Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines
Abstract
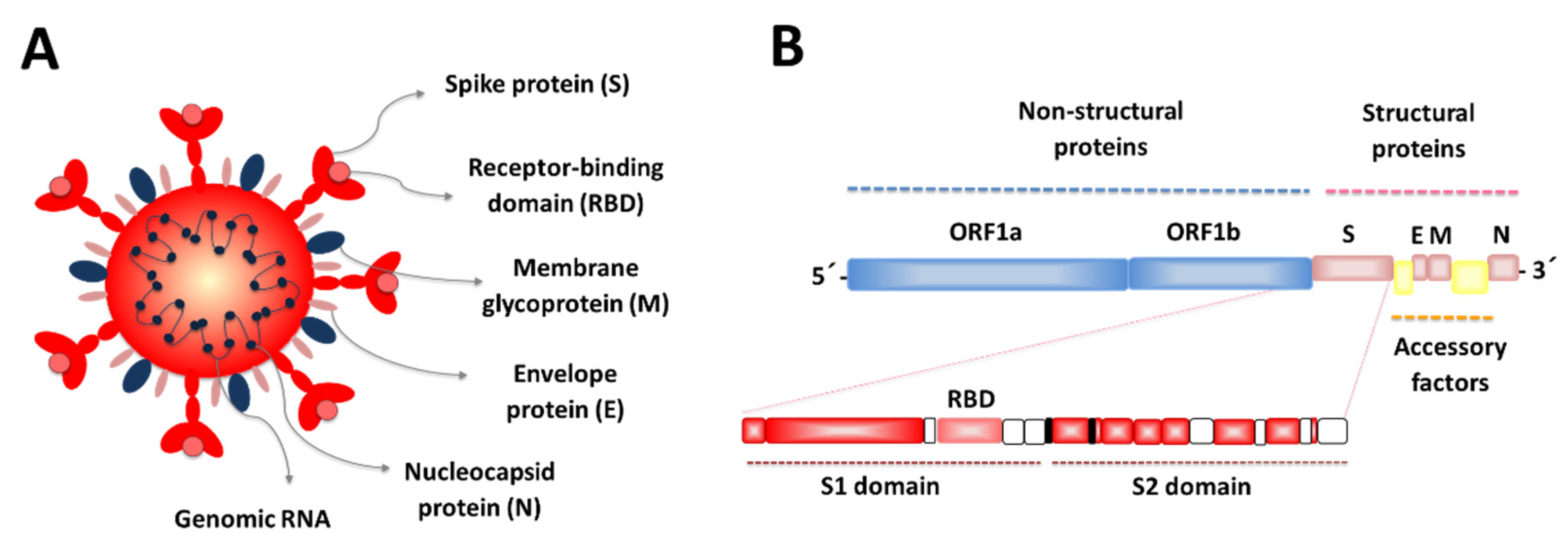
1. Introduction
2. SARS-CoV-2 Mucosal Infections
2.1. SARS-CoV-2 Respiratory Infection
2.2. SARS-CoV-2 Intestinal Infection
3. SARS-CoV-2 Immune Response and Vaccines
3.1. SARS-CoV-2 Immune Response
3.2. SARS-CoV-2 Vaccines
4. L. plantarum as Modulators of Antiviral Immune Responses in Mucosal Tissues
4.1. Modulation of Intestinal Antiviral Immune Responses by L. plantarum
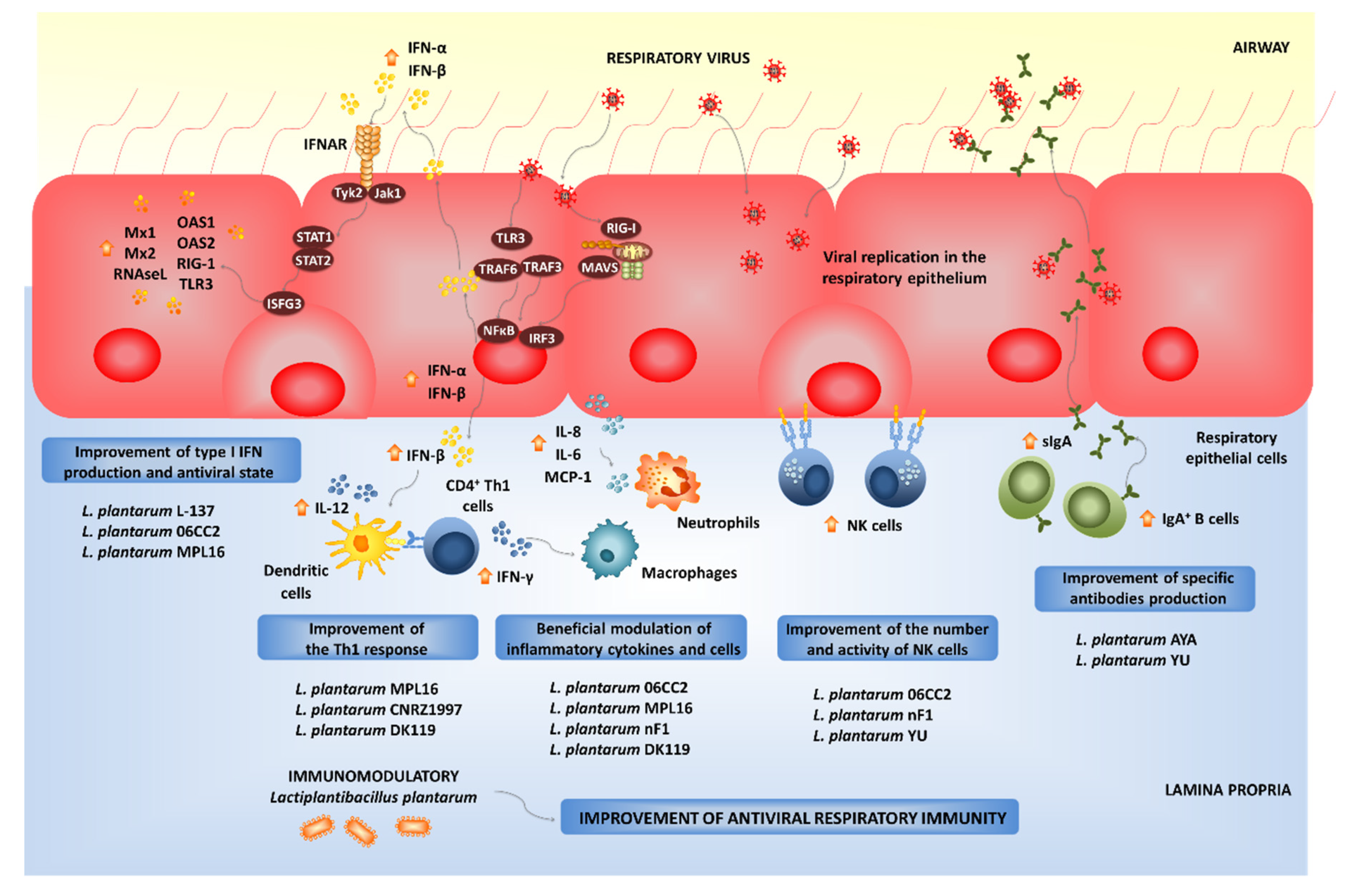
4.2. Modulation of Respiratory Antiviral Immune Responses by L. plantarum
5. L. plantarum as Platforms for Mucosal Vaccines Development
The Successful Expression of SARS-CoV-2 Antigen in L. plantarum
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- WHO. Weekly Operational Update on COVID-19-1. February 2021. Available online: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19 (accessed on 1 February 2021).
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Frederiksen, L.S.F.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817. [Google Scholar] [CrossRef]
- Moore, J.P.; Klasse, P.J. COVID-19 Vaccines:’’Warp Speed’’ Needs Mind Melds, Not Warped Minds. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Vintini, E.; Villena, J.; Raya, R.; Alvarez, S. Lactococcus lactis as an adjuvant and delivery vehicle of antigens against pneumococcal respiratory infections. Bioeng. Bugs 2010, 1, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Oliveira, M.L.; Ferreira, P.C.; Salva, S.; Alvarez, S. Lactic acid bacteria in the prevention of pneumococcal respiratory infection: Future opportunities and challenges. Int. Immunopharmacol. 2011, 11, 1633–1645. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e899. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Heurich, A.; Lavender, H.; Gierer, S.; Danisch, S.; Perin, P.; Lucas, J.M.; Nelson, P.S.; Pohlmann, S.; Soilleux, E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE 2012, 7, e35876. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, L.G. Pulmonary surfactant. Annu. Rev. Med. 1989, 40, 431–446. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Cooper, K.W.; Brann, D.H.; Farruggia, M.C.; Bhutani, S.; Pellegrino, R.; Tsukahara, T.; Weinreb, C.; Joseph, P.V.; Larson, E.D.; Parma, V.; et al. COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron 2020, 107, 219–233. [Google Scholar] [CrossRef]
- Walsh, K.A.; Jordan, K.; Clyne, B.; Rohde, D.; Drummond, L.; Byrne, P.; Ahern, S.; Carty, P.G.; O’Brien, K.K.; O’Murchu, E.; et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 2020, 81, 357–371. [Google Scholar] [CrossRef]
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Cardenas-Diaz, F.L.; Truitt, R.; Tan, L.H.; Dong, B.; Alysandratos, K.D.; et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial derived cells and cardiomyocytes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Huang, C.F.; Sun, P.T.; Tay, C.K. Bronchiolitis and Potential Pathophysiological Implications in Coronavirus Disease 2019 Acute Respiratory Distress Syndrome Patients With Near-Normal Respiratory Compliance. Crit. Care Med. 2020, 48, e993–e994. [Google Scholar] [CrossRef]
- Leung, W.K.; To, K.F.; Chan, P.K.; Chan, H.L.; Wu, A.K.; Lee, N.; Yuen, K.Y.; Sung, J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 2003, 125, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.; Cheng, V.C.; Wu, A.K.; Tang, B.S.; Chan, K.H.; Chu, C.M.; Wong, M.M.; Hui, W.T.; Poon, L.L.; Tse, D.M.; et al. Viral loads in clinical specimens and SARS manifestations. Emerg. Infect. Dis. 2004, 10, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Hung, I.F.; Tang, B.S.; Chu, C.M.; Wong, M.M.; Chan, K.H.; Wu, A.K.; Tse, D.M.; Chan, K.S.; Zheng, B.J.; et al. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2004, 38, 467–475. [Google Scholar] [CrossRef]
