Abstract
Gastrointestinal disorders (GIDs) are a common comorbidity in patients with neurodevelopmental disorders (NDDs), while anxiety-like behaviors are common among patients with gastrointestinal diseases. It is still unclear as to which microbes differentiate these two groups. This pilot study aims at proposing an answer by exploring both the bacteriome and the mycobiome in a cohort of 55 volunteers with NDD, GID or controls, while accounting for additional variables that are not commonly included such as probiotic intake and diet. Recruited participants answered a questionnaire and provided a stool sample using the Fisherbrand collection kit. Bacterial and fungal DNA was extracted using the Qiagen Stool minikit. Sequencing (16sRNA and ITS) and phylogenetic analyses were performed using the PE300 Illumina Miseq v3 sequencing. Statistical analysis was performed using the R package. Results showed a significant decrease in bacterial alpha diversity in both NDD and GID, but an increased fungal alpha diversity in NDD. Data pointed at a significant bacterial dysbiosis between the three groups, but the mycobiome dysbiosis is more pronounced in NDD than in GID. Fungi seem to be more affected by probiotics, diet and antibiotic exposure and are proposed to be the main key player in differentiation between NDD and GID dybiosis.
1. Introduction
Dysbiosis, or the alteration in the diversity and abundance of the gut microbial communities, has been consistently linked to modern diseases such as metabolic diseases [1,2], neurodegenerative diseases [3], cancers [2], autoimmune diseases [4] and even neuropsychiatric and neurodevelopmental disorders (NDD) such as Autism Spectrum Disorders (ASD) and Attention Deficit Hyperactivity Disorders (ADHD). Furthermore, it has been shown that a significant proportion of children with NDD often also suffer from gastrointestinal disorders (GID) such as celiac disease, irritable bowel syndrome or inflammatory bowel diseases (IBD) [5]. Interestingly, studies noticed that individuals with a GID also often develop anxiety-like behaviors [6,7,8]. This has been described as the gut–brain axis or GBA. A growing number of studies have greatly emphasized this bidirectional communication between the gut and the brain as well as the role of microbes and the metabolome in the pathogenesis of anxiety-like behaviors [9,10,11,12]. Microbiome alterations have been associated not only with ASD, ADHD, schizophrenia and anxiety, but also with Crohn’s diseases, ulcerative colitis and celiac disease [13,14,15]. These observations have led to the hypothesis that a general dysbiosis exists in both types of disorders, but while some features might be shared, others might certainly differentiate them.
Most studies that have investigated the dysbiosis in Autism and IBD have done it separately and have never reached a consensus on which microbial species are significantly affected and the relationship between the two types of disorders. In addition, replicating the results remained difficult, since the source of the sample (colon, stomach, mouth), lifestyle (diet, probiotic intake, antibiotic history) and method of collection are factors that affect both relative and absolute abundances of gut organisms and ultimately our understanding of gut composition. Moreover, despite the microbiome being a rich and complex ecosystem that includes not only bacteria but also fungi, protists, viruses and archaea, most studies to date have been devoted to the bacteriome while completely neglecting the mycobiome. Fungi are an integral part of the human flora and can be found in the gut as well as the skin, vagina, mouth and lungs [16,17,18]. Interestingly, recent studies have pointed to the importance of the mycobiome in ASD and a possible interaction between the bacteriome and mycobiome [16,19,20]
A variety of external factors have been described as modulators of the gut microbial composition, most of them pertain to lifestyle and include diet, physical activity, sleep, and stress [3,21,22,23,24]. Studies have identified a critical window for the gut microbiota maturation during infancy, and during which the drastic alterations of the gut microbiota could lead to further dysbiosis [25,26,27,28]. Processed carbohydrates such as white flour, white bread, white rice, pastries, pasta, sweets, breakfast cereals, added sugars, soda and snacks have been shown to greatly affect the microbiome composition [29]. Interestingly, this group of food seems to be craved by individuals with mood disorders.
In addition to lifestyle, probiotics and antibiotics have direct and indirect impacts on the growth of gut microbes. Probiotics intake has recently become a trend among individuals with health concerns due to marketing and increasing awareness of the importance of gut microbes and this can also directly affect the observed microbiome composition [30]. Indeed, studies have pointed to the important role of probiotics in re-establishing an eubiosis after a dysbiosis in diseases of the GBA [31,32,33]. A recent study by Lukacik et al. [34] has mentioned the possible role of early life exposure to antibiotics in the risk of developing autism. Slob et al. [35] showed in a twin study on 27,781 twins that early exposure to antibiotics significantly increased the risk of developing ADHD and ASD. This intriguing association between NDD and early exposure to antibiotics has been shown by a growing number of studies [34,36,37,38]. These three variables are not commonly taken into consideration when studying the gut microbiome.
In Qatar, the prevalence of ASD has been estimated at 1.81%, which is higher than world average, which is 0.6 to 1% [39]. However, no study has investigated the microbial signatures of individuals with NDD and/or GID in Qatar. Our previous questionnaire-based study assessed the awareness of both the general public and healthcare professionals in Qatar on the role of gut microbiome in NDD and GID and attempted to understand some of the dietary habits of 112 participants and their early exposure to antibiotics. The study showed an association between early exposure to antibiotics (before age 3) and developing an NDD [40]. Fifty-five of those participants volunteered to also donate a stool sample for the present analysis.
As the interest in the mechanisms underlying pathologies relating to the GBA is growing worldwide, comparing the gut composition of NDD and GID is essential to further understand the relationship between these two sets of disorders. In addition, exploring both the bacteriome and the mycobiome is becoming essential, as is the role of the external modulators.
The goal of this pilot study is therefore to analyze and compare both the gut bacteriome and mycobiome among individuals with NDD, GID, and non-NDD/non-GID controls (CTRL) of a small cohort in Qatar. This study attempts to identify microbial signatures of both bacteria and fungi in NDD and GID compared to controls, while taking into consideration antibiotic history at early age, diet and probiotic intake. Launched in 2018, this is the first study that compares both the bacteriome and mycobiome of individuals with NDD or GID with healthy individuals within the same cohort of a multiethnic population, while taking into consideration these external modulators of the gut microbiota.
2. Materials and Methods
2.1. Recruitment
Recruitment was performed using an Institutional Review Board (IRB)-approved flyer calling for volunteers, which was shared on social media and by email. Recruitment also happened during a seminar presentation of the project and during the Autism Family Day at a center for special needs in Qatar. Fifty-five participants were recruited according to the following inclusion criteria: 3 to 90 years old (however, only 3 to 57 volunteered) living in Qatar and experiencing one of the following conditions: ASD, ADHD (both included in NDD); Crohn’s disease OR ulcerative colitis OR celiac disease (all included in GID); or none of the previously mentioned conditions (CTRL). Exclusion criteria were: the participant or parent giving consent is able to read neither English nor Arabic. Participants were asked whether they have been taking antibiotics in the past month prior to stool collection, and none of them had. The recruited number of participants (N) per group were as follows: NDD: N = 15; GID: N = 13 and C: N = 27.
Consent forms were written in both English and Arabic. Non-cognitively impaired adults had a consent form to fill and sign. For children and cognitively impaired adults and children, the recruitment required consent from one or both parents and the child’s assent. Two different assent forms were designed and approved by the IRB: one for non-cognitively impaired children and one for the cognitively impaired children/adults. These forms aimed to ensure the participant understands that they will be providing a stool sample and how it will be used.
2.2. Questionnaire
A survey collected personal data for statistical correlation analyses: age, gender, height, weight, disease group (NDD, GID, CTRL), diet type, medical history (presence of other diseases, extensive use of antibiotic during first three years of age), ethnicity.
2.3. Sample Collection
Participants were offered a “commode specimen collection system” (Fisherbrand) to collect their own or their child’s stool sample at home. The Fisherbrand collection kit was chosen for its intuitive, safe and easy collection of a stool sample for both adults and kids without requiring the participant to handle the stool. The kit is placed on the toilet bowl and closed with a cap after defecation. Alphanumeric labels were added to each sample and to a corresponding survey upon reception.
2.4. DNA Extraction
QIAamp Fast DNA stool mini kit (Qiagen, Hilden, Germany) was used to extract DNA from fresh stool samples. Stool samples were vortexed and sonicated for 5 min prior to following the manufacturer’s protocol. Samples were lysed in InhibitEX Buffer, then incubated at 90 °C. After centrifugation at high speed, the bacterial genomic DNA was purified on the QIAamp Mini spin columns as follows: protein digestion by proteinase K occurred at 70 °C incubation then loaded on the QIAamp spin columns for silica membrane DNA binding, washing steps, and elution of DNA. Short centrifugation at high speed allowed adsorption of the DNA on the membrane. Two washing steps preceded the elution, which used a low-salt buffer suitable for direct use of the DNA for PCR reactions. Storage of purified DNA was done at −20 °C. DNA quality and quantity were assessed using a nanodrop spectrophotometer and measurements of its absorbance at 260 nm and 280 nm. The minimum concentration required was set to 100 ng with at least 20 µL of volume.
2.5. 16sRNA Sequencing and Phylogenetic Analysis
All amplified DNA samples were sent to CD genomics (Shirley, NY, USA), which performed 16sRNA sequencing and phylogenetic analyses using the PE300 Illumina Miseq v3 sequencing with a 600-cyle format. Standard analysis included operational taxonomic unit (OTU) clustering, alpha-diversity analysis, OTU analysis/species annotation (phylogenetic tree, Venn graph, Heatmap, and taxonomic tree), beta-diversity analysis (PCA analysis, PCoA analysis, unweighted UniFrac distance heatmap, UPGMA and NMDS analysis). CD genomics provided a full report with raw data excel files.
2.6. ITS Sequencing and Phylogenetic Analysis
Sequencing and analysis of the internal transcribed spacers (ITS) was used by CD genomics to analyze the fungal composition of samples. After testing the DNA quality required for ITS sequencing, only 35 (out of 55) samples were retained for fungal DNA analysis: NDD: N = 8; GID: N = 11; CTRL: N = 16. Standard phylogenetic analysis included the same analyses as for 16sRNA listed above and reports, and raw data were sent to us for further analyses.
2.7. Statistical Analyses
Data including age, gender, body mass index (BMI), history of probiotic use, history of antibiotic use in the first three years of life, and nature of diet were collected as described in Section 2.2. The , or diet, or both, in addition to basic covariates. A Student’s t-test was used to compare abundances among individuals based on their antibiotic use in the first three years of life. p-values < 0.05 were considered significant. All analyses were completed in R statistical package version 4.0.2.
3. Results
3.1. Characterisistics of the Studied Cohort
Table 1 depicts the three groups’ characteristics based on the data collected from the surveys. Besides the cosmopolitan recruited population, most participants with NDD were from the Middle East, while most participants with GID were caucasian. Although we did not have run a test on this, these interesting numbers are in concordance with previous studies pointing at racial differences in GID occurrence.

Table 1.
Characteristics of the studied cohort.
