Functional Analysis of an Acyltransferase-Like Domain from Polyunsaturated Fatty Acid Synthase in Thraustochytrium
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, Kits, and Primers
2.2. Sequence Analysis of the PUFA Synthase from Thraustochytrium
2.3. Cloning and Expression of AT-Like Domain in E. coli
2.4. Complementation of E. coli FabD Mutant
2.5. Expression of the AT-Like Domain in E. coli fadD Mutant
2.6. Free Fatty Acid Analysis
2.7. Expression of AT-Like Domain in E. coli SHuffle
2.8. In Vitro Thioesterase Assays
2.9. Site-Directed Mutagenesis
2.10. Statistical Analysis
3. Results
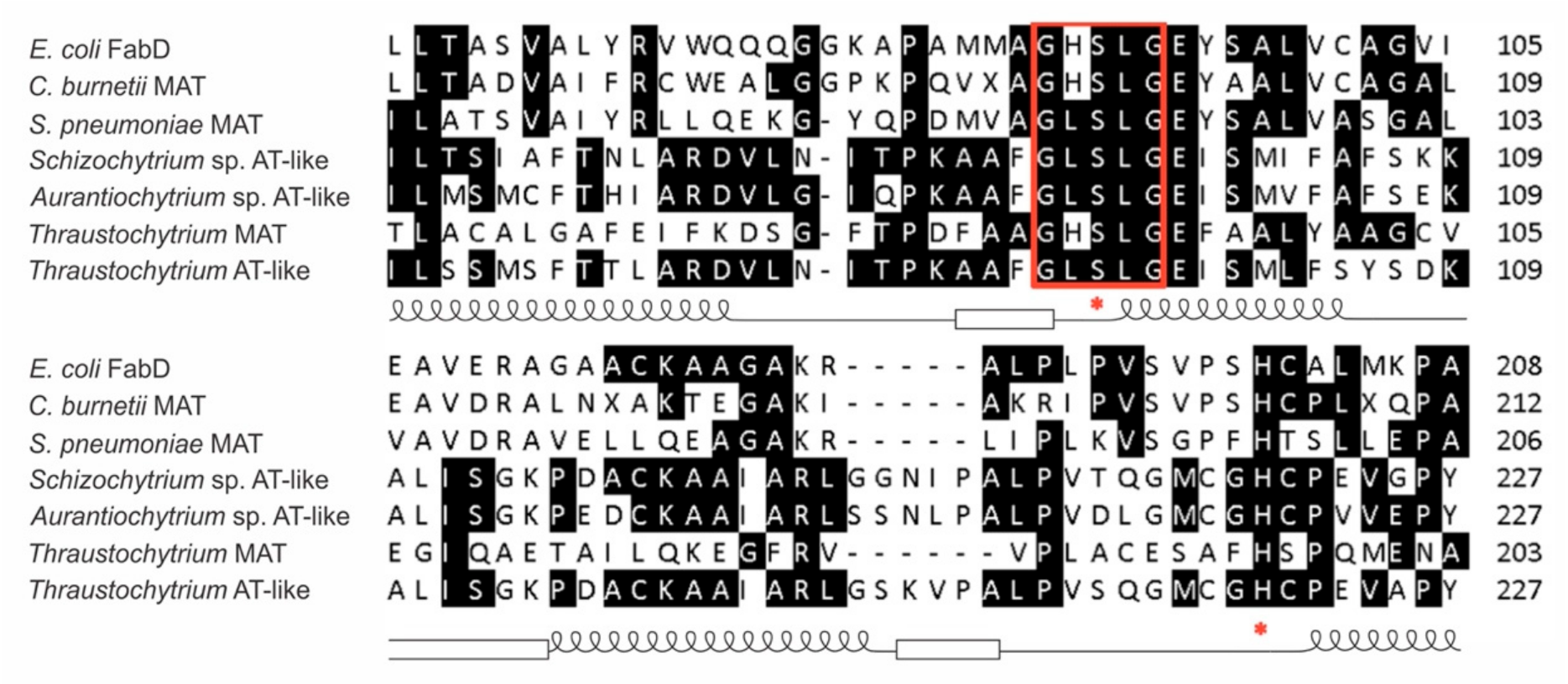
3.1. Sequence Analysis of a AT-Like Domain of the PUFA Synthase
3.2. Expression of the AT-Like Domain in an E. coli fabD Mutant
3.3. Expression of the AT-Like Domain in an E. coli tesAtesB Mutant
3.4. Expression of the AT-Like Domain in an E. coli fadD Mutant
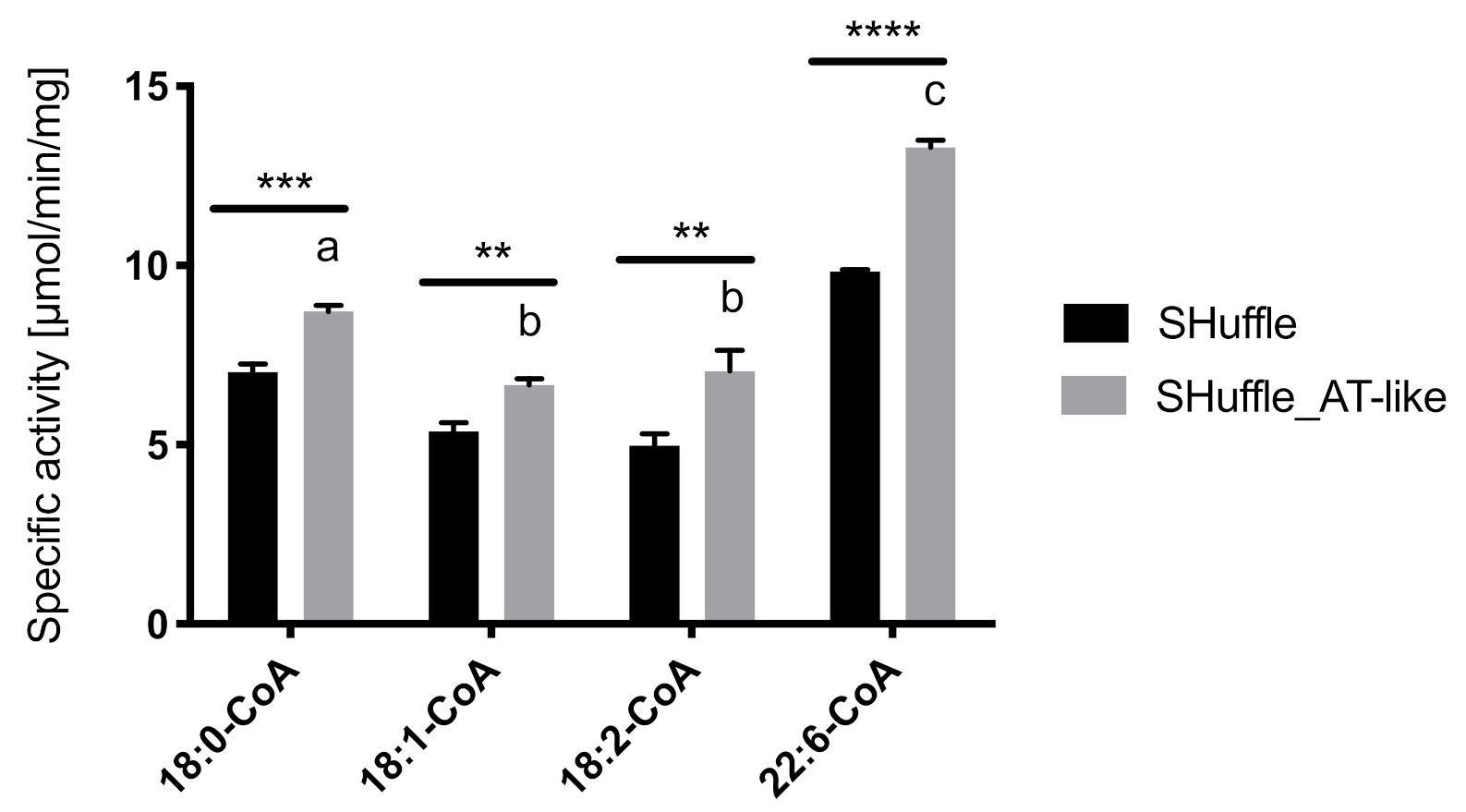
3.5. In Vitro Activity Assays of the AT-Like Domain
3.6. Mutagenesis Analysis of the AT-Like Domain
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Din, J.N.; Newby, D.E.; Flapan, A.D. Omega 3 fatty acids and cardiovascular disease—Fishing for a natural treatment. BMJ 2004, 328, 30–35. [Google Scholar] [CrossRef]
- Gomaa, A.M.; El-Aziz, E.A.A. Omega-3 fatty acids decreases oxidative stress, tumor necrosis factor-alpha, and interleukin-1 beta in hyperthyroidism-induced hepatic dysfunction rat model. Pathophysiology 2016, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.A.; Bevan, S.; Gachotte, D.J.; Larsen, C.M.; Moskal, W.A.; Merlo, P.A.O.; Sidorenko, L.V.; Hampton, R.E.; Stoltz, V.; Pareddy, D.; et al. Canola engineered with a microalgal polyketide synthase-like system produces oil enriched in docosahexaenoic acid. Nat. Biotechnol. 2016, 34, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Truksa, M.; Datla, N.; Vrinten, P.; Bauer, J.; Zank, T.; Cirpus, P.; Heinz, E.; Qiu, X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005, 23, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X. Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7,10,13,16,19): Two distinct pathways. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 181–186. [Google Scholar] [CrossRef]
- Voss, A.; Reinhart, M.; Sankarappa, S.; Sprecher, H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J. Biol. Chem. 1991, 266, 19995–20000. [Google Scholar] [CrossRef]