- Liu, C.L.; Lu, Y.T.; Peng, M.J.; Chen, P.J.; Lin, R.L.; Wu, C.L.; Kuo, H.T. Clinical and laboratory features of severe acute respiratory syndrome vis-a-vis onset of fever. Chest 2004, 126, 509–517. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Zhao, G.; Chu, H.; Wang, D.; Yan, H.H.; Poon, V.K.; Wen, L.; Wong, B.H.; Zhao, X.; et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017, 3, eaao4966. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Lau, S.K.; To, K.K.; Cheng, V.C.; Woo, P.C.; Yuen, K.Y. Middle East respiratory syndrome coronavirus: Another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015, 28, 465–522. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e1833. [Google Scholar] [CrossRef]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. Febs. Lett. 2002, 532, 107–110. [Google Scholar] [CrossRef]
- Zhao, D.; Yao, F.; Wang, L.; Zheng, L.; Gao, Y.; Ye, J.; Guo, F.; Zhao, H.; Gao, R. A Comparative Study on the Clinical Features of Coronavirus 2019 (COVID-19) Pneumonia With Other Pneumonias. Clin. Infect. Dis. 2020, 71, 756–761. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Tong, Z.D.; Wang, H.L.; Dai, Y.X.; Li, K.F.; Liu, J.N.; Wu, W.J.; Yuan, C.; Yu, M.L.; Li, P.; et al. Detection of Novel Coronavirus by RT-PCR in Stool Specimen from Asymptomatic Child, China. Emerg. Infect. Dis. 2020, 26, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes. Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wong, S.H.; Lui, R.N.; Sung, J.J. Covid-19 and the digestive system. J. Gastroenterol. Hepatol. 2020, 35, 744–748. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Yu, I.T.; Li, Y.; Wong, T.W.; Tam, W.; Chan, A.T.; Lee, J.H.; Leung, D.Y.; Ho, T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004, 350, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; Francois, B.; Seve, P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020, 19, 102567. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Renia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Neyt, K.; Lambrecht, B.N. The role of lung dendritic cell subsets in immunity to respiratory viruses. Immunol. Rev. 2013, 255, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Garbi, N.; Lambrecht, B.N. Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflügers Arch. 2017, 469, 561–572. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e1039. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Pere, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Zhou, R.; To, K.K.; Wong, Y.C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.Y.; Lau, T.T.; et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020, 53, 864–877.e865. [Google Scholar] [CrossRef]
- Ong, E.Z.; Chan, Y.F.Z.; Leong, W.Y.; Lee, N.M.Y.; Kalimuddin, S.; Mohideen, S.M.H.; Chan, K.S.; Tan, A.T.; Bertoletti, A.; Ooi, E.E.; et al. A Dynamic Immune Response Shapes COVID-19 Progression. Cell Host Microbe 2020, 27, 879–882.e872. [Google Scholar] [CrossRef]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020, 160, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shi, Y.; Gong, B.; Jiang, L.; Liu, X.; Yang, J.; Tang, J.; You, C.; Jiang, Q.; Long, B.; et al. Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ling, J.; Mo, P.; Zhang, Y.; Jiang, Q.; Ma, Z.; Cao, Q.; Hu, W.; Zou, S.; Chen, L.; et al. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Feng, Z.; Diao, B.; Wang, R.; Wang, G.; Wang, C.; Tan, Y.; Liu, L.; Wang, C.; Liu, Y.; Liu, Y.; et al. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. medRxiv 2020. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, R.S.; Qu, G.Q.; Wang, Y.Y.; Liu, P.; Zhu, Y.Z.; Fei, G.; Ren, L.; Zhou, Y.W.; Liu, L. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi 2020, 36, 21–23. