Abstract
Arsenic (As), a semimetal toxic for humans, is commonly associated with serious health problems. The most common form of massive and chronic exposure to As is through consumption of contaminated drinking water. This study aimed to isolate an As resistant bacterial strain to characterize its ability to oxidize As (III) when immobilized in an activated carbon batch bioreactor and to evaluate its potential to be used in biological treatments to remediate As contaminated waters. The diversity of bacterial communities from sediments of the As-rich Camarones River, Atacama Desert, Chile, was evaluated by Illumina sequencing. Dominant taxonomic groups (>1%) isolated were affiliated with Proteobacteria and Firmicutes. A high As-resistant bacterium was selected (Pseudomonas migulae VC-19 strain) and the presence of aio gene in it was investigated. Arsenite detoxification activity by this bacterial strain was determined by HPLC/HG/AAS. Particularly when immobilized on activated carbon, P. migulae VC-19 showed high rates of As(III) conversion (100% oxidized after 36 h of incubation). To the best of our knowledge, this is the first report of a P. migulae arsenite oxidizing strain that is promising for biotechnological application in the treatment of arsenic contaminated waters.
1. Introduction
Arsenic (As) is a metalloid present in the atmosphere, soil and water as the result of various natural processes, such as land erosion and volcanic emissions, as well as the consequence of anthropogenic activities [1]. This element is present in many natural environments, typically in the form of inorganic As combined with other elements, such as oxygenated and sulfate-rich mineral waters [2,3,4]. In neutral pH water, two major forms of inorganic As can be found: the reduced form as trivalent arsenite and the oxidized form as pentavalent arsenate. Both the reduced and oxidized forms of As are toxic, but arsenite is on the average 100 times more toxic and it is also more mobile than arsenate [5,6,7].
The toxicity of arsenite is due to its strong binding affinity for sulfhydryl groups in proteins, thus affecting the redox status of the cysteine residues present on active sites of many enzymes and impairing their activities [5,8]. Arsenite also reacts with dithiol groups of glutathione, glutaredoxin and thioredoxin, interfering with intracellular redox homeostasis, DNA synthesis and repair and protein folding [8]. On the other hand, since arsenate is a natural analogue of the phosphate molecule, it can compete with phosphate in various biological processes [5,8,9,10]. In addition, arsenate uncouples oxidative phosphorylation, disrupts ATP synthesis and impairs various ATP-dependent cellular processes, such as transport, glycolysis, the pentose phosphate pathway and signal transduction pathways [11]. The chronic exposure to As (for example in drinking water) may cause severe health problems. The International Agency for Research on Cancer, the United States Department of Health and Human Services and the United States Environmental Protection Agency have classified inorganic arsenic as carcinogenic for humans, increasing the risk of skin, liver, bladder and lung cancers [12,13,14,15].
As-contaminated drinking water has been identified in different parts of the world, including Bangladesh, China, India, Pakistan and the U.S.A., affecting millions of people due toxicity [2,16,17]. In Chile, the problem of chronic arsenicism affects around 50,000 persons, mainly in rural populations of the Atacama Desert [18,19]. The affected populations drink water from small waterfalls and rivers with arsenic contents greater than 1000 ug/L [20,21]. This situation greatly surpasses both the World Health Organization and the U.S. Environmental Protection Agency recommendations for As concentrations (up to 10 ug/L) [22].
As-removal by conventional technologies (electro/coagulation, filtration, lime softening, activated alumina adsorption, ion exchange, reverse osmosis, electrodialysis reversal and nanofiltration) is a process able to remove nearly 80–95% of the As. These methodologies generally make use of a two-step method involving the oxidation of As(III) followed by the adsorption of the As(V) produced [23,24,25,26]. However, the strong chemical oxidants used in these processes are high-priced and may generate secondary pollutants [3,16].
