An Actinobacterial Isolate, Streptomyces sp. YX44, Produces Broad-Spectrum Antibiotics That Strongly Inhibit Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of and Maintenance of the Organism
2.2. DNA Amplification by PCR and Determination of 16S rRNA Gene Sequence
2.3. Detection of Antimicrobial Activity of YX44
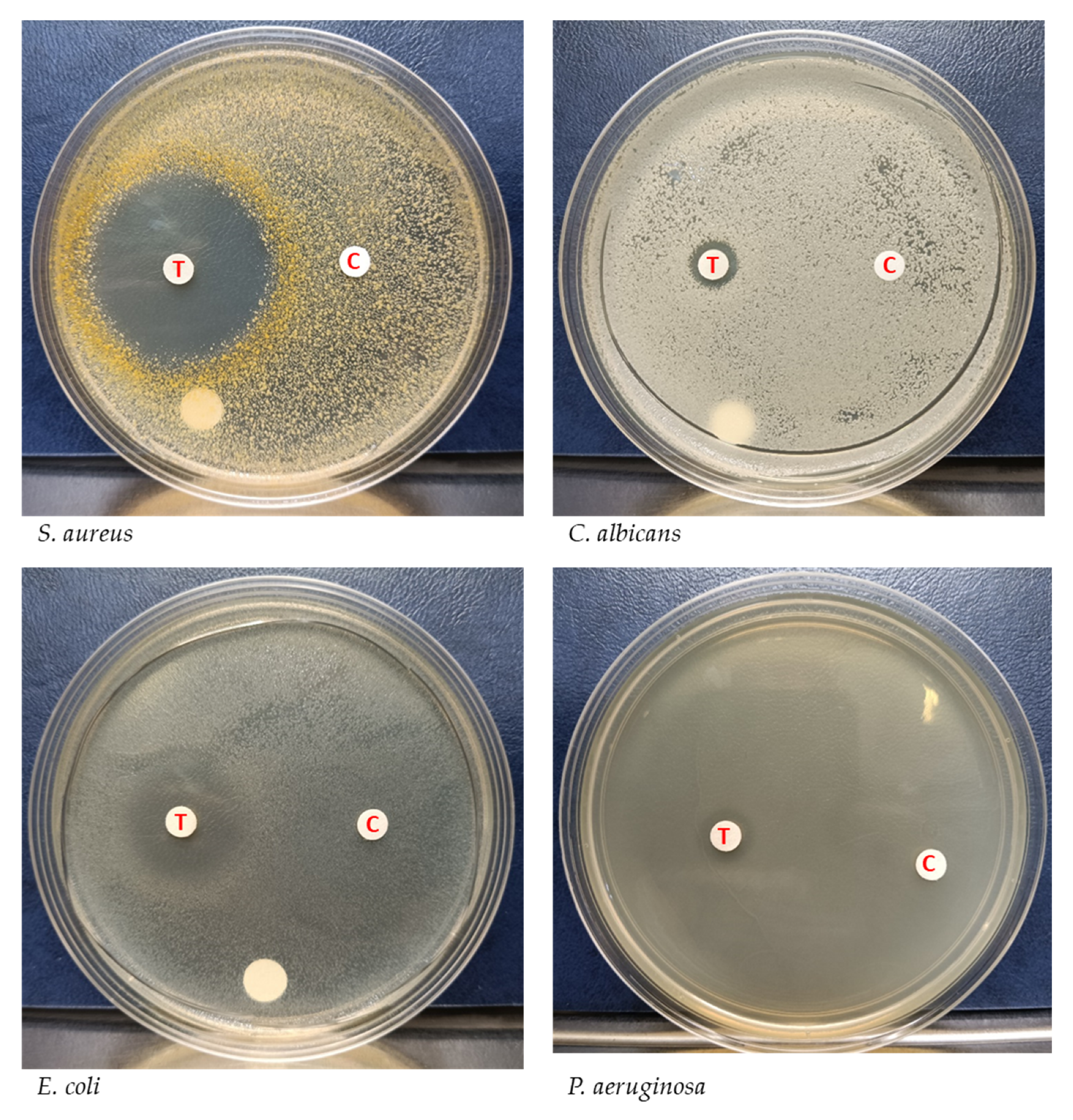
2.4. Inhibition of the Test Strains by YX44 Fermentation Broth
2.5. Characterization of the YX44 Antibacterial Products
2.6. HPLC and LC-MS/MS Analysis
2.7. ESI-MS
3. Results and Discussion
3.1. A New Actinobacterial Species, Streptomyces sp. YX44, Has Broad-Spectrum Antibacterial Activity in Solid Culture
3.2. Liquid Culture of YX44 Shows an Altered Antibiotic Profile
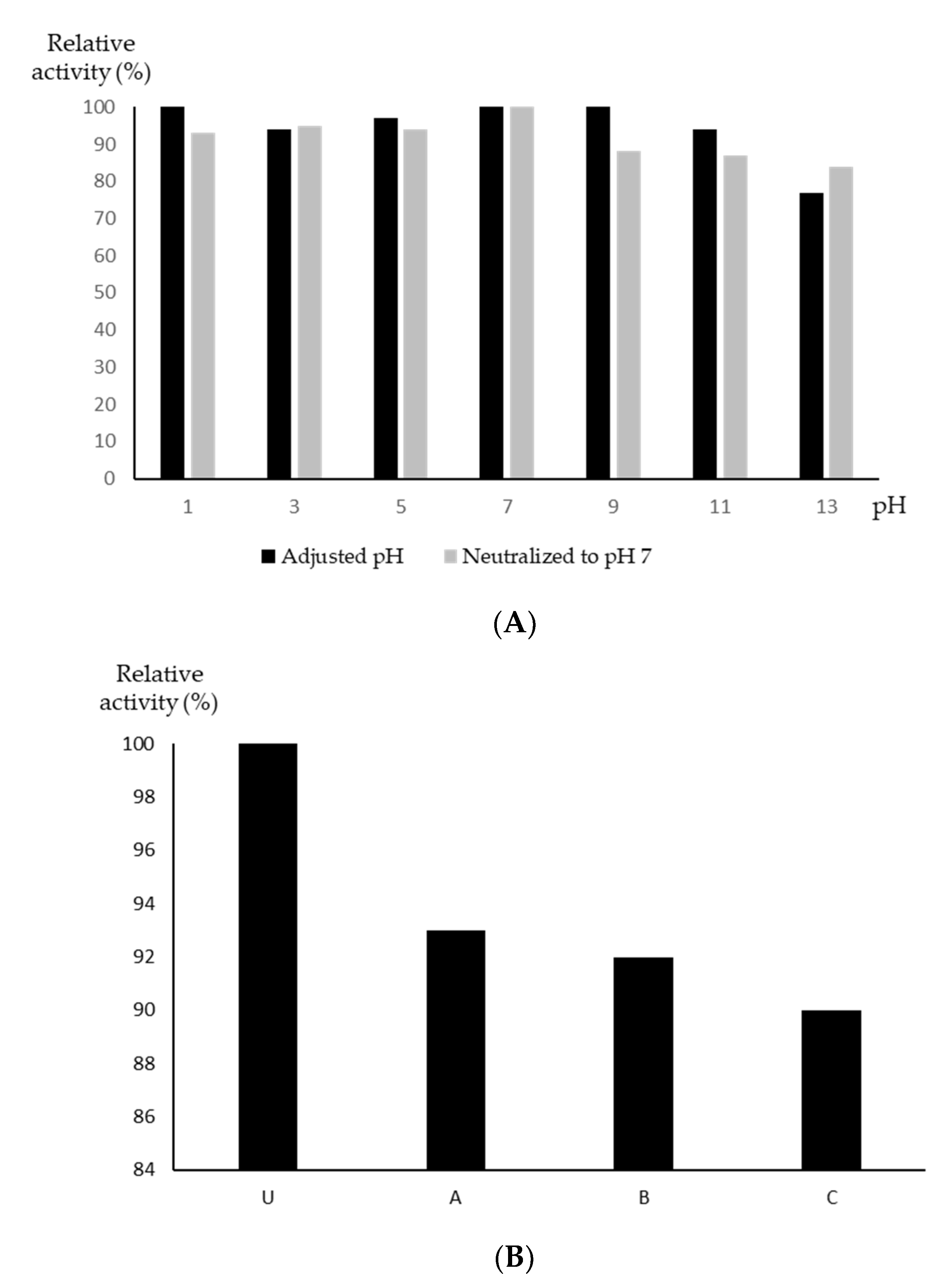
3.3. Characterization of the Streptomyces sp. YX44 Antibacterial Products
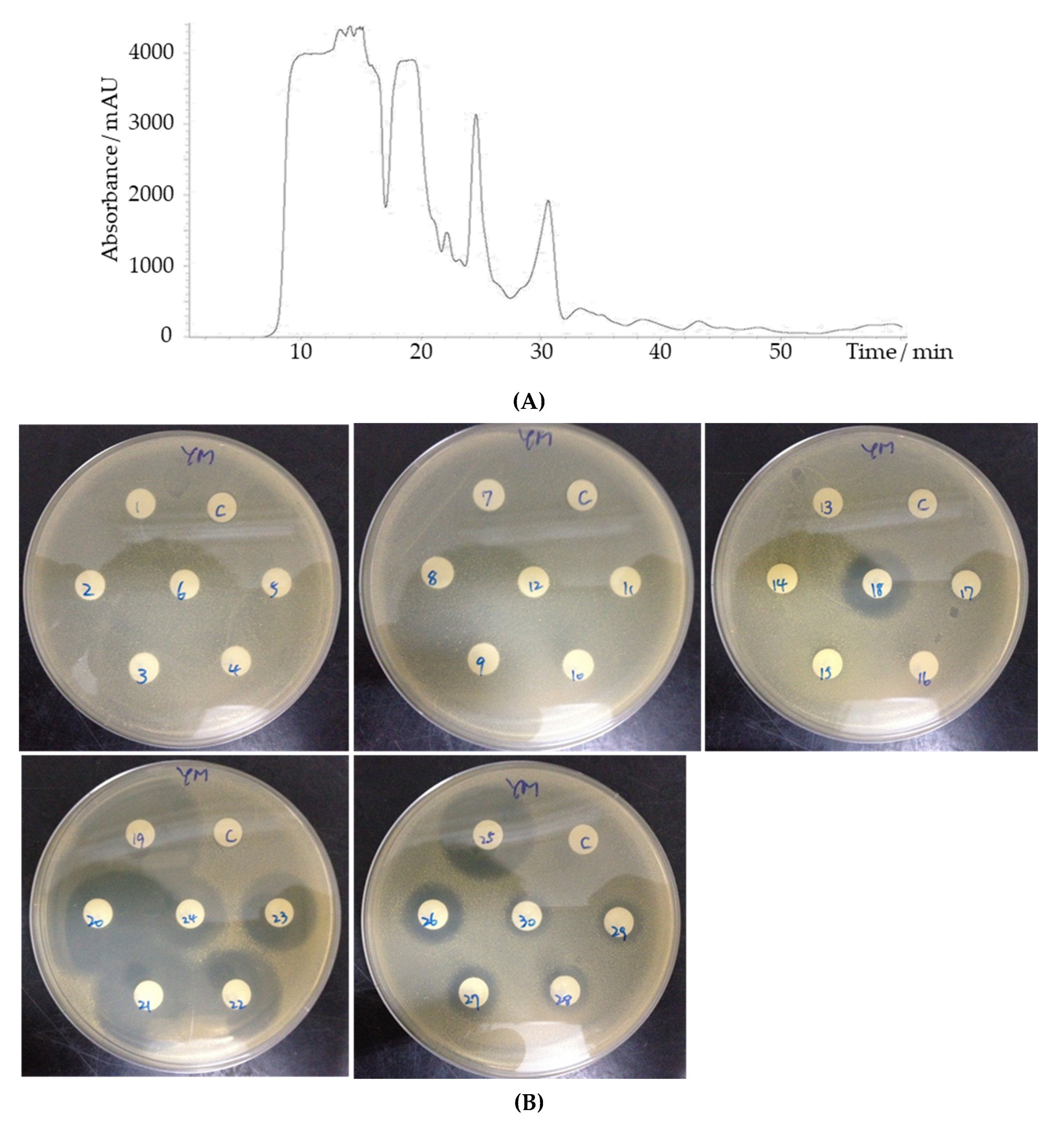
3.4. HPLC and LC-MS Analysis Revealed a Number of Candidates for the YX44 Antibacterial Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Waksman, S.A.; Henrici, A.T. The nomenclature and classification of the actinomycetes. J. Bacteriol. 1943, 46, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Labeda, D.P. Multilocus sequence analysis of phytopathogenic species of the genus Streptomyces. Int. J. Syst. Evol. Microbiol. 2011, 61, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Hain, T.; Ward-Rainey, N.; Kroppenstedt, R.M.; Stackebrandt, E.; Rainey, F.A. Discrimination of Streptomyces albidoflavus strains based on the size and number of 16S–23S ribosomal DNA intergenic spacers. Int. J. Syst. Bacteriol. 1997, 47, 202–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, X.C.; Miao, K.P.; Qian, K.X. Advances of genome and secondary metabolism in Streptomyces. Yi Chuan Xue Bao 2005, 32, 1221–1226. [Google Scholar] [PubMed]