3.2. Bacterial Microbiome in NDD, GID and CTRL
3.2.1. An Altered Bacterial Diversity in NDD and GID Groups
Observations at the phylum level note various phyla ratios that are significantly changed in NDD compared to CTRL and compared to GID (Table 2). In particular, the ratio of Firmicutes to Bacteroidetes is significantly decreased in NDD compared to CTRL (p = 0.045), but not significantly different between NDD and GID (p = 0.87, data not shown), which indicates that NDD and GID groups are closer in regard to this specific ratio. Another important ratio is the Firmicutes to Actinobacteria, which is significantly decreased in NDD compared to both CTRL and GID. Other significantly changed ratios are listed in the supplementary file.

Table 2.
Significant phyla ratio changes among neurodevelopmental disorder (NDD) and gastrointestinal disorder (GID) groups.
In the GID group, the ratios involving TM7—a recently described subgroup of uncultivable bacteria also called Sacharibacteria—with a decreased ratio of TM7/Actinobacteria (p = 0.041) compared to CTRL, and a decreased TM7/Firmicutes ratio (p = 0.044). These are in concordance with previous studies associating TM7 with IBD [41].
While ratios analyses can indicate a level of dysbiosis, these are not informative enough, and in fact, some ratios can still be similar while the abundance of both taxa involved is increased or decreased. Therefore, alpha diversity refers to the diversity within each sample; the richness is indicated by the number of observed species and the Chao1 index (estimation based on abundance). Both are represented in Figure 1. The representation in Figure 1A at the genus level points at a progressive shift from CTRL to GID to NDD, with shifts being more pronounced in NDD than GID. The Venn diagram confirms that the number of operational taxonomic units (OTUs) shared by the GID group and CTRL group is higher than NDD with CTRL, while both NDD and GID groups appear to be characterized by some unique OTUs (Figure 1B). The number of observed species is significantly decreased in both NDD and GID groups compared to the CTRL group, and the Chao1 index indicates that this decrease is more significant in NDD than in GID (Figure 1C,D).
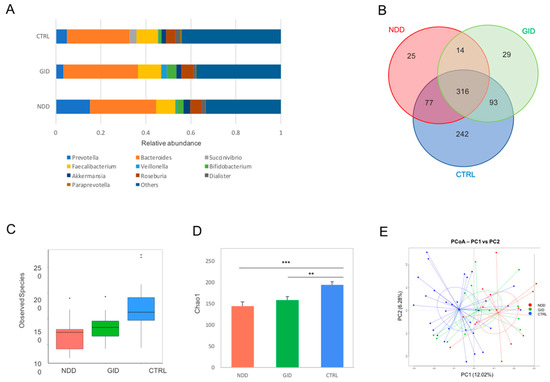
Figure 1.
Overview of bacterial diversity in NDD, GID and control (CTRL). Relative abundances of the 11 most significant bacterial genera (A); Venn diagram summarizing number of operational taxonomic units (OTUs) shared between different groups (B); alpha diversity indices including the number of observed species (C) and the Chao1 index (D); beta-diversity indicated by PCoA plot of unweighted UniFrac distance by groups (E). Significant difference of the NDD group compared to CTRL and GID group compared to CTRL was noted by * p < 0.05; ** p < 0.05; and *** p < 0.005.
The beta diversity indicated by the performed principal coordinated analysis (PCoA) based on the weighted UniFrac analysis of the OTU level revealed that the overall composition of the NDD bacteriome is different from that of the CTRL group, while the diversity of the GID group stands somewhere in between CTRL and NDD (Figure 1E).
3.2.2. Significant Taxa Abundance Variability in NDD Compared to CTRL
Table 3 and Table 4 depict the respective measurements of absolute abundance and relative abundance of taxa across phylogenetic levels in the NDD group compared to CTRL. Some differences in taxa are only significant when using relative abundance. For example, the significant decrease in Firmicutes in NDD is only observed while using relative abundance; the increase in the order of Clostridiales in NDD is also significant when only using relative abundance. Three more families, one more genus and three more species appear to be significant when using relative abundance.

Table 3.
Significant absolute abundances of bacterial taxa in NDD compared to CTRL.

Table 4.
Significant relative abundances of bacterial taxa in NDD compared to CTRL.
In addition, statistical models using basic covariates (BMI, gender, age) or including the intake of probiotics or the type of diet also influence the results and some taxa showing significant differences with basic covariates become non-significant once we considered the use of probiotics or the diet or both. For example, the species Bifidobacterieum bifidum and Veillonella dispar seem to have a significantly increased abundance in NDD, but not if probiotics intake and diet are considered. On the contrary, the increase in the family of Corynebacteriaceae and the genus of Odoribacter is only significant when considering relative abundance and the intake of probiotics and diet.
Therefore, the p-values of absolute abundance with the most stringent model (p-value 3), including basic covariates as well as probiotics and diet, will be considered in our following conclusion. However, it is still important to mention the abundances that do not take those into account, as well as the relative abundance for comparison purposes with other studies.
To conclude this section about bacteriome in NDD, the NDD group shows no significant changes at the phylum level and class level when considering absolute abundance and when including probiotic intake and diet in the covariates. As a result, these are the following taxa that are significantly changed in NDD compared to CTRL: orders of Enterobacteriales and RF32, the family of Enterobacteriaceae, genera of SMB53, Escherichia, Clostridium, Butyricicoccus, Enterococcus, Lactococcus, Veillonella, Coprococcus, which all increase except for Coprococcus, which decreases in NDD. Interestingly, the relative mean abundance of Prevotella (Figure 1A) suggest a possible increase in NDD. However, this is not significant with either of the models, even when using relative abundance (data not shown).
3.2.3. Comparing NDD Group to GID Group: Commonalities and Specificities in the Bacteriome
Table 5 shows that the GID group holds only a limited number of significant changes in bacterial abundances compared to CTRL. Interestingly, apart from SMB3, Echerichia coli and Anaerotruncus, which are increased in NDD compared to GID, and Odoribacter, which is decreased in NDD, no other significant changes are noticed between NDD and GID (Table 6). Table 4, Table 5 and Table 6 depict only a limited number of significant changes in GID compared to the CTRL group, as well as a limited number of significant changes in GID compared to the NDD group.

Table 5.
Significant relative abundances of bacterial taxa in GID compared to CTRL.

Table 6.
Significant absolute abundances of bacterial taxa in NDD compared to GID.
Added to the alpha and beta diversity data observed in Figure 1, this suggests that the bacterial diversity of the GID group lies somewhere between the CTRL and the NDD groups and that the bacterial dysbiosis observed in GID is less pronounced than in the NDD group.
3.3. The Fungal Microbiome or Mycobiome: A Neglected but Important Player
3.3.1. An Increased Fungal Diversity in the NDD Group
The fungal microbiome or mycobiome includes two main phyla, which are the Ascomycota and Basidiomycota. Other phyla are Chytridiomycota, Mucoromycota, Mortierellomycota. Saccharomyces genus is so preponderant that its abundance makes visualization of other genera difficult. For this reason, we have separated them: Figure 2 depicts the relative abundance of Saccharomyces in CTRL, NDD and GID groups and shows a significant increase of its abundance in the NDD group (p-value = 0.0044; Appendix A, Table A1).
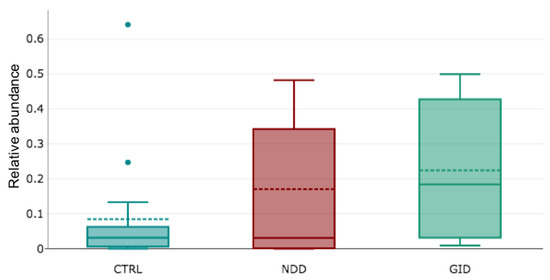
Figure 2.
Box plot of relative abundance of Saccharomyces genus in CTRL, NDD and GID groups.
Figure 3 shows the mean relative abundance of the 14 most significant fungal genera in NDD, CTRL and GID. Interestingly, 10 of those genera are unidentified: one of them being from the Chytridiomycota phylum, two others from the Basidiomycota phylum and the rest from the Ascomycota phylum. We note that the fungal diversity is actually increased in NDD compared to both CTRL and GID, which might indicate the overgrowth of certain fungal species (Figure 3A,B).
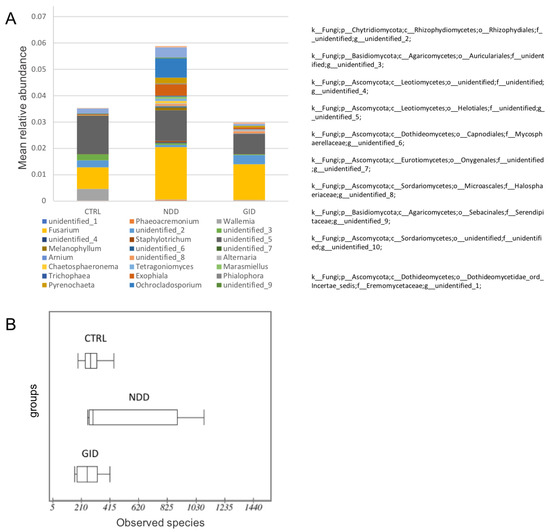
Figure 3.
Fungal alpha diversity in CTRL, NDD and GID. Mean relative abundances of 14 most significant fungal genera and the higher taxonomic classification of the unidentified genera (A); alpha diversity by the number of observed species (B).
3.3.2. An Increased Fungal Abundance in the NDD Group
Significant changes in abundance of the mycobiome community in NDD were observed at all taxa levels (Appendix A Table A1). Among the 28 genera that significantly changed, Saccharomycces is noted as one of the most significant genera for which the abundance increases in NDD (Appendix A Table A1). Interestingly, the sequencing noted 15 unidentified species that changed significantly in NDD (nine significantly increased; six significantly decreased). Most of the unidentified species are from the Ascomycota phylum (11 out of 15), two are from the Basidiomycota phylum, one is from the Mortierellomycota phylum and one is from the Chytridiomycota phylum.
In the previous section, p-values of bacterial abundances did not seem to greatly differ, as we included additonal variables into the model (probiotics intake and diet). However, fungi abundances seem to be greatly affected by the intake of probiotics and the type of diet, as including those variables resulted in the appearance/disappearance of a higher number of significantly different abundant taxa (Appendix A Table A1). For this reason, the following tables depict absolute abundance using the most stringent model.
Table 7 lists the 26 species with significantly increased abundance in NDD compared to CTRL and the 10 species with significantly decreased absolute abundance.

Table 7.
Significant absolute abundance in NDD compared to CTRL at the species level.
3.3.3. Mycobiome Is Key to Differentiate between NDD and GID Dysbiosis
As described in the previous section, the bacteriome of NDD and GID groups only showed significant differences in three genera and one species. However, the exploration of the mycobiome shows that, unlike for the bacteriome, NDD and GID show distinct fungal communities. Indeed, the regression analysis across all fungal taxa shows a significant change in abundance in NDD compared to GID in 1 Phylum, 4 classes, 4 orders, 5 families, 8 genera, and 10 species of fungi. Table 8 depicts all significant changes at each taxa level.