- Qiu, X.; Hong, H.; MacKenzie, S.L. Identification of a Δ4 Fatty Acid Desaturase from Thraustochytrium sp. Involved in the Biosynthesis of Docosahexanoic Acid by Heterologous Expression in Saccharomyces cerevisiae and Brassica juncea. J. Biol. Chem. 2001, 276, 31561–31566. [Google Scholar] [CrossRef]
- Metz, J.G.; Roessler, P.; Facciotti, D.; Levering, C.; Dittrich, F.; Lassner, M.; Valentine, R.; Lardizabal, K.; Domergue, F.; Yamada, A.; et al. Production of Polyunsaturated Fatty Acids by Polyketide Synthases in Both Prokaryotes and Eukaryotes. Science 2001, 293, 290–293. [Google Scholar] [CrossRef]
- Jiang, H.; Zirkle, R.; Metz, J.G.; Braun, L.; Richter, L.; Van Lanen, S.G.; Shen, B. The Role of Tandem Acyl Carrier Protein Domains in Polyunsaturated Fatty Acid Biosynthesis. J. Am. Chem. Soc. 2008, 130, 6336–6337. [Google Scholar] [CrossRef]
- Cronan, J.E.; Thomas, J. Chapter 17 Bacterial Fatty Acid Synthesis and its Relationships with Polyketide Synthetic Pathways. Methods Enzymol. 2009, 459, 395–433. [Google Scholar] [CrossRef]
- Qiu, X.; Xie, X.; Meesapyodsuk, D. Molecular mechanisms for biosynthesis and assembly of nutritionally important very long chain polyunsaturated fatty acids in microorganisms. Prog. Lipid Res. 2020, 79, 101047. [Google Scholar] [CrossRef]
- Zhao, X.; Dauenpen, M.; Qu, C.; Qiu, X. Genomic Analysis of Genes Involved in the Biosynthesis of Very Long Chain Polyunsaturated Fatty Acids in Thraustochytrium sp. Lipids 2016, 51, 1065–1075. [Google Scholar] [CrossRef]
- Meesapyodsuk, D.; Qiu, X. Biosynthetic mechanism of very long chain polyunsaturated fatty acids in Thraustochytrium sp. J. Lipid Res. 2016, 57, 1854–1864. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, X. Analysis of the biosynthetic process of fatty acids in Thraustochytrium. Biochimie 2018, 144, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Meesapyodsuk, D.; Qiu, X. Ketoacylsynthase Domains of a Polyunsaturated Fatty Acid Synthase in Thraustochytrium sp. Strain ATCC 26185 Can Effectively Function as Stand-Alone Enzymes in Escherichia coli. Appl. Environ. Microbiol. 2017, 83, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Meesapyodsuk, D.; Qiu, X. Functional analysis of the dehydratase domains of a PUFA synthase from Thraustochytrium in Escherichia coli. Appl. Microbiol. Biotechnol. 