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.C. SARS-CoV-2: A storm is raging. J. Clin. Invest. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.L.; Feng, Y.; Deng, Y.Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977.e973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Okba, N.M.A.; Muller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Jia, R.; Yi, C.; Gu, W.; Liu, P.; Dong, X.; Zhou, H.; Shang, B.; Cheng, S.; et al. Protective humoral immunity in SARS-CoV-2 infected pediatric patients. Cell Mol. Immunol. 2020, 17, 768–770. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020, 369, 818–823. [Google Scholar] [CrossRef]
- Afkhami, S.; Yao, Y.; Xing, Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol. Ther. Methods Clin. Dev. 2016, 3, 16030. [Google Scholar] [CrossRef] [PubMed]
- Samrat, S.K.; Tharappel, A.M.; Li, Z.; Li, H. Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development. Virus Res. 2020, 288, 198141. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, U.J.; Bukreyev, A.; Yang, L.; Lamirande, E.W.; Murphy, B.R.; Subbarao, K.; Collins, P.L. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. USA 2004, 101, 9804–9809. [Google Scholar] [CrossRef]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses: (Trends in Immunology 41, 355-359; 2020). Trends Immunol. 2020, 41, 545. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Vizoso-Pinto, M.G.; Kitazawa, H. Intestinal Innate Antiviral Immunity and Immunobiotics: Beneficial Effects against Rotavirus Infection. Front. Immunol. 2016, 7, 563. [Google Scholar] [CrossRef]
- Bradley, K.C.; Finsterbusch, K.; Schnepf, D.; Crotta, S.; Llorian, M.; Davidson, S.; Fuchs, S.Y.; Staeheli, P.; Wack, A. Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection. Cell Rep. 2019, 28, 245–256.e244. [Google Scholar] [CrossRef]
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory Antiviral Immunity and Immunobiotics: Beneficial Effects on Inflammation-Coagulation Interaction during Influenza Virus Infection. Front. Immunol. 2016, 7, 633. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. The Modulation of Mucosal Antiviral Immunity by Immunobiotics: Could They Offer Any Benefit in the SARS-CoV-2 Pandemic? Front. Physiol. 2020, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y.; et al. Lipoteichoic Acid Is Involved in the Ability of the Immunobiotic Strain Lactobacillus plantarum CRL1506 to Modulate the Intestinal Antiviral Innate Immunity Triggered by TLR3 Activation. Front. Immunol. 2020, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, L.; Garcia-Castillo, V.; Masumizu, Y.; Indo, Y.; Islam, M.A.; Suda, Y.; Garcia-Cancino, A.; Aso, H.; Takahashi, H.; Kitazawa, H.; et al. Efficient Selection of New Immunobiotic Strains With Antiviral Effects in Local and Distal Mucosal Sites by Using Porcine Intestinal Epitheliocytes. Front. Immunol. 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, L.; Kobayashi, H.; Iida, H.; Sato, N.; Nochi, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Transcriptomic Analysis of the Innate Antiviral Immune Response in Porcine Intestinal Epithelial Cells: Influence of Immunobiotic Lactobacilli. Front. Immunol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Gibson, C.J.; Bresee, J.S.; Glass, R.I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 2006, 12, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef]
- Hosoya, S.; Villena, J.; Shimazu, T.; Tohno, M.; Fujie, H.; Chiba, E.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; et al. Immunobiotic lactic acid bacteria beneficially regulate immune response triggered by poly(I:C) in porcine intestinal epithelial cells. Vet. Res. 2011, 42, 111. [Google Scholar] [CrossRef]