Several metabolic pathways are involved in bacterial arsenate reduction (arsABC and arrAB) and arsenite oxidation (aio and arx), both under aerobic and anaerobic conditions [6,8,12]. In particular, arsenite oxidase, enzyme catalyzing As(III) oxidation has been characterized in both autotrophic and heterotrophic bacteria, and contains two heterologous subunits: a large catalytic subunit (AioA) that contains the molybdenum cofactor together with a 3Fe–4S cluster, and a small subunit (AioB) that contains a Rieske 2Fe–2S cluster [4,8]. Microorganisms inducing oxidation of arsenite in aqueous phase into the less mobile and less toxic arsenate has been known for many years and members of no less than nine bacterial genera, including alpha, beta and gamma Proteobacteria; Deinocci (Thermus) and Crenarchaeota, have being involved in this process [27,28,29,30,31]. Thus, biological oxidation of arsenite has the potentiality to bioremediate As-contaminated waters, making it a promising alternative to the traditional chemical oxidants. Hence, bacteria capable to oxidize arsenite must be considered as at least part of the solution. Therefore, the aim of this study was to characterize natural community arsenic resistant of Camarones river and isolate a bacterial strain resistant to As and to evaluate its capability to oxidize arsenite in planktonic and sessile (biofilm) conditions. All this data is required to consider the possible use of an As-resistant selected bacterial strain to implement a biological treatment system to bioremediate arsenite contaminated water.
2. Materials and Methods
2.1. Sampling
Sediments samples were collected from the Camarones river (18°59′46.8″ S 69°46′33.0″ W), in triplicate, from the surface up to a depth of 10 cm using 15 cm long and 3 cm diameter sterile cores. After the collection, the sample was maintained at 4 °C while transported to the laboratory. The superficial layer, 2 cm, of each sample was mechanically homogenized within a laminar flow hood (ZHJH-C 1109C, Zhicheng, Korea) and stored at 4 °C in RNAlater (Invitrogen-Thermo Fisher Scientific, CA, USA) for further processing.
2.2. Analysis of the Bacterial Community Composition
Total DNA from 1 g of the upper 2 cm layer of the sediment sample (mix of the three replicas) was extracted using the UltraClean DNA extraction kit (MO BIO Laboratories Inc., Carlsbad, CA, USA) following the protocol provided by the manufacturer. DNA extracts were then purified. The quality and concentration of DNA was checked by UV/Vis spectroscopy (NanoDrop ND-1000, Peq-lab, Erlangen, Germany). The DNA was sequenced at the Greehey Children’s Cancer Research Institute Next Generation Sequencing Facility, UT Health Science Center, University of Texas System, San Antonio, TX, USA. Sequencing was achieved using the Illumina MiSeq technology with pair end reads, targeting the V1-V2 region of 16S rRNA with primers 27F/338R (forward primer: 5′-AGA GTT TGA TCM TGG CTC AG-3′, reverse primer: 5′-GCT GCC TCC CGT AGG AGT-3′).
The Mothur software v.1.33.3 (www.mothur.org (accessed on 10 December 2020)) was used for pre-processing the sequenced data, including quality check, removal of duplicates, alignments to SILVA database (http://www.arb-silva.de (accessed on 10 December 2020)), denoising and chimera removal. Then, Mothur was used for distances calculation and clustering of the sequences into OTUs with a distance of 0.03. For taxonomic assignment, the RDP classifier version 2.7 and SILVA database with a threshold of 0.8 were used. For statistical analysis, we used the R statistical software version 3.1.0 [32].
2.3. Isolation of Arsenic Resistant Bacterial Strains
One g of the top 2 cm layer of the sediment was inoculated into 50 mL chemically defined medium (CDM) [33] (pH 8.0) containing 100 mg L−1 Na3AsO3 (Sigma-Aldrich, San Luis, MO, USA) and incubated at 30 °C for 48 h, on a shaker adjusted at 130 rpm, in darkness, according to Campos et al. [34]. Bacteria were then isolated in and CDM agar plates supplemented with arsenite (0.5 mM). The plates were incubated at 30 °C, for 48 h, in darkness.