2017, 102, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Sun, K.; Meesapyodsuk, D.; Miao, Y.; Qiu, X. Distinct functions of two FabA-like dehydratase domains of polyunsaturated fatty acid synthase in the biosynthesis of very long-chain polyunsaturated fatty acids. Environ. Microbiol. 2020, 22, 3772–3783. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Satoh, Y.; Ogasawara, Y.; Maruyama, C.; Hamano, Y.; Ujihara, T.; Dairi, T. Control Mechanism for cis Double-Bond Formation by Polyunsaturated Fatty-Acid Synthases. Angew. Chem. Int. Ed. 2019, 58, 2326–2330. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Naka, M.; Ikeuchi, K.; Ohtsuka, M.; Kobayashi, K.; Satoh, Y.; Ogasawara, Y.; Maruyama, C.; Hamano, Y.; Ujihara, T.; et al. Control Mechanism for Carbon-Chain Length in Polyunsaturated Fatty-Acid Synthases. Angew. Chem. Int. Ed. 2019, 58, 6605–6610. [Google Scholar] [CrossRef] [PubMed]
- Torella, J.P.; Ford, T.J.; Kim, S.N.; Chen, A.M.; Way, J.C.; Silver, P.A. Tailored fatty acid synthesis via dynamic control of fatty acid elongation. Proc. Natl. Acad. Sci. USA 2013, 110, 11290–11295. [Google Scholar] [CrossRef]
- Semple, K.S.; Silbert, D.F. Mapping of the fabD locus for fatty acid biosynthesis in Escherichia coli. J. Bacteriol. 1975, 121, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Slabas, A. cDNA cloning of Brassica napus malonyl-CoA: ACP transacylase (MCAT) (fab D) and complementation of an E. coli MCAT mutant. FEBS Lett. 1998, 435, 204–206. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Han, C.; Hu, L.; Chen, K.; Shen, X.; Jiang, H. Characterization and inhibitor discovery of one novel malonyl-CoA: Acyl carrier protein transacylase (MCAT) from Helicobacter pylori. FEBS Lett. 2006, 580, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, F.; Moazen, F.; Rabbani, M.; Sadeghi, H.M.M. Optimization of the Expression of Reteplase in Escherichia coli TOP10 Using Arabinose Promoter. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e16676. [Google Scholar] [CrossRef]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Meesapyodsuk, D.; Chen, Y.; Ng, S.H.; Chen, J.; Qiu, X. Metabolic engineering of Pichia pastoris to produce ricinoleic acid, a hydroxy fatty acid of industrial importance. J. Lipid Res. 2015, 56, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Meesapyodsuk, D.; Qiu, X. A Peroxygenase Pathway Involved in the Biosynthesis of Epoxy Fatty Acids in Oat. Plant Physiol. 2011, 157, 454–463. [Google Scholar] [CrossRef][Green Version]