Table 8.
Significant changes in Fungal abundance in NDD compared to GID.
On the contrary, only 10 species are significantly increased in the GID compared to the CTRL group (Table 9), supporting the hypothesis of a GID mycobiome diversity that is closer to the CTRL group than the NDD group.

Table 9.
Fungi in GID compared to CTRL at the species level.
Several of our participants (three CTRL, two NDD and three GID) reported suffering from recurrent mucosal or dermal Candida infections (data not shown). Although the most reported type of candidiasis is by Candida Albicans, this species did not appear in this analysis as being significantly changed, neither in NDD nor in GID. However, the species Candida parapsilosis was found to be significantly increased (p = 0.0159) in GID compared to CTRL.
3.4. Exposure to Antibiotics at Early Age Is Associated with a Significant Fungal Dysbiosis
3.4.1. Effect of Recurrent Exposure to Antibiotics before 3 Years: An Independent t-Test
As our participants who provided stool samples also have answered a question regarding their exposure to recurrent courses of antibiotics before age 3, we were interested in exploring dysbiosis in those two groups: exposed (+ABX) or not exposed (−ABX). The independent T-test was run for both bacteria and fungi, using absolute and relative abundances, and at all taxa levels. Only results using absolute abundance are shown in Table 10. Interestingly, results indicate that exposure to ABX is associated with greater fungal dysbiosis than bacterial dysbiosis. Indeed, for bacteria, only one phylum, one class, one family, four genera and two species are significantly different. However, for fungi, 1 phylum, 2 classes, 4 orders, 6 families, 10 genera and 12 species show a significantly different abundance between the two groups.

Table 10.
Significant p-values using the t-test for +ABX vs. -ABX for bacteria and fungi at all taxa levels.
For this reason, to explore further, a regression analysis for the new variable ABX was performed, while accounting for the basic covariates, probiotic intake, diet and the group NDD or GID. Table 10 below shows the p-value for the use of antibiotics before 3Y in NDD group for both bacteria and fungi.
Table 10 shows the p-value for the use of antibiotics before 3Y in the GID group for both bacteria and fungi.
Whether in NDD or GID, antibiotics seem to be associated with more fungal dysbiosis than bacterial dysbiosis, as more taxa are affected in the fungi kingdom.
3.4.2. Regression Analysis for the Effect of ABX
The analysis noted that while taking into account the NDD group in addition to the basic covariates and probiotics and diet, the abundance of two bacterial families, eight bacterial genera and two bacterial species are significantly changed when exposed to ABX at an early age, and that two fungal phyla, 4 fungal orders, 4 fungal families, 11 fungal genera and 12 fungal species (two of them non-identified) are significantly affected as well (Table 11).

Table 11.
Significant changes in bacterial and fungal absolute abundance with ABX exposure in NDD.
Therefore, although the regression model results in some different taxa involved compared to the t-test, it still confirms that early ABX exposure does affect the fungi kingdom in NDD and maybe even more than bacteria.
The same pattern is noticed for GID (Table 12). Indeed, only two bacterial genera—Atopobium and Allobaculum—and one bacterial species—eggerthii—were affected by introducing the ABX exposure variable. However, 2 fungal phyla, 3 classes, 3 orders, 6 families, 11 genera and 13 species of fungi are significantly associated with early ABX exposure.

Table 12.
Significant changes in bacterial and fungal absolute abundance with ABX exposure in GID.
4. Discussion
4.1. From GID Group to NDD Group: What Do They Have in Common, and in What Ways Are They Different?
Several studies have shown that gut dysbiosis in both ASD and IBD is characterized by a decrease in biodiversity [42,43,44]. The present study also noted that the bacterial alpha diversity is significantly decreased in both NDD and GID groups compared to the CTRL group, but more significant for the NDD than the GID group. In the GID group, Streptococcus luteciae was the only significant species increasing compared to CTRL. A study by Liang et al. [45] also showed in a model of colitis-associated colorectal cancer that this species was increased during the process of carcinogenesis. However, E. coli was shown to be increased in our NDD cohort, while some studies showed the opposite [46]. As compared to the NDD group, the bacterial dysbiosis observed in GID only showed significant differences in three genera and one species, and all other taxa were not significantly different. Interestingly, this GID group also exhibited only a limited number of significant changes compared to the CTRL group. This suggests that the GID bacteriome is somewhere between the CTRL and the NDD and that only a limited number of taxa seem to differentiate between the groups.
This raised the following question: is there another gut component that presents more significant changes between the groups? This study provides preliminary data that support the following hypothesis: GID and NDD share a similar bacteriome dysbiosis but have a different mycobiome. Indeed, while the alpha diversity decreases for bacteria in both NDD and GID groups, it rather significantly increases for fungi in the NDD group (Figure 1C and Figure 3B). Moreover, the regression analysis across all fungal taxa showed a significant change in abundance in NDD compared to GID group across all taxa levels, with 1 Phylum, 4 classes, 4 orders, 5 families, 8 genera and 10 species of fungi. Interestingly, the comparison of Table 8 and Table 9 suggests that the GID group is characterized by an increased abundance in 10 fungal species compared to CTRL and eight different other species compared to NDD group, suggesting that the fungal dysbiosis in GID might be key to understanding both the development of GID and NDD.
One of the identified fungi that is significantly increased in GID is Saccharomyces cereviciae (S. Cereviciae). A recent study by Torres et al. [47] has identified antibodies against S. Cereviciae as one of the 51 biomarkers that can successfully predict the diagnosis of Crohn’s disease within 5 years with high accuracy. We also showed a significant increase in S. Cereviciae in the NDD group, as shown by other studies [33]. This suggests that the autoimmunity against this specific species might be a good predictor of those individuals in the NDD group who will also develop severe GID [48].
The presence of 15 unidentified fungal species whose abundance significantly changes in NDD not only emphasizes the possible crucial role of fungi in microbiome dysbiosis and pathogenesis in NDD but also testifies of the need for more studies on the mycobiome. Indeed, studies have shown that only a limited number of internal transcribed spacer DNA sequences are available on GenBank [49]. Recent findings suggest that there is competition between bacteria and fungi and that prolonged antibiotic use is associated with fungal infections [50,51]. Therefore, fungi seem to be key in understanding the spectrum going from simple gastrointestinal disorders to more complex neurodevelopmental disorders that combine with gastrointestinal disorders. Fungi might also be key in understanding the spectrum of symptomatology in ASD. While this study is only a pilot study with a relatively small sample size, it points to important directions to explore further to understanding pathogenesis of NDD and GID.
4.2. Need for Standard Measurements and Limitations of the Study
Data suggest that there is a need for establishing measurement standards in order to reveal the microbial communities that play a key role in pathogenesis of the diseases.
In addition to methods of extractions, sample origin and bacterial culture, data suggest that the use of relative vs. absolute abundance as well as including some factors related to lifestyle such as probiotic intake or diet can lead to large discrepancies between data obtained from various studies [52,53]. While the gold standard approach to accurately determine taxonomic shifts is to use absolute abundance [54], most studies replace this laborious requirement by the use of the relative abundance. In this study, both relative and absolute abundances were computed to allow for comparisons with other studies, and four different regression models were used to include some additional variables. Studies have shown that the delivery mode, exclusive breastfeeding, stress, smoking, sleeping and exercise are also important factors that have been associated with changes in microbiome composition and could also be used as additional variables [55]. Our study only looked at probiotic intake and diet. Some examples of the discrepancies found are as follows: the increase of the order of Clostridiales in NDD is only significant when using relative abundance. The species bifidum and dispar seem to have a significantly increased abundance in NDD, but not if probiotics intake and diet are considered. Studies have indeed shown that bifidum is actually decreased in children with ASD [56,57]. Similarly, the increase in the family of Corynebacteriaceae and the genus of Odoribacter is only significant when considering relative abundance and the intake of probiotics and diet. This emphasizes that not considering lifestyle in microbiome studies could lead to misinterpretations and be a barrier to finding microbial signatures.
While investigators have ensured that none of the participants were exposed to antibiotics a month before the stool collection, another limitation of this study to consider is that they did not ask participants whether they had been taking an anti-fungal in the past months prior to the stool collection. Although the intake of anti-fungals among children is not as common as intake of antibiotics, the observed decreased abundance of some fungal species in NDD must take that into account.
In addition, it is important to note that these observations among GID and NDD groups were performed on different age groups (Table 1). Indeed, most NDD participants were between 3 to 18 years old, and most GID participants were between 19 to 60 years old. Nevertheless, studies observed in healthy individuals that the gut microbiota is stabilized in an adult-like configuration after the first 3 years of life [28,58,59,60]. A study by Nagpal et al. [61] showed that the most significant change in microbiome composition happens during the window of 0 to 3 years after birth. Furthermore, the decline in microbiome diversity that occurs with age has been described by studies to be a consequence of several lifestyle changes, including nutrition [62]. Since age and lifestyle are confounding factors, it is very difficult to separate the effect of aging from the dietary change effect on the gut microbiome. Due to these reasons, this pilot study did not consider the age variable in its statistics but considered an aspect of the diet.
4.3. Effect of Probiotics and Diet
Fermented foods, such as cultured milk products and yogurt, provide the body with ingestible microorganisms that may modulate the intestinal homeostasis and treat or prevent IBD [63]. Consumption of probiotics was reported to increase the abundance of Bifidobacteria and/or Lactobacilli and to reduce the counts of E. coli and Helicobacter pylori. Furthermore, they regulate inflammation by reducing the proinflammatory cytokine IL-6, and increasing the anti-inflammatory cytokine IL-10. Probiotics were also found to increase the total serum IgA, which potentiates the humoral immune response. In addition, they inhibit the adhesion of pathogens to the intestinal mucosa and promote the production of SCFA [64].
In this study, it was observed that higher proportions of the NDD group consumed a diet with medium to high processed carbohydrates as compared to the GID and CTRL groups. It has been well established that carbohydrates can modify the gut microbiome [65,66]. Artificial sweeteners, often present in processed food, have also been shown to alter the gut microbiota [67,68]. A growing number of studies have shown the importance of dietary fibers for the growth of beneficial microbes [69,70,71,72].
Unfortunately, the questionnaire was not specific enough, nor was the sample size large enough, to allow for more in-depth evaluation of the effect of these two factors (probiotic and diet). Only 9 out of 55 participants had a diet low in processed food, which made it difficult to investigate the effect of processed food on bacteria and fungi.
Nevertheless, the p-values of fungi taxa abundance were greatly affected by adding probiotic intake to the regression model (Appendix A Table A1). As the questionnaire did not specifically ask for which type of probiotic participants were taking, the assumption is that they used bacterial probiotics, as most probiotics sold over the counter are bacterial. Together, these data suggest, probably, that there is an interaction between bacteria and fungi that influences fungal abundance. Indeed, bacteria and fungi have been shown to interact in various ways, including cell contact, quorum sensing, pH changes and use of metabolites by-products [73].