- Ishizuka, T.; Kanmani, P.; Kobayashi, H.; Miyazaki, A.; Soma, J.; Suda, Y.; Aso, H.; Nochi, T.; Iwabuchi, N.; Xiao, J.Z.; et al. Immunobiotic Bifidobacteria Strains Modulate Rotavirus Immune Response in Porcine Intestinal Epitheliocytes via Pattern Recognition Receptor Signaling. PLoS ONE 2016, 11, e0152416. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Chiba, E.; Vizoso-Pinto, M.G.; Tomosada, Y.; Takahashi, T.; Ishizuka, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H. Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC Microbiol. 2014, 14, 126. [Google Scholar] [CrossRef]
- Morelli, M.; Ogden, K.M.; Patton, J.T. Silencing the alarms: Innate immune antagonism by rotavirus NSP1 and VP3. Virology 2015, 479–480, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Zelaya, H.; Clua, P.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Immunobiotic Lactobacillus strains reduce small intestinal injury induced by intraepithelial lymphocytes after Toll-like receptor 3 activation. Inflamm. Res. 2016, 65, 771–783. [Google Scholar] [CrossRef]
- Villena, J.; Chiba, E.; Tomosada, Y.; Salva, S.; Marranzino, G.; Kitazawa, H.; Alvarez, S. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C). BMC Immunol. 2012, 13, 53. [Google Scholar] [CrossRef]
- Wang, K.; Ran, L.; Yan, T.; Niu, Z.; Kan, Z.; Zhang, Y.; Yang, Y.; Xie, L.; Huang, S.; Yu, Q.; et al. Anti-TGEV Miller Strain Infection Effect of Lactobacillus plantarum Supernatant Based on the JAK-STAT1 Signaling Pathway. Front. Microbiol. 2019, 10, 2540. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Kinoshita, N.; Hiroi, T.; Ohta, N.; Fukuyama, S.; Park, E.J.; Kiyono, H. Autocrine IL-15 mediates intestinal epithelial cell death via the activation of neighboring intraepithelial NK cells. J. Immunol. 2002, 169, 6187–6192. [Google Scholar] [CrossRef]
- Zhou, R.; Wei, H.; Sun, R.; Tian, Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J. Immunol. 2007, 178, 4548–4556. [Google Scholar] [CrossRef]
- Zhou, R.; Wei, H.; Sun, R.; Zhang, J.; Tian, Z. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl. Acad. Sci. USA 2007, 104, 7512–7515. [Google Scholar] [CrossRef]
- Kim, K.; Lee, G.; Thanh, H.D.; Kim, J.H.; Konkit, M.; Yoon, S.; Park, M.; Yang, S.; Park, E.; Kim, W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J. Dairy Sci. 2018, 101, 5702–5712. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Yi, D.Y.; Jo, S.; Lee, Y.M.; Kim, J.H.; Kim, W.; Park, M.R.; Yoon, S.M.; Kim, Y.; Yang, S.; et al. Effect of a new Lactobacillus plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine 2020, 99, e22192. [Google Scholar] [CrossRef] [PubMed]
- Le Bon, A.; Tough, D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 2002, 14, 432–436. [Google Scholar] [CrossRef]
- Montoya, M.; Schiavoni, G.; Mattei, F.; Gresser, I.; Belardelli, F.; Borrow, P.; Tough, D.F. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 2002, 99, 3263–3271. [Google Scholar] [CrossRef]
- Yoo, J.K.; Baker, D.P.; Fish, E.N. Interferon-beta modulates type 1 immunity during influenza virus infection. Antivir. Res. 2010, 88, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Raya Tonetti, F.; Arce, L.; Salva, S.; Alvarez, S.; Takahashi, H.; Kitazawa, H.; Vizoso-Pinto, M.G.; Villena, J. Immunomodulatory Properties of Bacterium-Like Particles Obtained From Immunobiotic Lactobacilli: Prospects for Their Use as Mucosal Adjuvants. Front. Immunol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Le Bon, A.; Schiavoni, G.; D’Agostino, G.; Gresser, I.; Belardelli, F.; Tough, D.F. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 2001, 14, 461–470. [Google Scholar] [CrossRef]