2.4. Tolerance Levels to Arsenic (TLA)
The Tolerance levels to arsenic (TLA) for the isolates was determined by the agar dilution technique, on Luria Bertani (LB) agar (Becton Dickinson and Company, Maryland, MO, USA) plates supplemented with different concentrations of sodium arsenite (0.5–100 mM) (Sigma-Aldrich, San Luis, MO, USA) or sodium arsenate (0.5–1000 mM) (Sigma-Aldrich, San Luis, USA). Each plate was inoculated with cell suspensions from fresh pre-cultures at a final density of approximately 107 colony forming units (CFU) mL−1 and incubated for 24 h at 25 °C. To be used as control, LB agar plates were seeded with bacteria but without the metalloid. The criterion to consider bacterial strains as resistant or sensitive to As(III) or (As(V) was the one described by Rokbani et al. [35]. The bacterial strain capable to tolerate the highest arsenite and arsenate concentrations was selected to perform all the following experiments.
2.5. Identification of the Selected Strain and Aio Gene Detection Based on Molecular Characterization
The As resistant bacterial strain selected was identified after polymerase chain reaction (PCR) amplification of its 16S rRNA gen. The DNA was extracted using the InstaGen Matrix kit (BIO-RAD, Hercules, CA, USA) following the directions of the manufacturer. PCR was done using 16S rDNA bacteria universal primers GM3 (AGAGTTTGATCMTGGC) and GM4 (TACCTTGTTACGACTT). The PCR procedure followed the description of Urdiain et al. [36]. To detect the arsenite oxidase, a PCR analysis was performed using degenerated primer which target the large subunit of arsenite oxidase 69F (5′-TGYA TYGT NGGN TGYG GNTA YMA-3′) and 1374R (5′-TANC CYTC YTGR TGNC CNCC-3′), which target the large subunit of arsenite oxidase, according to Valenzuela et al. [21]. Sequencing was conducted by means of the Sanger’s method using an ABI PRISM 3500 xL Genetic Analizer (Applied Biosystems, Foster City, USA). The sequences were analysed by means of Basic Local Alignment Search Tools (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST (accessed on 10 December 2020)) to obtain sequences with the greatest significant alignment.
2.6. Effect of As(III) and As(V) on the Growth of the Selected Bacterial Strain
The selected bacterial strain (~104 CFU mL−1) was inoculated into 50 mL CDM medium supplemented with 0.5 mM As(III) or As(V) and then incubated under aerobic conditions at 30 °C under agitation (130 rpm) for 48 h. A control was implemented without the addition of As and incubated under the same conditions described above. The experiments were performed in triplicate and growth was determined recording the number of CFU at regular time intervals. The curves obtained were analysed, making use of the mathematical modelling of Gompertz [37]. Graphs and models were made using GraphPad Prism version 5.0 (GraphPad software, San Diego, CA, USA).
2.7. Attachment of the Selected Strain to Inert Support (Biofilm Formation)
The biofilm was formed placing sterile activated carbon and CDM (5:100 w/v) in 250 mL flasks. Then, an inoculum (105 CFU mL−1) of the selected strain was added and cultured at 30 °C, with agitation (130 rpm), for 72 h under aerobic conditions, in the presence or absence of 0.5 mM As(III). The experiments were performed in triplicate and biofilm formation was evaluated by total cell counts using fluorescence microscopy and by scanning electron microscopy (SEM).
2.8. Scanning Electron Microscopy (SEM)
Samples of activated carbon were fixed using 2.5% glutaraldehyde (Sigma-Aldrich, San Luis, USA). Samples were dehydrated with ethanol and critical point dried using CO2. Then, they were coated with gold using an Edwards S150B sputter coater (Edwards High Vacuum International, West Sussex, UK) [38]. Samples were analysed using a JEOL JSM 6380LV SEM (JEOL, Tokyo, Japan).
2.9. Fluorescence Microscopy
One g of activated carbon samples were sonicated in 10 mL phosphate-buffered saline (PBS) (pH 7.0) in an ultrasonic bath (Elma/S30, Singen, Germany) adjusted at 100 W for 2 min, fixed in formaldehyde solution (4% w/v) and then filtered using white polycarbonate membranes (0.2 μm, Ø47 mm). Then, 4,6-diamino-2-phenylin doldihydrochloride dilactate (DAPI) (Sigma-Aldrich, San Luis, MO, USA) staining (1 mg mL−1) was added [39]. Samples were taken in triplicates and biofilms were evaluated on the basis of bacterial cells counted, using a Motic BS-310 epifluorescence microscope equipped with a MotiCam Pro 285A digital camera (Motic, Richmond, Canada).