As an increase in the abundance of S. cereviciae was noted in both GID and NDD groups, it is important to mention that this species is often named S. boulardii, a probiotic, that has a beneficial effect on several strains of enteropathogenic bacteria [32,74,75,76]. The present data question again whether cereviciae and boulardii are the same species [76].
4.4. Effect of Early Antibiotic Exposure
Slob et al. [35] showed in a cohort of 27,781 twins that early exposure to antibiotics significantly increased the risk of developing ADHD and ASD. As our previous study also showed a significant association between early exposure to recurrent courses of antibiotics developing an NDD [40], we decided to explore this question further and run both an independent t-test and a regression model to investigate the taxa that could be affected by early antibiotic exposure. While the sample size is relatively small, this preliminary exploration aims principally at drawing future directions of investigations. One of the genera that were affected is Prevotella. Interestingly, Figure 1A suggests a possible increase in NDD of the relative mean abundance of Prevotella. However, this is not significant with either of the models, even when using relative abundance. Nevertheless, the regression analysis using relative abundance and all covariates—including diet and probiotics—for the NDD group, showed a significant decrease in Prevotella in NDD participants who were exposed to recurrent courses of antibiotics before 3 years. Our limited sample size might explain why Prevotella did not show up in the significantly decreased genera in NDD, as shown by a high number of studies [77,78,79,80,81].
Results also suggest that early ABX exposure is associated with greater fungal dysbiosis than bacterial dysbiosis, which again suggests the existence of essential interactions between bacteria and fungi kingdoms. Indeed, studies show that antibiotics, although directly targeting bacteria, by creating an imbalance in bacteriome, can lead to fungal overgrowth of certain species that might be later involved in the production of immune response modulators [36,40,82].
The effect of antibiotics on gut microbiota depends on the type of antibiotic, timing and duration of consumption, and microbiome modulatory factors such as age, travel, underlying illness, antibiotic resistance pattern and diet. The administration of antibiotics during childhood usually consists of short courses of relatively narrow spectrum agents for respiratory tract and oropharyngeal infections [83]. The abundance of Clostridiales and Ruminococcus was reduced as a result of postnatal exposure to antibiotics. However, the exposed and non-exposed groups had a similar overall number of species and diversity after 1 year of life [84]. This effect can be magnified due to the excessive use of antibiotics or underlying gastrointestinal conditions [84]. Thus, antibiotics, diet and environment may substantially impact the development of the gut microbiome of the developing child. Therefore, antibiotics consumption may indirectly modulate the intestinal immune homeostasis by shifting the structure of the gut microbiota [85].
4.5. Important Taxa to Explore Further
The Firmicutes/Bacteroidetes (F/B) ratio is believed to play an important role in maintaining gut homeostasis (Stojanov et al., 2020). In fact, studies have shown an increase in this ratio. Contradicting results have been published earlier, some showing a decrease in this ratio due to a decrease of Bacteroidetes in ASD [86,87,88,89] and others showing an increase [90]. In GID, Firmicutes, which is normally the most dominant phylum in the microbiota of healthy guts, was observed at significantly lower levels, leading to an observed decrease in the F/B ratio [14,16]. In this study, the ratio of Firmicutes/Bacteroidetes was significantly decreased in NDD compared to CTRL (p = 0.045), and this was due to a decrease in Firmicutes. Interestingly, this same ratio in GID is significantly different from neither CTRL (p-value 3 = 0.402) nor NDD groups (p-value 3 = 0.87, data not shown), supporting the hypothesis that the “dysbiotic window” is extremely narrow and that small changes in bacteriome composition could result in association with different disorders. Many studies have explored the F/B ratio as acetate and propionate are mainly produced by Bacteroidetes, whereas Firmicutes contribute primarily to the production of butyrate [91,92,93]. Acetate, propionate and butyrate are short-chain fatty acids that have been shown to be essential for the development the immune system as well as neurodevelopment [64,91,92,93,94].
While the focus is often made on the F/B ratio, a change in the F/B ratio is often the result of concomitant changes in the levels of other phyla like Proteobacteria and Actinobacteria. Observing ratios can inform of the general dynamics between phyla. Therefore, the R package was used to investigate changes of all possible ratios at all taxa levels to explore the significant change in any other ratio. Table 1 shows only the significant ratio changes at the phylum level. While these ratios were not previously described, they are worth mentioning because of their significance. We noticed indeed a significant decrease in the Firmicutes/Actinobacteria ratio in NDD compared to both CTRL and GID, and this is due to an increase in Actinobacteria and a decrease in Firmicutes in the NDD group (data not shown).
Indeed, Actinobacteria, despite their lower abundance, also play an important role in the maintenance of gut homeostasis [95], and this is confirmed by studies on the probiotic role of Bifidobacteria, which is a class of Actinobacteria. Interestingly, the TM7 phylum or Saccharibacteria have been shown to be an obligate epibiont parasite of diverse Actinobacteria [96], and changes in TM7 might be due to changes in Actinobacteria. In the GID group, the ratios involving the Saccharibacteria TM7 are decreased: TM7/Actinobacteria (p = 0.041) and TM7/Firmicutes (p = 0.044). A study has investigated the association of TM7 with IBD and noted a genetic alteration for an antibiotic resistance gene of TM7 species in IBD participants [41].
Other taxa levels have shown significant changes in abundance and have also been mentioned by other studies, such as the orders of Enterobacteriales [86] and RF32 [97] and the family of Enterobacteriaceae and Butyricicoccus [86]. Butyricicoccus pullicaecorum is a butyrate-producing bacterium that has been shown by other studies to be significantly decreased in NDD compared to CTRL [98]. However, this current study shows that it is significantly increased in NDD compared to CTRL. Interestingly, a recent exploration of the gut microbiome of autistic infants in various age groups also found a higher relative abundance of Butyricicoccus pullicaecorum at 3 years old [55]. This observation emphasizes the importance of considering all factors that might influence the gut microbiome composition in statistical analyses, including age, and other factors mentioned [55]. Coprococcus is butyric acid-producing bacterium that has been shown to have a protective role. Its decrease in NDD shown in this present study has also been observed by a number of other studies [81,90,99].
Among the 28 fungal genera that significantly changed, Saccharomycces and its species Saccharomycces cereviciae are noted as one of the most significantly changed genus and species with an increased abundance in NDD (Table A1). While several studies have confirmed the same [33], some others have shown that the probiotic yeast Saccharomyces boulardii efficiently reduced anxiety-related symptoms in ASD [32,100,101]. Some studies consider S. boulardii as a strain of S. cereviciae [76], which would contradict our data. This raises again the question of whether S. boulardii is a distinct species from S. cereviciae [32,76].
4.6. Importance of the Gut Microbiome in Immunity Development
The human gut bacteriome contributes to the health of the host by producing short-chain fatty acids (SCFAs) such as butyrate, propionate and acetate. These are considered as a major source of energy for the intestinal epithelia in addition to their role in enhancing the integrity of the mucosal barrier [92,102]. With the decreased bacterial biodiversity observed in both GID and NDD, lower amounts of these SCFAs should be expected in future metabolomics explorations.
Moreover, the gut microbiota, including fungi, plays an essential role in modulating both the innate and adaptive arms of the immune response through various mechanisms [103,104,105,106]. by downregulating the secretion of the pro-inflammatory cytokines [107] and increasing levels of inflammatory markers such as lactoferrin are expected to be found in these groups.
Therefore, it is not surprising that auto-immunity is often mentioned to explain IBD, celiac disease and also ASD or ADHD [108,109]
Further metabolomics explorations are needed to determine the presence of immune alterations in NDD and GID and whether those can be reversible by targeting bacterial and/or the fungal gut dysbiosis.
5. Conclusions
In recent years, the gut microbiota has been considered as an important actor of the gut–brain axis, making it possible to take new steps in understanding neurological diseases and neurodevelopmental disorders as well as gastrointestinal disorders. While most studies, including large-scale projects such as the Human Microbiome project, were exclusively focusing on the bacteriome, the mycobiome was neglected. In this study, we unravel how, unlike for bacterial dysbiosis, the fungal dysbiosis in NDD is such that its diversity is increased while the abundance of specific taxa is decreased. The opposite pattern was observed with bacterial dysbiosis in NDD, with a decrease in alpha diversity and an increase in abundance of a high number of taxa.
While the GID group elicits only a small number of significant differences in bacterial abundances compared to CTRL and compared to NDD, the mycobiome seem to be the component that differentiates the GID and the NDD group. We identified 10 fungal species that increase in GID compared to CTRL and 8 other species that are increased in GID compared to NDD. Our model in Figure 4 summarizes these findings.
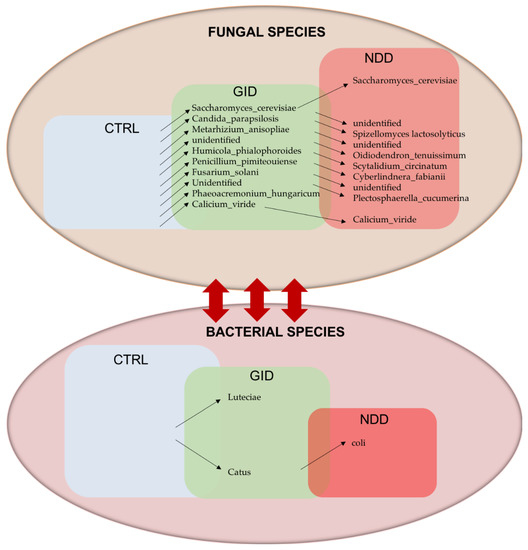
Figure 4.
Fungal species as key players in GID and NDD. Arrows up show the increasing abundance of species, and arrows down show the decreasing abundance of species between two consecutive boxes. Height of the boxes CTRL, GID and NDD simulates the increasing alpha diversity for fungi and the decreasing alpha diversity for bacteria. The double arrow shows the interaction between bacteria and fungi.
Interestingly, probiotic intake, diet and antibiotic exposure had a greater effect on fungal abundances than bacterial abundances, suggesting the presence of strong interactions between both kingdoms. These interactions will be key in further understanding the pathogenesis of various diseases of the gut–brain axis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9040741/s1, Table S1: Ratios in GID compared to CTRL: top 5 most significant ratios at each level; Table S2: Bacterial Ratios in NDD compared to GID: top 5 most significant ratios at each level; Video S1: Video Abstract: Bacterial and Fungal Dysbiosis in disorders of the Gut-Brain axis.