- Takeda, S.; Takeshita, M.; Kikuchi, Y.; Dashnyam, B.; Kawahara, S.; Yoshida, H.; Watanabe, W.; Muguruma, M.; Kurokawa, M. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: Alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int. Immunopharmacol. 2011, 11, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, T.; Takeda, S.; Takeshita, M.; Arima, Y.; Tsend-Ayush, C.; Oyunsuren, T.; Sugita, C.; Yoshida, H.; Watanabe, W.; Kurokawa, M. Augmentation of T helper type 1 immune response through intestinal immunity in murine cutaneous herpes simplex virus type 1 infection by probiotic Lactobacillus plantarum strain 06CC2. Int. Immunopharmacol. 2016, 39, 320–327. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Chiba, E.; Tomosada, Y.; Vizoso-Pinto, M.G.; Salva, S.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Immunobiotic Lactobacillus rhamnosus improves resistance of infant mice against respiratory syncytial virus infection. Int. Immunopharmacol. 2013, 17, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Tsukida, K.; Chiba, E.; Marranzino, G.; Alvarez, S.; Kitazawa, H.; Aguero, G.; Villena, J. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation-coagulation interactions. Int. Immunopharmacol. 2014, 19, 161–173. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; Takahashi, H.; Kitazawa, H.; Villena, J. Alveolar Macrophages Are Key Players in the Modulation of the Respiratory Antiviral Immunity Induced by Orally Administered Lacticaseibacillus rhamnosus CRL1505. Front. Immunol. 2020, 11, 568636. [Google Scholar] [CrossRef] [PubMed]
- Kechaou, N.; Chain, F.; Gratadoux, J.J.; Blugeon, S.; Bertho, N.; Chevalier, C.; Le Goffic, R.; Courau, S.; Molimard, P.; Chatel, J.M.; et al. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl. Environ. Microbiol. 2013, 79, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Yoshikai, Y.; Tsuru, T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006, 136, 3069–3073. [Google Scholar] [CrossRef]
- Maeda, N.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Kase, T.; Yoshikai, Y. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009, 9, 1122–1125. [Google Scholar] [CrossRef]
- Arimori, Y.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Shidara, O.; Ichikawa, H.; Yoshikai, Y. Daily intake of heat-killed Lactobacillus plantarum L-137 enhances type I interferon production in healthy humans and pigs. Immunopharmacol. Immunotoxicol. 2012, 34, 937–943. [Google Scholar] [CrossRef]
- Hirose, Y.; Yamamoto, Y.; Yoshikai, Y.; Murosaki, S. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J. Nutr. Sci. 2013, 2, e39. [Google Scholar] [CrossRef]
- Lee, A.; Lee, Y.J.; Yoo, H.J.; Kim, M.; Chang, Y.; Lee, D.S.; Lee, J.H. Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity. Nutrients 2017, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chung, W.C.; Chun, S.H.; Han, J.H.; Song, M.J.; Lee, K.W. Enhancing the natural killer cell activity and anti-influenza effect of heat-treated Lactobacillus plantarum nF1-fortified yogurt in mice. J. Dairy Sci. 2018, 101, 10675–10684. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Bae, J.Y.; Yoo, K.; Kim, H.; Kim, I.H.; Park, M.S.; Lee, I. Effects of heat-killed Lactobacillus plantarum against influenza viruses in mice. J. Microbiol. 2018, 56, 145–149. [Google Scholar] [CrossRef]
- Park, M.K.; Ngo, V.; Kwon, Y.M.; Lee, Y.T.; Yoo, S.; Cho, Y.H.; Hong, S.M.; Hwang, H.S.; Ko, E.J.; Jung, Y.J.; et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Kunitoh-Asari, A.; Hayakawa, K.; Imai, S.; Kasuya, K.; Abe, K.; Adachi, Y.; Fukudome, S.; Takahashi, Y.; Hachimura, S. Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS ONE 2014, 9, e86416. [Google Scholar] [CrossRef] [PubMed]
- Vilander, A.C.; Dean, G.A. Adjuvant Strategies for Lactic Acid Bacterial Mucosal Vaccines. Vaccines 2019, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- LeCureux, J.S.; Dean, G.A. Lactobacillus Mucosal Vaccine Vectors: Immune Responses against Bacterial and Viral Antigens. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Wyszynska, A.; Kobierecka, P.; Bardowski, J.; Jagusztyn-Krynicka, E.K. Lactic acid bacteria--20 years exploring their potential as live vectors for mucosal vaccination. Appl. Microbiol. Biotechnol. 2015, 99, 2967–2977. [Google Scholar] [CrossRef]
