA14_51010 gene of PA14 deletion on pEX18Tc; Tc r | This study |
| pUCP20-PPA14_51020-lacZ | PA14_51020 promoter of PA14 on a promoterless lacZ fusion vector; Amp r | This study |
| pMMB67EH-PA14_51020 | PA14_51020 gene driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-operon (PA14_51020-PA14_51010) | The operon (PA14_51020-PA14_51010) driven by tac promoter on pMMB67EH; Amp r | This study |
| pMMB67EH-PA14_51010 (RES-A) | PA14_51010 gene with simultaneous replacement of the R, E, S residues with A residues driven by tac promoter on pMMB67EH; Amp r | This study |
| Primer | Sequence 5′–3′ | Purpose |
| EcoRI-PA14_51020-up-F | CCGGAATTCGCTGGAGTTGCTGACCG | PA14_51020, PA14_51010 deletion |
| KpnI-PA14_51020-up-R | CGGGGTACCGCCCCAATTGCTCGC | PA14_51020, PA14_51010 deletion |
| KpnI-PA14_51020-down-F | CGGGGTACCACGCCTGTCACGGAAAAG | PA14_51020 deletion |
| HindIII-PA14_51020-down-F | CCCAAGCTTTCGCCGAAGCCTCTTGC | PA14_51020 deletion |
| EcoRI-PA14_51010-up-F | CCGGAATTCTCAGCAGCATCCGTCGCGAT | PA14_51010 deletion |
| KpnI-PA14_51010-up-R | CGGGGTACCCTTCCGCCCCTCGCTTCCTG | PA14_51010 deletion |
| KpnI-PA14_51010-down-F | CGGGGTACCGAGCTGTTCCTGGTGG | PA14_51020 and PA14_51010 deletion |
| HindIII-PA14_51010-down-R | CCCAAGCTTGACGCACTTCCTCTTCC | PA14_51020 and PA14_51010 deletion |
| SmaI-PPA14_51020-F | TCCCCCGGGGGCAATGGGCCGATCGAATC | PA14_51020 promoter cloning |
| BamHI-PPA14_51020-R | CGCGGATCCCCCCAATTGCTCGCGCGCGG | PA14_51020 promoter cloning |
| EcoRI-PA14_51010-F | CCGGAATTCTCCGACACCACAGGAAGCGA | PA14_51010 cloning |
| HindIII-PA14_51010-R | CCCAAGCTTTCATTTATCATCATCATCTTTGTAATCCGCCGGATGCGGCA | PA14_51010 cloning |
| BamHI-PA14_51020-F | CGCGGATCCACGCAGCTCGAACTGGCCGG | PA14_51020 cloning |
| HindIII-PA14_51020-R | CCCAAGCTTTCAGTGGTGGTGGTGGTGGTGGACCTTGCCGCGGATCGCAT | PA14_51020 cloning |
| EcoRI-GST-F | CCGGAATTCTTTAAGAAGGAGATATAATGTCCCCTATACTAGGTTA | PA14_51020 cloning |
| BamHI-GST-R | CGCGGATCCACCAGAACCACTAGTTGAAC | PA14_51020 cloning |
| BamHI-PA14_40220-F | CGCGGATCCGCTCGTTTCACCGGTAGCGG | PA14_40220 cloning |
| HindIII-PA14_40220-R | CCCAAGCTTCTATTTATCATCATCATCTTTGTAATCGGCGTCGCGCCGA | PA14_40220 cloning |
| BamHI-PA14_21710-F | CGCGGATCCACGCTCTGATGGGAGCGGAG | PA14_21710 cloning |
| HindIII-PA14_21710-R | CCCAAGCTTTCATTTATCATCATCATCTTTGTAATCGCCGGTGAAGCTGGCT | PA14_21710 cloning |
| EcoRI-PA14_28120-F | CCGGAATTCCCGCCAGCCTGTACGCACAA | PA14_28120 cloning |
| BamHI-PA14_28120-R | CGCGGATCCTCATTTATCATCATCATCTTTGTAATCGCCTCGCGCCAGT | PA14_28120 cloning |
| EcoRI-PA14_28790-F | CCGGAATTCCAGCATATGCGGGAGCTGTT | PA14_28790 cloning |
| HindIII-PA14_28790-R | CCCAAGCTTTCATTTATCATCATCATCTTTGTAATCGTGAGTACCAGCCC | PA14_28790 cloning |
| EcoRI-PA14_60050-F | CCGGAATTCGAGCTCGGCAACCAGGCGAG | PA14_60050 cloning |
| HindIII-PA14_60050-R | CCCAAGCTTTCATTTATCATCATCATCTTTGTAATCTCGTTGGGGCAGGT | PA14_60050 cloning |
| EcoRI-PA14_71340-F | CCGGAATTCCCCCGCTCCACCCTTTCCCA | PA14_71340 cloning |
| HindIII-PA14_71340-R | CCCAAGCTTTCATTTATCATCATCATCTTTGTAATCTTGAGGTTGCT | PA14_71340 cloning |
| EMSA-upstream-F | GTTTTTCTCTCTATCACGCC | EMSA |
| EMSA-upstream-R | CCCCAATTGCTCGCGCGCGG | EMSA |
| EMSA-inside-F | CGGGCTGGAAGGTGGAGCGG | EMSA |
| EMSA-inside-R | CGCGGGCGTGAACAGGGCGA | EMSA |
| PA14_51010-F | GAGCCAAGCCTGTTCTAC | qRT-PCR |
| PA14_51010-R | CAGGACACAACGGTAATACG | qRT-PCR |
| PA14_51020-F | CACTCCCAACCATCAC | qRT-PCR |
| PA14_51020-R | AGGTATTCCAGCACAT | qRT-PCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Li, S.; Li, H.; Jin, Y.; Bai, F.; Cheng, Z.; Wu, W. Identification of a Toxin–Antitoxin System That Contributes to Persister Formation by Reducing NAD in Pseudomonas aeruginosa. Microorganisms 2021, 9, 753. https://doi.org/10.3390/microorganisms9040753
Zhou J, Li S, Li H, Jin Y, Bai F, Cheng Z, Wu W. Identification of a Toxin–Antitoxin System That Contributes to Persister Formation by Reducing NAD in Pseudomonas aeruginosa. Microorganisms. 2021; 9(4):753. https://doi.org/10.3390/microorganisms9040753
Chicago/Turabian StyleZhou, Jingyi, Shouyi Li, Haozhou Li, Yongxin Jin, Fang Bai, Zhihui Cheng, and Weihui Wu. 2021. "Identification of a Toxin–Antitoxin System That Contributes to Persister Formation by Reducing NAD in Pseudomonas aeruginosa" Microorganisms 9, no. 4: 753. https://doi.org/10.3390/microorganisms9040753
APA StyleZhou, J., Li, S., Li, H., Jin, Y., Bai, F., Cheng, Z., & Wu, W. (2021). Identification of a Toxin–Antitoxin System That Contributes to Persister Formation by Reducing NAD in Pseudomonas aeruginosa. Microorganisms, 9(4), 753. https://doi.org/10.3390/microorganisms9040753