- Smith, S.; Tsai, S.-C. The type I fatty acid and polyketide synthases: A tale of two mega synthases. Nat. Prod. Rep. 2007, 24, 1041–1072. [Google Scholar] [CrossRef]
- Berge, R.K.; Farstad, M. Long-chain fatty acyl-CoA hydrolase from rat liver mitochondria. Methods Enzymol. 1981, 71, 234–242. [Google Scholar] [CrossRef]
- Kraut, J. Serine Proteases: Structure and Mechanism of Catalysis. Annu. Rev. Biochem. 1977, 46, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Guilbe, M.; Oyola-Robles, D.; Schreiter, E.R.; Baerga-Ortiz, A. Structure, Activity, and Substrate Selectivity of the Orf6 Thioesterase from Photobacterium profundum. J. Biol. Chem. 2013, 288, 10841–10848. [Google Scholar] [CrossRef]
- Heckman, K.L.; Pease, L.R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2007, 2, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Reikofski, J.; Tao, B.Y. Polymerase chain reaction (PCR) techniques for site-directed mutagenesis. Biotechnol. Adv. 1992, 10, 535–547. [Google Scholar] [CrossRef]
- Orikasa, Y.; Tanaka, M.; Sugihara, S.; Hori, R.; Nishida, T.; Ueno, A.; Morita, N.; Yano, Y.; Yamamoto, K.; Shibahara, A.; et al. pfaBproducts determine the molecular species produced in bacterial polyunsaturated fatty acid biosynthesis. FEMS Microbiol. Lett. 2009, 295, 170–176. [Google Scholar] [CrossRef][Green Version]
- Rao, L.; Xue, Y.; Zheng, Y.; Lu, J.R.; Ma, Y. A Novel Alkaliphilic Bacillus Esterase Belongs to the 13th Bacterial Lipolytic Enzyme Family. PLoS ONE 2013, 8, e60645. [Google Scholar] [CrossRef] [PubMed]
- Serre, L.; Verbree, E.C.; Dauter, Z.; Stuitje, A.R.; Derewenda, Z.S. The Escherichia coli Malonyl-CoA: Acyl Carrier Protein Transacylase at 1.5-Å Resolution. Crystal Structure of a Fatty Acid Synthase Component. J. Biol. Chem. 1995, 270, 12961–12964. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.C.; Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.; Gary, E.; Hillerich, B.; Yao, Z.; Carlier, P.R.; Totrov, M.; et al. Structural genomics for drug design against the pathogen Coxiella burnetii. Proteins Struct. Funct. Bioinform. 2015, 83, 2124–2136. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, K.H.; Park, J.K.; Jeong, K.-W.; Kim, Y.; Kim, E.E. New design platform for malonyl-CoA-acyl carrier protein transacylase. FEBS Lett. 2010, 584, 1240–1244. [Google Scholar] [CrossRef]