- Cortes-Perez, N.G.; Lefevre, F.; Corthier, G.; Adel-Patient, K.; Langella, P.; Bermudez-Humaran, L.G. Influence of the route of immunization and the nature of the bacterial vector on immunogenicity of mucosal vaccines based on lactic acid bacteria. Vaccine 2007, 25, 6581–6588. [Google Scholar] [CrossRef]
- Kuczkowska, K.; Overland, L.; Rocha, S.D.C.; Eijsink, V.G.H.; Mathiesen, G. Comparison of eight Lactobacillus species for delivery of surface-displayed mycobacterial antigen. Vaccine 2019, 37, 6371–6379. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, J.; Guo, Y.; Yang, W.; Ye, L.; Shi, C.; Liu, Y.; Yang, G.; Wang, C. Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing HN of Newcastle disease virus and DC- targeting peptide fusion protein. J. Biotechnol. 2015, 216, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Li, Q.Y.; Ata, E.B.; Jiang, Y.L.; Huang, H.B.; Shi, C.W.; Wang, J.Z.; Wang, G.; Kang, Y.H.; Liu, J.; et al. Immune response characterization of mice immunized with Lactobacillus plantarum expressing spike antigen of transmissible gastroenteritis virus. Appl. Microbiol. Biotechnol. 2018, 102, 8307–8318. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, J.; Xu, X.; Tang, Y.; Xu, W.; Yang, W.; Jiang, Y.; Yang, G.; Ding, Z.; Cong, Y.; et al. Dendritic cell-targeted recombinantLactobacilli induce DC activation and elicit specific immune responses against G57 genotype of avian H9N2 influenza virus infection. Vet. Microbiol. 2018, 223, 9–20. [Google Scholar] [CrossRef]
- Huang, K.Y.; Yang, G.L.; Jin, Y.B.; Liu, J.; Chen, H.L.; Wang, P.B.; Jiang, Y.L.; Shi, C.W.; Huang, H.B.; Wang, J.Z.; et al. Construction and immunogenicity analysis of Lactobacillus plantarum expressing a porcine epidemic diarrhea virus S gene fused to a DC-targeting peptide. Virus Res. 2018, 247, 84–93. [Google Scholar] [CrossRef]
- Grangette, C.; Muller-Alouf, H.; Goudercourt, D.; Geoffroy, M.C.; Turneer, M.; Mercenier, A. Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect. Immun. 2001, 69, 1547–1553. [Google Scholar] [CrossRef]
- Mathiesen, G.; Overland, L.; Kuczkowska, K.; Eijsink, V.G.H. Anchoring of heterologous proteins in multiple Lactobacillus species using anchors derived from Lactobacillus plantarum. Sci. Rep. 2020, 10, 9640. [Google Scholar] [CrossRef]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Du, S.; Ye, F.; Liu, C.; Liu, H.; Wang, M.; Li, Y.; et al. In vitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe 2014, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Perez, N.G.; Azevedo, V.; Alcocer-Gonzalez, J.M.; Rodriguez-Padilla, C.; Tamez-Guerra, R.S.; Corthier, G.; Gruss, A.; Langella, P.; Bermudez-Humaran, L.G. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. J. Drug Target. 2005, 13, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kuczkowska, K.; Mathiesen, G.; Eijsink, V.G.; Oynebraten, I. Lactobacillus plantarum displaying CCL3 chemokine in fusion with HIV-1 Gag derived antigen causes increased recruitment of T cells. Microb. Cell Fact. 2015, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Geng, N.; Zhou, D.; Qu, Y.; Shi, M.; Xu, Y.; Liu, K.; Liu, Y.; Liu, J. Oral Immunization of Chickens With Recombinant Lactobacillus plantarum Vaccine Against Early ALV-J Infection. Front. Immunol. 2019, 10, 2299. [Google Scholar] [CrossRef]
- Narita, J.; Okano, K.; Kitao, T.; Ishida, S.; Sewaki, T.; Sung, M.H.; Fukuda, H.; Kondo, A. Display of alpha-amylase on the surface of Lactobacillus casei cells by use of the PgsA anchor protein, and production of lactic acid from starch. Appl. Environ. Microbiol. 2006, 72, 269–275. [Google Scholar] [CrossRef]