Author Contributions
Conceptualization, G.B., N.A.Y., D.Z.; methodology, G.B., N.A.Y., D.Z.; I.L., A.S., D.A.-A., K.P., Z.B., M.S., N.M.; software, N.A.Y., I.L.; validation, G.B., N.A.Y.; formal analysis, G.B., N.A.Y., D.Z.; I.L., Z.B., D.A.-A., A.S., K.P., M.S., N.M.; investigation, G.B., N.A.Y., D.Z.; I.L., Z.B., D.A.-A., A.S., K.P., M.S., N.M.; data curation, G.B., N.A.Y., I.L., Z.B., D.A.-A., A.S., K.P., M.S., N.M., D.Z., A.C.; writing—original draft preparation, G.B., I.L., A.C., D.Z., N.A.Y.; writing—review and editing, G.B., N.A.Y., D.Z.; I.L., A.C., Z.B., D.A.-A., A.S., K.P., M.S., N.M.; visualization, G.B., N.A.Y., I.L., A.C.; supervision, G.B., N.A.Y., D.Z.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Qatar National Research Funds, grant number 5727002821. and the APC was funded by Weill Cornell Medicine—Qatar Distributed eLibrary.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Weill Cornell Medicine Qatar (protocol code 17-00026, first approved on 30 November 2017, reviewed on 18 November 2018, reviewed on 27 November 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We would like to thank Marco Ameduri, Senior Associate Dean for Premedical Education and Education City Collaborative Curricular Affairs, for allowing the lab-based experiments to take place in the Premedical Microbiology Lab, and Rachid Bendriss, Rachid Bendriss, Associate Dean for Foundation, Student Outreach and Educational Development Programs, for funding part of the recruitment phase. The publication of this article was funded by the Weill Cornell Medicine—Qatar Distributed eLibrary.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Significant changes in abundance of the mycobiome community in NDD are observed at all taxa levels.
Table A1.
Significant changes in abundance of the mycobiome community in NDD are observed at all taxa levels.
| Level | NDD Absolute Abundance Compared to CTRL | Name | Basic Covariates | Adding Probiotics | Adding Diet | Adding Probiotics and Diet | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | R-Squared | Beta | SEbeta | p-Value | p-Value 1 | p-Value 2 | p-Value 3 | |||
| Phylum | Chytridiomycota | k__Fungi;p__Chytridiomycota; | 14 | 0.4210 | −1.8554 | 0.5119 | 0.0028 | 0.0019 | 0.0029 | 0.0013 |
| Class | Rhizophydiomycetes | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes; | 14 | 0.4610 | −1.7178 | 0.4920 | 0.0036 | 0.0249 | NS | 0.0111 |
| Orbiliomycetes | k__Fungi;p__Ascomycota;c__Orbiliomycetes; | 14 | 0.2538 | 0.7096 | 0.3110 | 0.0387 | NS | NS | NS | |
| Pezizomycetes | k__Fungi;p__Ascomycota;c__Pezizomycetes; | 14 | 0.1742 | −1.3952 | 0.6216 | 0.0415 | 0.0144 | 0.0378 | 0.0073 | |
| Ustilaginomycetes | k__Fungi;p__Basidiomycota;c__Ustilaginomycetes; | 14 | 0.1186 | 1.6869 | 0.7778 | 0.0478 | NS | 0.0538 | NS | |
| Spizellomycetes | k__Fungi;p__Chytridiomycota;c__Spizellomycetes; | 12 | 0.2570 | −1.8647 | 0.7718 | NS | 0.0325 | NS | 0.0496 | |
| Agaricomycetes | k__Fungi;p__Basidiomycota;c__Agaricomycetes; | 12 | 0.7201 | −1.1365 | 0.4985 | NS | 0.0417 | NS | NS | |
| Eurotiomycetes | k__Fungi;p__Ascomycota;c__Eurotiomycetes; | 13 | 0.1476 | 0.7961 | 0.3702 | NS | NS | 0.0509 | NS | |
| unidentified | k__Fungi;p__Ascomycota;c__unidentified; | 11 | 0.2480 | −1.7915 | 0.7848 | NS | NS | NS | 0.0433 | |
| Family | Alphamycetaceae | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes;o__Rhizophydiales;f__Alphamycetaceae; | 14 | 0.3128 | −1.1437 | 0.3700 | 0.0080 | 0.0042 | 0.0510 | |
| unidentified | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__unidentified;f__unidentified; | 14 | 0.4182 | 1.3737 | 0.4703 | 0.0112 | 0.0264 | 0.0180 | 0.0499 | |
| unidentified | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes;o__Rhizophydiales;f__unidentified; | 14 | 0.3771 | −1.3958 | 0.5048 | 0.0152 | 0.0215 | 0.0164 | 0.0183 | |
| Nectriaceae | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Hypocreales;f__Nectriaceae; | 14 | 0.3147 | 1.6213 | 0.6036 | 0.0177 | NS | 0.0253 | NS | |
| Valsaceae | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Diaporthales;f__Valsaceae; | 14 | 0.3067 | 1.1744 | 0.4458 | 0.0196 | NS | 0.0239 | NS | |
| Sordariales_fam_Incertae_sedis | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Sordariales_fam_Incertae_sedis; | 14 | 0.3809 | 0.2410 | 0.0986 | 0.0284 | NS | NS | NS | |
| Entolomataceae | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Agaricales;f__Entolomataceae; | 14 | 0.2524 | −1.8388 | 0.7595 | 0.0296 | 0.0316 | 0.0306 | 0.0255 | |
| Glomerellaceae | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Glomerellales;f__Glomerellaceae; | 14 | 0.2860 | 1.5580 | 0.6711 | 0.0358 | NS | 0.0406 | NS | |
| Ustilaginaceae | k__Fungi;p__Basidiomycota;c__Ustilaginomycetes;o__Ustilaginales;f__Ustilaginaceae; | 14 | 0.1186 | 1.6869 | 0.7778 | 0.0478 | NS | 0.0538 | NS | |
| unidentified | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Auriculariales;f__unidentified; | 12 | 0.3450 | −2.1705 | 0.6456 | NS | 0.0057 | NS | 0.0124 | |
| unidentified | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__Helotiales;f__unidentified; | 12 | 0.4483 | −0.7760 | 0.2780 | NS | 0.0163 | NS | 0.0160 | |
| Herpotrichiellaceae | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales;f__Herpotrichiellaceae; | 12 | 0.2111 | 2.3400 | 0.9128 | NS | 0.0248 | NS | 0.0304 | |
| Halosphaeriaceae | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Microascales;f__Halosphaeriaceae; | 12 | 0.2201 | 1.7823 | 0.7234 | NS | 0.0298 | NS | 0.0246 | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Branch06;f__unidentified; | 12 | 0.3074 | −1.8874 | 0.7661 | NS | 0.0298 | NS | 0.0428 | |
| unidentified | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Onygenales;f__unidentified; | 12 | 0.2042 | 1.1675 | 0.4939 | NS | 0.0358 | NS | 0.0266 | |
| Tetragoniomycetaceae | k__Fungi;p__Basidiomycota;c__Tremellomycetes;o__Trichosporonales;f__Tetragoniomycetaceae; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Omphalotaceae | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Agaricales;f__Omphalotaceae; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Dipodascaceae | k__Fungi;p__Ascomycota;c__Saccharomycetes;o__Saccharomycetales;f__Dipodascaceae; | 12 | 0.0943 | −0.1554 | 0.0662 | NS | 0.0368 | NS | 0.0438 | |
| Cucurbitariaceae | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Cucurbitariaceae; | 12 | 0.1366 | 1.5240 | 0.6699 | NS | 0.0421 | NS | 0.0469 | |
| Sordariales_fam_Incertae_sedis | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Sordariales_fam_Incertae_sedis; | 13 | 0.3350 | 0.2445 | 0.1040 | NS | NS | 0.0351 | NS | |
| Plectosphaerellaceae | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Glomerellales;f__Plectosphaerellaceae; | 11 | 0.3344 | −1.5347 | 0.6468 | NS | NS | NS | 0.0370 | |
| unidentified | k__Fungi;p__Ascomycota;c__unidentified;o__unidentified;f__unidentified; | 11 | 0.2480 | −1.7915 | 0.7848 | NS | NS | NS | 0.0433 | |
| Order | Rhizophydiales | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes;o__Rhizophydiales; | 14 | 0.4610 | −1.7178 | 0.4920 | 0.0036 | 0.0249 | 0.0017 | 0.0111 |
| unidentified | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__unidentified; | 14 | 0.4182 | 1.3737 | 0.4703 | 0.0112 | 0.0264 | 0.0180 | 0.0499 | |
| Diaporthales | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Diaporthales; | 14 | 0.3451 | 1.4422 | 0.4998 | 0.0120 | 0.0534 | 0.0146 | NS | |
| Orbiliales | k__Fungi;p__Ascomycota;c__Orbiliomycetes;o__Orbiliales; | 14 | 0.3375 | 0.4525 | 0.1919 | 0.0334 | NS | 0.0520 | NS | |
| Pezizales | k__Fungi;p__Ascomycota;c__Pezizomycetes;o__Pezizales; | 14 | 0.1964 | −1.3952 | 0.6162 | 0.0400 | 0.0149 | 0.0352 | 0.0070 | |
| Ustilaginales | k__Fungi;p__Basidiomycota;c__Ustilaginomycetes;o__Ustilaginales; | 14 | 0.1186 | 1.6869 | 0.7778 | 0.0478 | NS | 0.0538 | NS | |
| Auriculariales | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Auriculariales; | 12 | 0.3452 | −2.1724 | 0.6460 | NS | 0.0056 | NS | 0.0124 | |
| Branch06 | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Branch06; | 12 | 0.3074 | −1.8874 | 0.7661 | NS | 0.0298 | NS | 0.0428 | |
| Spizellomycetales | k__Fungi;p__Chytridiomycota;c__Spizellomycetes;o__Spizellomycetales; | 12 | 0.2570 | −1.8647 | 0.7718 | NS | 0.0325 | NS | 0.0496 | |
| Chaetothyriales | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales; | 12 | 0.1421 | 2.1636 | 0.9841 | NS | 0.0483 | NS | NS | |
| Leotiomycetes_ord_Incertae_sedis | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__Leotiomycetes_ord_Incertae_sedis; | 11 | 0.1224 | −2.1509 | 0.8922 | NS | NS | NS | 0.0346 | |
| unidentified | k__Fungi;p__Ascomycota;c__unidentified;o__unidentified; | 11 | 0.2480 | −1.7915 | 0.7848 | NS | NS | NS | 0.0433 | |
| Genus | Fusarium | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Hypocreales;f__Nectriaceae;g__Fusarium; | 14 | 0.4268 | 1.8384 | 0.5123 | 0.0030 | 0.0358 | 0.0051 | NS |
| Saccharomyces | k__Fungi;p__Ascomycota;c__Saccharomycetes;o__Saccharomycetales;f__Saccharomycetaceae;g__Saccharomyces; | 14 | 0.4150 | 1.1286 | 0.3333 | 0.0044 | 0.0474 | 0.0069 | NS | |
| Betamyces | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes;o__Rhizophydiales;f__Alphamycetaceae;g__Betamyces; | 14 | 0.3128 | −1.1437 | 0.3700 | 0.0080 | 0.0042 | 0.0510 | ||
| unidentified | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__unidentified;f__unidentified;g__unidentified; | 14 | 0.4182 | 1.3737 | 0.4703 | 0.0112 | 0.0264 | 0.0180 | 0.0499 | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Diaporthales;f__Valsaceae;g__unidentified; | 14 | 0.3207 | 1.9895 | 0.7075 | 0.0139 | 0.0166 | NS | ||