- August, P.R.; Tang, Y.J.; Yoon, S.; Ning, R.; Müller, T.; Yu, T.M.; Hoffmann, D.; Kim, C.-G.; Zhang, X.; Hutchinson, C.R.; et al. Biosynthesis of the Ansamycin Antibiotic Rifamycin: Deductions from the Molecular Analysis of the Rif Biosynthetic Gene Cluster of Amycolatopsis mediterranei S699. Chem. Biol. 1998, 5, 69–79. [Google Scholar] [CrossRef]
- Whicher, J.R.; Florova, G.; Sydor, P.K.; Singh, R.; Alhamadsheh, M.; Challis, G.L.; Reynolds, K.A.; Smith, J.L. Structure and Function of the RedJ Protein, a Thioesterase from the Prodiginine Biosynthetic Pathway in Streptomyces coelicolor. J. Biol. Chem. 2011, 286, 22558–22569. [Google Scholar] [CrossRef]
- Dumchin, M. Redefining the Future of Perioperative Nursing Education: A Conceptual Framework. AORN J. 2010, 92, 87–100. [Google Scholar] [CrossRef]
- Arts, M.T.; Brett, M.T.; Kainz, M. Biomedical and Life Sciences; Springer: Washington, DC, USA, 2009; pp. 214–217. [Google Scholar]
- Pazirandehs, M.; Subrahmanyam, S.C.; Wakilg, J. Site-Directed Mutagenesis Studies on the Recombinant Thioesterase Domain of Chicken Fatty Acid Synthase Expressed in Escherichia coli. J. Biol. Chem. 1991, 266, 20946–20952. [Google Scholar] [CrossRef]
- Herskowitz, I. Functional inactivation of genes by dominant negative mutations. Nat. Cell Biol. 1987, 329, 219–222. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, G.; Qin, Z.; Wu, C.; Lv, M.; Liao, S.; Qi, N.; Xie, M.; Cai, J. Functional characterizations of malonyl-CoA: Acyl carrier protein transacylase (MCAT) in Eimeria tenella. Mol. Biochem. Parasitol. 2012, 184, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lennen, R.M.; Pfleger, B.F. Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol. 2012, 30, 659–667. [Google Scholar] [CrossRef]
- Steen, E.J.; Kang, Y.; Bokinsky, G.; Hu, Z.; Schirmer, A.; McClure, A.; Del Cardayre, S.B.; Keasling, J.D. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nat. Cell Biol. 2010, 463, 559–562. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Agrawal, A.; San, K.