- Liu, Y.S.; Liu, Q.; Jiang, Y.L.; Yang, W.T.; Huang, H.B.; Shi, C.W.; Yang, G.L.; Wang, C.F. Surface-Displayed Porcine IFN-lambda3 in Lactobacillus plantarum Inhibits Porcine Enteric Coronavirus Infection of Porcine Intestinal Epithelial Cells. J. Microbiol. Biotechnol. 2020, 30, 515–525. [Google Scholar] [CrossRef]
- Wang, M.; Du, S.; Xu, W.; Song, L.; Hao, P.; Jin, N.; Ren, L.; Li, C. Construction and optimization of Lactobacillus plantarum expression system expressing glycoprotein 5 of porcine reproductive and respiratory syndrome virus. Int. J. Biol. Macromol. 2020, 143, 112–117. [Google Scholar] [CrossRef]
- Ye, T.; Yue, Y.; Fan, X.; Dong, C.; Xu, W.; Xiong, S. M cell-targeting strategy facilitates mucosal immune response and enhances protection against CVB3-induced viral myocarditis elicited by chitosan-DNA vaccine. Vaccine 2014, 32, 4457–4465. [Google Scholar] [CrossRef]
- Takahashi, K.; Yano, A.; Watanabe, S.; Langella, P.; Bermudez-Humaran, L.G.; Inoue, N. M cell-targeting strategy enhances systemic and mucosal immune responses induced by oral administration of nuclease-producing L. lactis. Appl. Microbiol. Biotechnol. 2018, 102, 10703–10711. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Firdous, J.; Badruddoza, A.Z.M.; Reesor, E.; Azad, M.; Hasan, A.; Lim, M.; Cao, W.; Guillemette, S.; Cho, C.S. M cell targeting engineered biomaterials for effective vaccination. Biomaterials 2019, 192, 75–94. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yang, W.T.; Shi, S.H.; Li, Y.J.; Zhao, L.; Shi, C.W.; Zhou, F.Y.; Jiang, Y.L.; Hu, J.T.; Gu, W.; et al. Immunogenicity of recombinant Lactobacillus plantarum NC8 expressing goose parvovirus VP2 gene in BALB/c mice. J. Vet. Sci. 2017, 18, 159–167. [Google Scholar] [CrossRef]
- Xu, Y.G.; Guan, X.T.; Liu, Z.M.; Tian, C.Y.; Cui, L.C. Immunogenicity in Swine of Orally Administered Recombinant Lactobacillus plantarum Expressing Classical Swine Fever Virus E2 Protein in Conjunction with Thymosin alpha-1 as an Adjuvant. Appl. Environ. Microbiol. 2015, 81, 3745–3752. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Cong, Y.L.; Huang, H.B.; Wang, Q.; Cai, R.P.; Ye, L.P.; Hu, J.T.; Zhou, J.Y.; et al. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarumNC8 expressing hemagglutinin in BALB/c mice. Virology 2014, 464–465, 166–176. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Zhang, X.K.; Liu, Y.Y.; Zhang, L.J.; Ye, L.P.; Hu, J.T.; Xing, X.; Qi, C.; et al. Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus Res. 2016, 211, 46–57. [Google Scholar] [CrossRef]
- Yang, W.T.; Shi, S.H.; Yang, G.L.; Jiang, Y.L.; Zhao, L.; Li, Y.; Wang, C.F. Cross-protective efficacy of dendritic cells targeting conserved influenza virus antigen expressed by Lactobacillus plantarum. Sci. Rep. 2016, 6, 39665. [Google Scholar] [CrossRef]
- Yang, W.T.; Yang, G.L.; Zhao, L.; Jin, Y.B.; Jiang, Y.L.; Huang, H.B.; Shi, C.W.; Wang, J.Z.; Wang, G.; Kang, Y.H.; et al. Lactobacillus plantarum displaying conserved M2e and HA2 fusion antigens induces protection against influenza virus challenge. Appl. Microbiol. Biotechnol. 2018, 102, 5077–5088. [Google Scholar] [CrossRef]
- Bo, F.; Yang, W.T.; Shonyela, S.M.; Jin, Y.B.; Huang, K.Y.; Shao, L.N.; Wang, C.; Zhou, Y.; Li, Q.Y.; Jiang, Y.L.; et al. Immune responses of mice inoculated with recombinant Lactobacillus plantarum NC8 expressing the fusion gene HA2 and 3M2e of the influenza virus and protection against different subtypes of influenza virus. Virus Res. 2019, 263, 64–72. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Tavakol, S.; Alavijeh, M.S.; Seifalian, A.M. COVID-19 Vaccines on Clinical Trials and their Mode of Action for Immunity against the Virus. Curr. Pharm. Des. 2020. [Google Scholar] [CrossRef]