| unidentified | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes;o__Rhizophydiales;f__unidentified;g__unidentified; | 14 | 0.3771 | −1.3958 | 0.5048 | 0.0152 | 0.0215 | 0.0164 | 0.0183 | |
| Staphylotrichum | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Sordariales_fam_Incertae_sedis;g__Staphylotrichum; | 14 | 0.5841 | 0.7721 | 0.2800 | 0.0154 | 0.0454 | 0.0210 | NS | |
| Engyodontium | k__Fungi;p__Ascomycota;c__Ascomycota_cls_Incertae_sedis;o__Ascomycota_ord_Incertae_sedis;f__Ascomycota_fam_Incertae_sedis;g__Engyodontium; | 14 | 0.3469 | −0.7645 | 0.2794 | 0.0161 | NS | 0.0116 | NS | |
| Colletotrichum | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Glomerellales;f__Glomerellaceae;g__Colletotrichum; | 14 | 0.2860 | 1.5580 | 0.6711 | 0.0358 | NS | 0.0406 | NS | |
| Neonectria | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Hypocreales;f__Nectriaceae;g__Neonectria; | 14 | 0.1046 | 1.6340 | 0.7143 | 0.0382 | NS | 0.0533 | NS | |
| Melanophyllum | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Agaricales;f__Agaricaceae;g__Melanophyllum; | 14 | 0.2073 | 1.7648 | 0.7733 | 0.0386 | 0.0156 | 0.0468 | 0.0190 | |
| unidentified | k__Fungi;p__Mucoromycota;c__Mucoromycetes;o__Mucorales;f__Lichtheimiaceae;g__unidentified; | 14 | 0.0957 | 0.0873 | 0.0391 | 0.0423 | NS | 0.0463 | NS | |
| unidentified | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Auriculariales;f__unidentified;g__unidentified; | 11 | 0.3016 | −2.0728 | 0.6945 | NS | 0.0057 | NS | 0.0124 | |
| unidentified | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__Helotiales;f__unidentified;g__unidentified; | 11 | 0.4276 | −0.8382 | 0.2949 | NS | 0.0163 | NS | 0.0160 | |
| Mortierella | k__Fungi;p__Mortierellomycota;c__Mortierellomycetes;o__Mortierellales;f__Mortierellaceae;g__Mortierella; | 11 | 0.4528 | −1.7326 | 0.6124 | NS | 0.0222 | NS | 0.0164 | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Microascales;f__Halosphaeriaceae;g__unidentified; | 11 | 0.2080 | 1.9764 | 0.7597 | NS | 0.0298 | NS | 0.0246 | |
| unidentified | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Capnodiales;f__Mycosphaerellaceae;g__unidentified; | 11 | 0.1891 | 1.1674 | 0.4489 | NS | 0.0210 | NS | 0.0247 | |
| unidentified | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Onygenales;f__unidentified;g__unidentified; | 11 | 0.2070 | 1.3142 | 0.5135 | NS | 0.0358 | NS | 0.0266 | |
| Arnium | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Lasiosphaeriaceae;g__Arnium; | 11 | 0.0973 | 2.1493 | 0.9062 | NS | 0.0277 | NS | 0.0370 | |
| Chaetosphaeronema | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Phaeosphaeriaceae;g__Chaetosphaeronema; | 11 | 0.0922 | 2.4429 | 1.0354 | NS | 0.0273 | NS | 0.0379 | |
| Tetragoniomyces | k__Fungi;p__Basidiomycota;c__Tremellomycetes;o__Trichosporonales;f__Tetragoniomycetaceae;g__Tetragoniomyces; | 11 | 0.0954 | 2.4512 | 1.0611 | NS | NS | NS | 0.0413 | |
| Marasmiellus | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Agaricales;f__Omphalotaceae;g__Marasmiellus; | 11 | 0.0954 | 2.4512 | 1.0611 | NS | 0.0366 | NS | 0.0413 | |
| Trichophaea | k__Fungi;p__Ascomycota;c__Pezizomycetes;o__Pezizales;f__Pyronemataceae;g__Trichophaea; | 11 | 0.0954 | 2.4512 | 1.0611 | NS | 0.0366 | NS | 0.0413 | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Branch06;f__unidentified;g__unidentified; | 11 | 0.2450 | −1.9076 | 0.8332 | NS | 0.0298 | NS | 0.0428 | |
| unidentified | k__Fungi;p__Ascomycota;c__unidentified;o__unidentified;f__unidentified;g__unidentified; | 11 | 0.2480 | −1.7915 | 0.7848 | NS | NS | NS | 0.0433 | |
| Exophiala | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales;f__Herpotrichiellaceae;g__Exophiala; | 11 | 0.0584 | 2.3498 | 1.0471 | NS | 0.0383 | NS | 0.0464 | |
| Pyrenochaeta | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Cucurbitariaceae;g__Pyrenochaeta; | 11 | 0.0663 | 1.6738 | 0.7547 | NS | 0.0435 | NS | 0.0486 | |
| Plectosphaerella | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Glomerellales;f__Plectosphaerellaceae;g__Plectosphaerella; | 11 | 0.1903 | −1.3601 | 0.6277 | NS | NS | NS | 0.0531 | |
| Species | Saccharomyces_cerevisiae | k__Fungi;p__Ascomycota;c__Saccharomycetes;o__Saccharomycetales;f__Saccharomycetaceae;g__Saccharomyces;s__Saccharomyces_cerevisiae; | 14 | 0.4150 | 1.1286 | 0.3333 | 0.0044 | 0.0474 | 0.0069 | NS |
| Betamyces_americae-meridionalis | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes;o__Rhizophydiales;f__Alphamycetaceae;g__Betamyces;s__Betamyces_americae-meridionalis; | 14 | 0.3128 | −1.1437 | 0.3700 | 0.0080 | NS | 0.0042 | 0.0510 | |
| unidentified | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__unidentified;f__unidentified;g__unidentified;s__unidentified; | 14 | 0.4182 | 1.3737 | 0.4703 | 0.0112 | 0.0264 | 0.0180 | 0.0499 | |
| Peziza_nivalis | k__Fungi;p__Ascomycota;c__Pezizomycetes;o__Pezizales;f__Pezizaceae;g__Peziza;s__Peziza_nivalis; | 14 | 0.2416 | 0.4322 | 0.1520 | 0.0130 | 0.0267 | 0.0145 | 0.0260 | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Diaporthales;f__Valsaceae;g__unidentified;s__unidentified; | 14 | 0.3207 | 1.9895 | 0.7075 | 0.0139 | NS | 0.0166 | NS | |
| Fusarium_oxysporum | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Hypocreales;f__Nectriaceae;g__Fusarium;s__Fusarium_oxysporum; | 14 | 0.3128 | 1.4464 | 0.5171 | 0.0143 | NS | 0.0163 | NS | |
| unidentified | k__Fungi;p__Chytridiomycota;c__Rhizophydiomycetes;o__Rhizophydiales;f__unidentified;g__unidentified;s__unidentified; | 14 | 0.3771 | −1.3958 | 0.5048 | 0.0152 | 0.0215 | 0.0164 | 0.0183 | |
| Staphylotrichum_coccosporum | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Sordariales_fam_Incertae_sedis;g__Staphylotrichum;s__Staphylotrichum_coccosporum; | 14 | 0.5851 | 0.8130 | 0.2963 | 0.0158 | 0.0449 | 0.0214 | NS | |
| Engyodontium_album | k__Fungi;p__Ascomycota;c__Ascomycota_cls_Incertae_sedis;o__Ascomycota_ord_Incertae_sedis;f__Ascomycota_fam_Incertae_sedis;g__Engyodontium;s__Engyodontium_album; | 14 | 0.3469 | −0.7645 | 0.2794 | 0.0161 | NS | 0.0116 | NS | |
| Chaetomium_erectum | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Chaetomiaceae;g__Chaetomium;s__Chaetomium_erectum; | 14 | 0.2427 | 1.8071 | 0.7522 | 0.0307 | 0.0143 | 0.0378 | 0.0176 | |
| Neonectria_candida | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Hypocreales;f__Nectriaceae;g__Neonectria;s__Neonectria_candida; | 14 | 0.1046 | 1.6340 | 0.7143 | 0.0382 | NS | 0.0533 | NS | |
| Melanophyllum_haematospermum | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Agaricales;f__Agaricaceae;g__Melanophyllum;s__Melanophyllum_haematospermum; | 14 | 0.2073 | 1.7648 | 0.7733 | 0.0386 | 0.0156 | 0.0468 | 0.0190 | |
| unidentified | k__Fungi;p__Mucoromycota;c__Mucoromycetes;o__Mucorales;f__Lichtheimiaceae;g__unidentified;s__unidentified; | 14 | 0.0957 | 0.0873 | 0.0391 | 0.0423 | NS | 0.0463 | NS | |
| Spizellomyces_dolichospermus | k__Fungi;p__Chytridiomycota;c__Spizellomycetes;o__Spizellomycetales;f__Spizellomycetaceae;g__Spizellomyces;s__Spizellomyces_dolichospermus; | 14 | 0.2033 | −1.7153 | 0.7748 | 0.0439 | 0.0455 | 0.0501 | 0.0442 | |
| Hypochnicium_bombycinum | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Corticiales;f__Corticiaceae;g__Hypochnicium;s__Hypochnicium_bombycinum; | 14 | 0.1286 | 0.7133 | 0.3367 | 0.0525 | NS | NS | NS | |
| Microascus_paisii | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Microascales;f__Microascaceae;g__Microascus;s__Microascus_paisii; | 14 | 0.1093 | 0.7894 | 0.3730 | 0.0527 | NS | 0.0442 | NS | |
| unidentified | k__Fungi;p__Ascomycota;c__Leotiomycetes;o__Helotiales;f__unidentified;g__unidentified;s__unidentified; | 12 | 0.4483 | −0.7760 | 0.2780 | NS | 0.0163 | NS | 0.0160 | |
| unidentified | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Auriculariales;f__unidentified;g__unidentified;s__unidentified; | 12 | 0.3450 | −2.1705 | 0.6456 | NS | 0.0057 | NS | 0.0124 | |
| unidentified | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Capnodiales;f__Mycosphaerellaceae;g__unidentified;s__unidentified; | 12 | 0.2411 | 1.1072 | 0.4170 | NS | 0.0210 | NS | 0.0247 | |
| unidentified | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Phaeosphaeriaceae;g__Chaetosphaeronema;s__unidentified; | 12 | 0.1657 | 2.3941 | 0.9530 | NS | 0.0273 | NS | NS | |
| Arnium_arizonense | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Lasiosphaeriaceae;g__Arnium;s__Arnium_arizonense; | 12 | 0.1686 | 2.0909 | 0.8350 | NS | 0.0277 | NS | 0.0370 | |
| Chaetomium_grande | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Sordariales;f__Chaetomiaceae;g__Chaetomium;s__Chaetomium_grande; | 12 | 0.5311 | −1.3565 | 0.5441 | NS | 0.0283 | NS | NS | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Microascales;f__Halosphaeriaceae;g__unidentified;s__unidentified; | 12 | 0.2197 | 1.7837 | 0.7239 | NS | 0.0298 | NS | 0.0246 | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Branch06;f__unidentified;g__unidentified;s__unidentified; | 12 | 0.3074 | −1.8874 | 0.7661 | NS | 0.0298 | NS | NS | |
| Phialophora_mustea | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales;f__Herpotrichiellaceae;g__Phialophora;s__Phialophora_mustea; | 12 | 0.1589 | 2.3167 | 0.9793 | NS | 0.0357 | NS | 0.0404 | |
| unidentified | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Onygenales;f__unidentified;g__unidentified;s__unidentified; | 12 | 0.2042 | 1.1675 | 0.4939 | NS | 0.0358 | NS | 0.0266 | |
| Exophiala_dermatitidis | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales;f__Herpotrichiellaceae;g__Exophiala;s__Exophiala_dermatitidis; | 12 | 0.1551 | 2.2601 | 0.9609 | NS | 0.0366 | NS | 0.0413 | |