-Y. Efficient free fatty acid production in Escherichia coli using plant acyl-ACP thioesterases. Metab. Eng. 2011, 13, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.G.; Kuner, J.; Rosenzweig, B.; Lippmeier, J.C.; Roessler, P.; Zirkle, R. Biochemical characterization of polyunsaturated fatty acid synthesis in Schizochytrium: Release of the products as free fatty acids. Plant Physiol. Biochem. 2009, 47, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Ogasawara, Y.; Satoh, Y.; Maruyama, C.; Hamano, Y.; Dairi, T. Off-Loading Mechanism of Products in Polyunsaturated Fatty Acid Synthases. ACS Chem. Biol. 2020, 15, 651–656. [Google Scholar] [CrossRef] [PubMed]

| Source | FFA Content [mg/L] | |
|---|---|---|
| ∆fadD | ∆fadD_AT-Like | |
| Intracellular | 3.43 ± 0.22 a | 4.98 ± 0.003 b |
| Extracellular | 1.29 ± 0.02 a | 1.77 ± 0.13 b |
| Protein | µmol TNB/min/mg Protein |
|---|---|
| Shuffle control | 10.32 ± 0.24 a |
| Shuffle_AT-like | 13.40 ± 0.08 b |
| Shuffle_AT-like S96A | 11.00 ± 0.17 c |
| Shuffle_AT-like H220A | 11.03 ± 0.14 c |
| Source | FFA Content [mg/L] | |||
|---|---|---|---|---|
| ∆fadD | ∆fadD_AT-Like | ∆fadD_AT-Like S96A | ∆fadD_AT-Like H220A | |
| Intracellular | 3.43 ± 0.22 a | 4.98 ± 0.003 b | 1.75 ± 0.10 c | 2.88 ± 0.18 d |
| Extracellular | 1.29 ± 0.02 a | 1.77 ± 0.13 b | 1.32 ± 0.03 a | 1.05 ± 0.05 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almendáriz-Palacios, C.; Meesapyodsuk, D.; Qiu, X. Functional Analysis of an Acyltransferase-Like Domain from Polyunsaturated Fatty Acid Synthase in Thraustochytrium. Microorganisms 2021, 9, 626. https://doi.org/10.3390/microorganisms9030626
Almendáriz-Palacios C, Meesapyodsuk D, Qiu X. Functional Analysis of an Acyltransferase-Like Domain from Polyunsaturated Fatty Acid Synthase in Thraustochytrium. Microorganisms. 2021; 9(3):626. https://doi.org/10.3390/microorganisms9030626
Chicago/Turabian StyleAlmendáriz-Palacios, Carla, Dauenpen Meesapyodsuk, and Xiao Qiu. 2021. "Functional Analysis of an Acyltransferase-Like Domain from Polyunsaturated Fatty Acid Synthase in Thraustochytrium" Microorganisms 9, no. 3: 626. https://doi.org/10.3390/microorganisms9030626
APA StyleAlmendáriz-Palacios, C., Meesapyodsuk, D., & Qiu, X. (2021). Functional Analysis of an Acyltransferase-Like Domain from Polyunsaturated Fatty Acid Synthase in Thraustochytrium. Microorganisms, 9(3), 626. https://doi.org/10.3390/microorganisms9030626