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Raeber, M.E.; Adamo, S.; Weigang, S.; Emmenegger, M.; et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Du, S.; Liu, H.; Zhang, Y.; Wang, C.; Rong, F.; Jin, N. Evaluation of immunomodulatory activity of two potential probiotic Lactobacillus strains by in vivo tests. Anaerobe 2015, 35, 22–27. [Google Scholar] [CrossRef]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Li, X.; Tian, M.; Du, S.; Guo, H.; Liu, C.; Zhu, N.; et al. Inhibition of Staphylococcus aureus adherence to Caco-2 cells by lactobacilli and cell surface properties that influence attachment. Anaerobe 2012, 18, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.Y.; Li, C.; Qin, Y.Q.; Yin, R.L.; Du, S.W.; Ye, F.; Liu, H.F.; Wang, M.P.; Sun, Y.; Li, X.; et al. Lactobacilli reduce chemokine IL-8 production in response to TNF-alpha and Salmonella challenge of Caco-2 cells. Biomed. Res. Int. 2013, 2013, 925219. [Google Scholar] [CrossRef]
- Wang, M.; Fu, T.; Hao, J.; Li, L.; Tian, M.; Jin, N.; Ren, L.; Li, C. A recombinant Lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020, 160, 736–740. [Google Scholar] [CrossRef]
- Guo, M.; Tao, W.; Flavell, R.A.; Zhu, S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021, 15, 1–15. [Google Scholar] [CrossRef]



| Virus | Antigen and Expression Cassette | Location of the Antigen | Route of Administration | Efficacy and Safety of Vaccine | Reference |
|---|---|---|---|---|---|
| HPV-16 | Signal peptide and the first 15 amino acids of lactococcal Usp45 protein, the E7 mutant protein of HPV-16 E7mm and the cell wall anchor of L. plantarum protein containing anchor motif LPQTXE | Cell wall anchoring | - | The effcicacy or the safety of the experimental vaccine based on L. plantarum was not reported. | [139] |
| ALV-J | Anchorin Poly-γ-glutamate synthase A (pgsA) and the ALV-J gp85 | Bacterial surface | Oral immunization | Pre-clinical stage. Immunization of chickens stimulated humoral immunity and improved protection against ALV-J challenge. No adverse effects were detected. | [141] |
| PEDV | Anchorin pgsA, the spike protein of PEDV and target peptide DCpep (FYPSYHSTPQRP) | Bacterial surface | Oral immunization | Pre-clinical stage. Immunization of mice stimulated humoral and cellular immunity. No adverse effects were detected. | [135] |
| TGEV | Anchorin pgsA, the spike protein of TGEV and target peptide DCpep (FYPSYHSTPQRP) | Bacterial surface | Oral immunization | Pre-clinical stage. Immunization of mice stimulated humoral and cellular immunity. No adverse effects were detected. | [133] |
| NDV | Hemagglutinin–neuraminidase protein and target peptide DCpep | Intracellular | Oral immunization | Pre-clinical stage. Immunization of chickens stimulated humoral and cellular immunity and improved protection agains NDV challenge. No adverse effects were detected. | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villena, J.; Li, C.; Vizoso-Pinto, M.G.; Sacur, J.; Ren, L.; Kitazawa, H. Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines. Microorganisms 2021, 9, 683. https://doi.org/10.3390/microorganisms9040683
Villena J, Li C, Vizoso-Pinto MG, Sacur J, Ren L, Kitazawa H. Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines. Microorganisms. 2021; 9(4):683. https://doi.org/10.3390/microorganisms9040683
Chicago/Turabian StyleVillena, Julio, Chang Li, Maria Guadalupe Vizoso-Pinto, Jacinto Sacur, Linzhu Ren, and Haruki Kitazawa. 2021. "Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines" Microorganisms 9, no. 4: 683. https://doi.org/10.3390/microorganisms9040683
APA StyleVillena, J., Li, C., Vizoso-Pinto, M. G., Sacur, J., Ren, L., & Kitazawa, H. (2021). Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines. Microorganisms, 9(4), 683. https://doi.org/10.3390/microorganisms9040683