- Challis, G.L.; Hopwood, D.A. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. USA 2003, 100, 14555–14561. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. Recent advances in engineering the central carbon metabolism of industrially important bacteria. Microb. Cell Factories 2012, 11, 50. [Google Scholar] [CrossRef]
- Dulon, M.; Haamann, F.; Peters, C.; Schablon, A.; Nienhaus, A. MRSA prevalence in European healthcare settings: A review. BMC Infect. Dis. 2011, 11, 138. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Seipke, R.F. Strain-Level Diversity of Secondary Metabolism in Streptomyces albus. PLoS ONE 2015, 10, e0116457. [Google Scholar] [CrossRef]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Marine bacteria: Potential sources for compounds to overcome antibiotic resistance. Appl. Microbiol. Biotechnol. 2013, 97, 4763–4773. [Google Scholar] [CrossRef]
- Blanco, G. Comparative analysis of a cryptic thienamycin-like gene cluster identified in Streptomyces flavogriseus by genome mining. Arch. Microbiol. 2012, 194, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, Q.; Bu, Q.; Guo, Y.; Liu, S.; Liu, Y.; Du, Y.; Li, Y. Genome Mining-Directed Activation of a Silent Angucycline Biosynthetic Gene Cluster in Streptomyces chattanoogensis. Chembiochem 2015, 16, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Mehling, A.; Wehmeier, U.F.; Piepersberg, W. Nucleotide sequences of streptomycete 16S ribosomal DNA: Towards a specific identification system for streptomycetes using PCR. Microbiology 1995, 141, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Colemont, L.J.; Phillips, S.F.; Brown, M.L.; Thomforde, G.M.; Chapman, N.; Zinsmeister, A.R. Human gastric emptying and colonic filling of solids characterized by a new method. Am. J. Physiol. 1989, 257, G284–G290. [Google Scholar] [CrossRef]