| Dioszegia_fristingensis | k__Fungi;p__Basidiomycota;c__Tremellomycetes;o__Tremellales;f__Bulleribasidiaceae;g__Dioszegia;s__Dioszegia_fristingensis; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Pyrenochaeta_keratinophila | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Cucurbitariaceae;g__Pyrenochaeta;s__Pyrenochaeta_keratinophila; | 12 | 0.1551 | 1.7072 | 0.7258 | NS | 0.0366 | NS | 0.0413 | |
| Exophiala_oligosperma | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales;f__Herpotrichiellaceae;g__Exophiala;s__Exophiala_oligosperma; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Alternaria_hungarica | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Pleosporaceae;g__Alternaria;s__Alternaria_hungarica; | 12 | 0.1551 | 0.5971 | 0.2539 | NS | 0.0366 | NS | 0.0413 | |
| Peziza_buxea | k__Fungi;p__Ascomycota;c__Pezizomycetes;o__Pezizales;f__Pezizaceae;g__Peziza;s__Peziza_buxea; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Tetragoniomyces_uliginosus | k__Fungi;p__Basidiomycota;c__Tremellomycetes;o__Trichosporonales;f__Tetragoniomycetaceae;g__Tetragoniomyces;s__Tetragoniomyces_uliginosus; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| unidentified | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Leptosphaeriaceae;g__Leptosphaeria;s__unidentified; | 12 | 0.1551 | 2.3152 | 0.9843 | NS | 0.0366 | NS | 0.0413 | |
| unidentified | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Agaricales;f__Omphalotaceae;g__Marasmiellus;s__unidentified; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Exophiala_phaeomuriformis | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales;f__Herpotrichiellaceae;g__Exophiala;s__Exophiala_phaeomuriformis; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| unidentified | k__Fungi;p__Ascomycota;c__Pezizomycetes;o__Pezizales;f__Pyronemataceae;g__Trichophaea;s__unidentified; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Aspergillus_ochraceus | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Eurotiales;f__Aspergillaceae;g__Aspergillus;s__Aspergillus_ochraceus; | 12 | 0.1551 | 1.4435 | 0.6137 | NS | 0.0366 | NS | 0.0413 | |
| Mortierella_zonata | k__Fungi;p__Mortierellomycota;c__Mortierellomycetes;o__Mortierellales;f__Mortierellaceae;g__Mortierella;s__Mortierella_zonata; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| unidentified | k__Fungi;p__Ascomycota;c__Eurotiomycetes;o__Chaetothyriales;f__Herpotrichiellaceae;g__Phialophora;s__unidentified; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Microascus_albonigrescens | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Microascales;f__Microascaceae;g__Microascus;s__Microascus_albonigrescens; | 12 | 0.1551 | 2.3156 | 0.9845 | NS | 0.0366 | NS | 0.0413 | |
| Coniophora_olivacea | k__Fungi;p__Basidiomycota;c__Agaricomycetes;o__Boletales;f__Coniophoraceae;g__Coniophora;s__Coniophora_olivacea; | 12 | 0.1522 | 2.3083 | 0.9868 | NS | 0.0374 | NS | 0.0420 | |
| Mortierella_amoeboidea | k__Fungi;p__Mortierellomycota;c__Mortierellomycetes;o__Mortierellales;f__Mortierellaceae;g__Mortierella;s__Mortierella_amoeboidea; | 12 | 0.3633 | −1.2981 | 0.5824 | NS | 0.0457 | NS | 0.0549 | |
| unidentified | k__Fungi;p__Mortierellomycota;c__Mortierellomycetes;o__Mortierellales;f__Mortierellaceae;g__Mortierella;s__unidentified; | 12 | 0.3202 | −1.1002 | 0.5135 | NS | 0.0534 | NS | 0.0140 | |
| unidentified | k__Fungi;p__Basidiomycota;c__Wallemiomycetes;o__Wallemiales;f__Wallemiales_fam_Incertae_sedis;g__Wallemia;s__unidentified; | 12 | 0.6577 | −1.1381 | 0.5338 | NS | 0.0544 | NS | NS | |
| unidentified | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Branch06;f__unidentified;g__unidentified;s__unidentified; | 11 | 0.2450 | −1.9076 | 0.8332 | NS | NS | NS | 0.0428 | |
| unidentified | k__Fungi;p__Ascomycota;c__unidentified;o__unidentified;f__unidentified;g__unidentified;s__unidentified; | 11 | 0.2480 | −1.7915 | 0.7848 | NS | NS | NS | 0.0433 | |
| Plectosphaerella_cucumerina | k__Fungi;p__Ascomycota;c__Sordariomycetes;o__Glomerellales;f__Plectosphaerellaceae;g__Plectosphaerella;s__Plectosphaerella_cucumerina; | 11 | 0.1903 | −1.3601 | 0.6277 | NS | NS | NS | 0.0531 | |
| unidentified | k__Fungi;p__Ascomycota;c__Dothideomycetes;o__Pleosporales;f__Phaeosphaeriaceae;g__Chaetosphaeronema;s__unidentified; | 11 | 0.0922 | 2.4429 | 1.0354 | NS | NS | NS | 0.0379 | |
References
- Hand, T.W.; Vujkovic-Cvijin, I.; Ridaura, V.K.; Belkaid, Y. Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol. Metab. 2016, 27, 831–843. [Google Scholar] [CrossRef]
- Sun, J.; Chang, E.B. Exploring gut microbes in human health and disease: Pushing the envelope. Genes Dis. 2014, 1, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Lee, M.; Krishnamurthy, J.; Susi, A.; Sullivan, C.; Gorman, G.H.; Hisle-Gorman, E.; Erdie-Lalena, C.R.; Nylund, C.M. Association of Autism Spectrum Disorders and Inflammatory Bowel Disease. J. Autism Dev. Disord. 2018, 48, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Garrett, V.D.; Brantley, P.J.; Jones, G.N.; McKnight, G.T. The relation between daily stress and Crohn’s disease. J. Behav. Med. 1991, 14, 87–96. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2010, 23, 255-e119. [Google Scholar] [CrossRef]
- Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis. Can. J. Psychiatry 2016, 61, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; A Olson, C.; Hsiao, T.C.F.C.A.O.E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Vendrik, K.E.W.; Ooijevaar, R.E.; De Jong, P.R.C.; Laman, J.D.; Van Oosten, B.W.; Van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Moos, W.H.; Faller, U.V.; Harpp, D.N.; Kanara, I.; Pernokas, J.; Powers, W.R.; Steliou, K. Microbiota and Neurological Disorders: A Gut Feeling. BioResearch Open Access 2016, 5, 137–145. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in. Crime Justice Am. 2012, 417–421. [Google Scholar] [CrossRef]
- Enaud, R.; Vandenborght, L.-E.; Coron, N.; Bazin, T.; Prevel, R.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Lamireau, T.; Delhaes, L. The Mycobiome: A Neglected Component in the Microbiota-Gut-Brain Axis. Microorg. 2018, 6, 22. [Google Scholar] [CrossRef]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal Fungi in Health and Disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Ding, C. The Role of Copper Homeostasis at the Host-Pathogen Axis: From Bacteria to Fungi. Int. J. Mol. Sci. 2019, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Oever, J.T.; Netea, M.G. The bacteriome-mycobiome interaction and antifungal host defense. Eur. J. Immunol. 2014, 44, 3182–3191. [Google Scholar] [CrossRef] [PubMed]
- Al Theyab, A.; Almutairi, T.; Al-Suwaidi, A.M.; Bendriss, G.; McVeigh, C.; Chaari, A. Epigenetic Effects of Gut Metabolites: Exploring the Path of Dietary Prevention of Type 1 Diabetes. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome–gut–brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Stilling, R.; Dinan, T.G.; Cryan, J.F. Microbiome to Brain: Unravelling the Multidirectional Axes of Communication. Adv. Exp. Med. Biol. 2016, 874, 301–336. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLOS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Haartsen, R.; Jones, E.J.; Johnson, M.H. Human brain development over the early years. Curr. Opin. Behav. Sci. 2016, 10, 149–154. [Google Scholar] [CrossRef]
- Yang, I.; Corwin, E.J.; Brennan, P.A.; Jordan, S.; Murphy, J.R.; Dunlop, A. The Infant Microbiome. Nurs. Res. 2016, 65, 76–88. [Google Scholar] [CrossRef]
- Rook, G.A.; Lowry, C.A.; Raison, C.L. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Res. 2015, 1617, 47–62. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Bressan, P.; Kramer, P. Bread and Other Edible Agents of Mental Disease. Front. Hum. Neurosci. 2016, 10, 130. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef]
- Abdellatif, B.; McVeigh, C.; Bendriss, G.; Chaari, A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Zou, R.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zheng, H. Dysbiosis of Gut Fungal Microbiota in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2021, 51, 267–275. [Google Scholar] [CrossRef]
- Łukasik, J.; Patro-Gołąb, B.; Horvath, A.; Baron, R.; Szajewska, H. Sawanti Working Group Early Life Exposure to Antibiotics and Autism Spectrum Disorders: A Systematic Review. J. Autism Dev. Disord. 2019, 49, 3866–3876. [Google Scholar] [CrossRef]
- A Slob, E.M.; Brew, B.K.; Vijverberg, S.J.H.; Dijs, T.; Beijsterveldt, C.E.M.V.; Koppelman, G.H.; Bartels, M.; Dolan, C.V.; Larsson, H.; Lundström, S.; et al. Early-life antibiotic use and risk of attention-deficit hyperactivity disorder and autism spectrum disorder: Results of a discordant twin study. Int. J. Epidemiol. 2020, dyaa168. [Google Scholar] [CrossRef]
- Heijtz, R.D. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal Med. 2016, 21, 410–417. [Google Scholar] [CrossRef]
- Hamad, A.F.; Alessi-Severini, S.; Mahmud, S.M.; Brownell, M.; Kuo, I.F. Early childhood antibiotics use and autism spectrum disorders: A population-based cohort study. Int. J. Epidemiol. 2018, 47, 1497–1506. [Google Scholar] [CrossRef]
- Jaureguiberry, M.S.; Venturino, A. Nutritional and environmental contributions to Autism Spectrum Disorders: Focus on nutrigenomics as complementary therapy. Int. J. Vitam. Nutr. Res. 2020, 1–19. [Google Scholar] [CrossRef]
- Alshaban, F. Prevalence of Autism Spectrum Disorders in Qatar. Qatar Found. Annu. Res. Forum Proc. 2011, 2011, BMP64. [Google Scholar] [CrossRef]