- Manteca, A.; Yague, P. Streptomyces Differentiation in Liquid Cultures as a Trigger of Secondary Metabolism. Antibiotics 2018, 7, 41. [Google Scholar] [CrossRef]
- Čihák, M.; Kameník, Z.; Šmídová, K.; Bergman, N.; Benada, O.; Kofroňová, O.; Petříčková, K.; Bobek, J. Secondary Metabolites Produced during the Germination of Streptomyces coelicolor. Front. Microbiol. 2017, 8, 2495. [Google Scholar] [CrossRef]
- Spinelli, E.; Requena, T.; Caruso, M.; Parisi, A.; Capozzi, L.; Difato, L.; Normanno, G. Fate of Methicillin-resistant Staphylococcus aureus (MRSA) under simulated acidic conditions of the human stomach. Food Sci. Nutr. 2020, 8, 4739–4745. [Google Scholar] [CrossRef]
- Erah, P.O.; Goddard, A.F.; Barrett, D.A.; Shaw, P.N.; Spiller, R.C. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: Relevance to the treatment of Helicobacter pylori infection. J. Antimicrob. Chemother. 1997, 39, 5–12. [Google Scholar] [CrossRef]
- Burian, A.; Erdogan, Z.; Jandrisits, C.; Zeitlinger, M. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology 2012, 90, 281–287. [Google Scholar] [CrossRef]
- Cyphert, E.L.; Wallat, J.D.; Pokorski, J.K.; Von Recum, H.A. Erythromycin Modification That Improves Its Acidic Stability while Optimizing It for Local Drug Delivery. Antibiotics 2017, 6, 11. [Google Scholar] [CrossRef]
- Gagnaire, J.; Verhoeven, P.O.; Grattard, F.; Rigaill, J.; Lucht, F.; Pozzetto, B.; Berthelot, P.; Botelho-Nevers, E. Epidemiology and clinical relevance of Staphylococcus aureus intestinal carriage: A systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 2017, 15, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Acton, D.S.; Plat-Sinnige, M.J.T.; Van Wamel, W.; De Groot, N.; Van Belkum, A. Intestinal carriage of Staphylococcus aureus: How does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.; Munckhof, W.J. Antibiotics currently used in the treatment of infections caused by Staphylococcus aureus. Intern. Med. J. 2005, 35, S3–S16. [Google Scholar] [CrossRef] [PubMed]
- Sabath, L.D.; Toftegaard, I. Rapid microassays for clindamycin and gentamicin when present together and the effect of pH and of each on the antibacterial activity of the other. Antimicrob. Agents Chemother. 1974, 6, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Hamm, P.S.; Caimi, N.A.; Northup, D.E.; Valdez, E.W.; Buecher, D.C.; Dunlap, C.A.; Labeda, D.P.; Lueschow, S.; Porras-Alfaro, A. Western Bats as a Reservoir of Novel Streptomyces Species with Antifungal Activity. Appl. Environ. Microbiol. 2017, 83, e03057–e03116. [Google Scholar] [CrossRef] [PubMed]
- Voytsekhovskaya, I.V.; Axenov-Gribanov, D.V.; Murzina, S.A.; Pekkoeva, S.N.; Protasov, E.S.; Gamaiunov, S.V.; Timofeyev, M.A. Estimation of antimicrobial activities and fatty acid composition of actinobacteria isolated from water surface of underground lakes from Badzheyskaya and Okhotnichya caves in Siberia. PeerJ 2018, 6, e5832. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Yang, Z.-W.; Asem, M.D.; Fang, B.-Z.; Salam, N.; Alkhalifah, D.H.M.; Hozzein, W.N.; Nie, G.-X.; Li, W.-J. Streptomyces desertarenae sp. nov., a novel actinobacterium isolated from a desert sample. Antonie Leeuwenhoek 2018, 112, 367–374. [Google Scholar]
- Wiegand, C.; Abel, M.; Ruth, P.; Elsner, P.; Hipler, U.-C. pH influence on antibacterial efficacy of common antiseptic substances. Ski. Pharmacol. Physiol. 2015, 28, 147–158. [Google Scholar] [CrossRef]
- Bartek, I.L.; Reichlen, M.J.; Honaker, R.W.; Leistikow, R.L.; Clambey, E.T.; Scobey, M.S.; Hinds, A.B.; Born, S.E.; Covey, C.R.; Schurr, M.J.; et al. Antibiotic Bactericidal Activity Is Countered by Maintaining pH Homeostasis in Mycobacterium smegmatis. mSphere 2016, 1. [Google Scholar] [CrossRef]






| Primer | Sequences |
|---|---|
| 16S rRNA R | AGTTTGATCCTGGCTCAGGA |
| 16S rRNA F | ATTACCGCGGCTGCTGGCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-L.; Huang, T.-W.; Wang, Y.-X.; Liu, C.-P.; Kirby, R.; Chu, C.-M.; Huang, C.-H. An Actinobacterial Isolate, Streptomyces sp. YX44, Produces Broad-Spectrum Antibiotics That Strongly Inhibit Staphylococcus aureus. Microorganisms 2021, 9, 630. https://doi.org/10.3390/microorganisms9030630
Chang T-L, Huang T-W, Wang Y-X, Liu C-P, Kirby R, Chu C-M, Huang C-H. An Actinobacterial Isolate, Streptomyces sp. YX44, Produces Broad-Spectrum Antibiotics That Strongly Inhibit Staphylococcus aureus. Microorganisms. 2021; 9(3):630. https://doi.org/10.3390/microorganisms9030630
Chicago/Turabian StyleChang, Tien-Lin, Tzu-Wen Huang, Ying-Xuan Wang, Chang-Pan Liu, Ralph Kirby, Chien-Ming Chu, and Chih-Hung Huang. 2021. "An Actinobacterial Isolate, Streptomyces sp. YX44, Produces Broad-Spectrum Antibiotics That Strongly Inhibit Staphylococcus aureus" Microorganisms 9, no. 3: 630. https://doi.org/10.3390/microorganisms9030630
APA StyleChang, T.-L., Huang, T.-W., Wang, Y.-X., Liu, C.-P., Kirby, R., Chu, C.-M., & Huang, C.-H. (2021). An Actinobacterial Isolate, Streptomyces sp. YX44, Produces Broad-Spectrum Antibiotics That Strongly Inhibit Staphylococcus aureus. Microorganisms, 9(3), 630. https://doi.org/10.3390/microorganisms9030630