- Bendriss, G.; Al-Ali, D.; Shafiq, A.; Laswi, I.; Mhaimeed, N.; Salameh, M.; Burney, Z.; Pillai, K.; Chaari, A.; Zakaria, D.; et al. Targeting the gut microbiome: A brief report on the awareness, practice, and readiness to engage in clinical interventions in Qatar. Qatar Med. J. 2021, 2020, 47. [Google Scholar] [CrossRef]
- Kuehbacher, T.; Rehman, A.; Lepage, P.; Hellmig, S.; Fölsch, U.R.; Schreiber, S.; Ott, S.J. Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J. Med. Microbiol. 2008, 57, 1569–1576. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutr. 2019, 11, 521. [Google Scholar] [CrossRef]
- Ma, B.; Liang, J.; Dai, M.; Wang, J.; Luo, J.; Zhang, Z.; Jing, J. Altered Gut Microbiota in Chinese Children With Autism Spectrum Disorders. Front. Cell. Infect. Microbiol. 2019, 9, 40. [Google Scholar] [CrossRef]
- Gong, D.; Gong, X.; Wang, L.; Yu, X.; Dong, Q. Involvement of Reduced Microbial Diversity in Inflammatory Bowel Disease. Gastroenterol. Res. Pr. 2016, 2016, 6951091. [Google Scholar] [CrossRef]
- Liang, X.; Li, H.; Tian, G.; Li, S. Dynamic microbe and molecule networks in a mouse model of colitis-associated colorectal cancer. Sci. Rep. 2014, 4, 4985. [Google Scholar] [CrossRef]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association Between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef]
- Torres, J.; Hu, J.; Seki, A.; Eisele, C.; Nair, N.; Huang, R.; Tarassishin, L.; Jharap, B.; Cote-Daigneault, J.; Mao, Q.; et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut 2019, 69, 42–51. [Google Scholar] [CrossRef]
- Russo, A.; Krigsman, A.; Jepson, B.; Wakefield, A. Generalized Autoimmunity of ANCA and ASCA Related to Severity of Disease in Autistic Children with GI Disease. Immunol. Immunogenet. Insights 2009, 1, III.S3073. [Google Scholar] [CrossRef]
- Brock, P.M.; Döring, H.; Bidartondo, M.I. How to know unknown fungi: The role of a herbarium. New Phytol. 2008, 181, 719–724. [Google Scholar] [CrossRef]
- Dollive, S.; Chen, Y.-Y.; Grunberg, S.; Bittinger, K.; Hoffmann, C.; Vandivier, L.; Cuff, C.; Lewis, J.D.; Wu, G.D.; Bushman, F.D. Fungi of the Murine Gut: Episodic Variation and Proliferation during Antibiotic Treatment. PLOS ONE 2013, 8, e71806. [Google Scholar] [CrossRef]
- Noverr, M.C.; Noggle, R.M.; Toews, G.B.; Huffnagle, G.B. Role of Antibiotics and Fungal Microbiota in Driving Pulmonary Allergic Responses. Infect. Immun. 2004, 72, 4996–5003. [Google Scholar] [CrossRef]
- Rapid Range Shifts of Species Associated with High Levels of Climate Warming—European Environment Agency. 2011. Available online: https://value.eea.europa.eu/data-and-maps/indicators/distribution-of-animal-species-1/chen-et-al.-2011-rapid (accessed on 27 February 2021).
- Gouba, N.; Raoult, D.; Drancourt, M. Plant and Fungal Diversity in Gut Microbiota as Revealed by Molecular and Culture Investigations. PLOS ONE 2013, 8, e59474. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.T.; Marotz, C.; Washburne, A.; Silverman, J.; Zaramela, L.S.; Edlund, A.; Zengler, K.; Knight, R. Establishing microbial composition measurement standards with reference frames. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Laue, H.E.; Korrick, S.A.; Baker, E.R.; Karagas, M.R.; Madan, J.C. Prospective associations of the infant gut microbiome and microbial function with social behaviors related to autism at age 3 years. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Iebba, V.; Aloi, M.; Civitelli, F.; Cucchiara, S. Gut Microbiota and Pediatric Disease. Dig. Dis. 2011, 29, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low Relative Abundances of the Mucolytic Bacterium Akkermansia muciniphila and Bifidobacterium spp. in Feces of Children with Autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a Healthy Human Gut Microbiome: Current Concepts, Future Directions, and Clinical Applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2010, 108, 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Health Aging 2018, 4, 267–285. [Google Scholar] [CrossRef]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Shen, J.; Zuo, Z.-X.; Mao, A.-P. Effect of Probiotics on Inducing Remission and Maintaining Therapy in Ulcerative Colitis, Crohnʼs Disease, and Pouchitis. Inflamm. Bowel Dis. 2014, 20, 21–35. [Google Scholar] [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Asghari, G.; Farhadnejad, H.; Teymoori, F.; Mirmiran, P.; Tohidi, M.; Azizi, F. High dietary intake of branched-chain amino acids is associated with an increased risk of insulin resistance in adults. J. Diabetes 2017, 10, 357–364. [Google Scholar] [CrossRef]
- Kandeel, W.A.; Meguid, N.A.; Bjørklund, G.; Eid, E.M.; Farid, M.; Mohamed, S.K.; Wakeel, K.E.; Chirumbolo, S.; Elsaeid, A.; Hammad, D.Y. Impact of Clostridium Bacteria in Children with Autism Spectrum Disorder and Their Anthropometric Measurements. J. Mol. Neurosci. 2020, 70, 897–907. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nat. Cell Biol. 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Miclotte, L.; Van De Wiele, T. Food processing, gut microbiota and the globesity problem. Crit. Rev. Food Sci. Nutr. 2019, 60, 1769–1782. [Google Scholar] [CrossRef]
- Blum, W.E.; Zechmeister-Boltenstern, S.; Keiblinger, K.M. Does Soil Contribute to the Human Gut Microbiome? Microorganisms 2019, 7, 287. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hogan, D.A.; Mylonakis, E. Medically important bacterial–fungal interactions. Nat. Rev. Genet. 2010, 8, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, R.; Sabbatini, S.; Roselletti, E.; Perito, S.; Monari, C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.S.; Nardi, R.M.D.; Arantes, R.M.E.; Rosa, C.A.; Neves, M.J.; Nicoli, J.R. Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J. Gen. Appl. Microbiol. 2005, 51, 83–92. [Google Scholar] [CrossRef]
- Kühle, A.V.D.A.; Jespersen, L. The Taxonomic Position of Saccharomyces boulardii as Evaluated by Sequence Analysis of the D1/D2 Domain of 26S rDNA, the ITS1-5.8S rDNA-ITS2 Region and the Mitochondrial Cytochrome-c Oxidase II Gene. Syst. Appl. Microbiol. 2003, 26, 564–571. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Lozupone, C.; Kang, D.-W.; Adams, J.B. Gut bacteria in children with autism spectrum disorders: Challenges and promise of studying how a complex community influences a complex disease. Microb. Ecol. Health Dis. 2015, 26, 26914. [Google Scholar] [CrossRef]
- Ercolini, D.; Francavilla, R.; Vannini, L.; De Filippis, F.; Capriati, T.; Di Cagno, R.; Iacono, G.; De Angelis, M.; Gobbetti, M. From an imbalance to a new imbalance: Italian-style gluten-free diet alters the salivary microbiota and metabolome of African celiac children. Sci. Rep. 2015, 5, 18571. [Google Scholar] [CrossRef]
- Son, J.S.; Zheng, L.J.; Rowehl, L.M.; Tian, X.; Zhang, Y.; Zhu, W.; Litcher-Kelly, L.; Gadow, K.D.; Gathungu, G.; Robertson, C.E.; et al. Comparison of Fecal Microbiota in Children with Autism Spectrum Disorders and Neurotypical Siblings in the Simons Simplex Collection. PLOS ONE 2015, 10, e0137725. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLOS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Esaiassen, E.; Fjalstad, J.W.; Juvet, L.K.; Anker, J.N.V.D.; Klingenberg, C. Antibiotic exposure in neonates and early adverse outcomes: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 1858–1870. [Google Scholar] [CrossRef]
- E Dawson-Hahn, E.; Mickan, S.; Onakpoya, I.; Roberts, N.; Kronman, M.; Butler, C.C.; Thompson, M.J. Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: A review of systematic reviews. Fam. Pr. 2017, 34, 511–519. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-Del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota—Gut—Brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Heal. Dis. 2012, 23, 23. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Cross, K.L.; Campbell, J.H.; Balachandran, M.; Campbell, A.G.; Cooper, S.J.; Griffen, A.; Heaton, M.; Joshi, S.; Klingeman, D.; Leys, E.; et al. Targeted isolation and cultivation of uncultivated bacteria by reverse genomics. Nat. Biotechnol. 2019, 37, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sun, T.; Wu, F.; Li, F.; Liu, Y.; Li, W.; Dai, N.; Tan, L.; Li, T.; Song, Y. Correlation of gut microbiota and neurotransmitters in a rat model of post-traumatic stress disorder. J. Tradit. Chin. Med Sci. 2020, 7, 375–385. [Google Scholar] [CrossRef]
- Bojović, K.; Bajić, S.S.; Milutinović, D.V.; Tomić, M.; Golić, N.; Tolinački, M. Gut Microbiota Dysbiosis Associated With Altered Production of Short Chain Fatty Acids in Children with Neurodevelopmental Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 223. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kobliner, V.; Mumper, E.; Baker, S.M. Reduction in obsessive compulsive disorder and self-injurious behavior with sac-charomyces boulardii in a child with autism: A case report. Integr. Med. 2018, 17, 38–41. [Google Scholar]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Boushey, H.A. The microbiome in asthma. J. Allergy Clin. Immunol. 2015, 135, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Leonardi, I.D.I.I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Longo, M.N.L.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Tokman, H.; Uysal, H.; Demiryas, S.; Karakullukcu, A.; Saribas, S.; Cokugras, H.; Kocazeybek, B. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopathol. 2019, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Enstrom, A.M.; A Van De Water, J.; Ashwood, P. Autoimmunity in autism. Curr. Opin. Investig. Drugs 2009, 10, 463–473. [Google Scholar]
- McLean, M.H.; Dieguez, D.; Miller, L.M.; A Young, H. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut 2015, 64, 332–341. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).