2.10. Oxidation of As(III) to As(V) by the Selected Strain under Planktonic and Immobilized Conditions
The oxidation of As by the selected strain under planktonic and immobilized conditions was studied using 500 mL glass bottles, as batch bioreactors, containing 250 mL CDM plus 0.5 mM sodium arsenite (NaH2AsO3). The free cells (planktonic) bioreactor was inoculated with 106 CFU mL−1 of the selected strain. The immobilized cells bioreactor was inoculated with 10 g of a biofilm (108 cells/g) of the selected strain obtained after 72 h previous incubation on active carbon at 25 °C [40]. Both batch bioreactors were incubated under aerobic conditions at 25 °C during 48 h. The experiments were performed in triplicate and oxidation of As(III) to As(V) was determined from culture supernatants aseptically removed from the reactors. One mL supernatant samples were filtered through a sterile 0.22 μm pore size filter (Corning Inc., New York, NY, USA) and As-species were detected by means of high-performance liquid chromatography coupled to arsine gaseous formation performing the detection by atomic absorption (HPLC/HG/QAAS) [19]. The quantitative determination of both arsenic species (As(III) and As(V)) was performed using an atomic absorption spectrometer (AAnalyst 100, PerkinElmer, Norwalk, CT, USA), equipped with a commercial hydride generation system (FIAS-100, PerkinElmer, Norwalk, CT, USA) and a quartz cell (15 cm length, 1 cm i.d.) associated to an acetylene flame served as atomiser. An externally controlled by An EDL power supply system (EDL System 2, PerkinElmer, Norwalk, CT, USA) allowed to control an electrode-less arsenic lamp used as emission source [19].
2.11. Statistical Analysis
Bacterial biotransformation and growth rates (K) of the models that best conformed to the kinetics of bacterial growth were analysed through Student’s t tests using the MINITAB software (version 15, USA). P values < 0.05 were considered as statistically significant.
3. Results
3.1. Sequencing Data and Diversity Estimates
The sequencing analysis of the universal V1-V2 region of the 16S rRNA for Bacteria Domain produced a total of 295,530 sequences. After quality check within the SILVA database and removing chimeras, 294,104 (99.4%) high quality sequences remained (Table 1). The Shannon diversity index and non-parametric Chao1 and ACE richness estimators are reported in Table 1, showing a normal diversity.

Table 1.
Sequencing information, diversity index (H’) and estimator of richness (Chao1 and ACE) obtained after Illumina sequencing.
3.2. Composition of the Bacterial Community
The OTUs retrieved from the Camarones river superficial sediment sample showed the presence of a total of 17 different bacterial phyla. The dominant taxonomic groups (abundance ≥ 1%) were affiliated to Proteobacteria and Firmicutes (76.6% and 21.9%, respectively) (Figure 1). Within Proteobacteria, the most abundant class was Gammaproteobacteria (73.43%) and another four classes were also present, including two classes representing minor components (0.44% and 0.2%) (Table 2). Among the phyla whose abundance was ≤1%, OTUs affiliated to fifteen different phyla, with abundances varying from 0.46% (Acidobacteria) to 0.02%, were identified (Table 2).
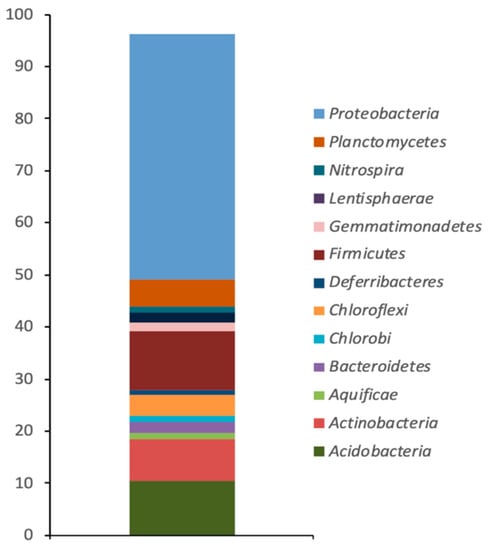
Figure 1.
Relative abundance of sequences (percentage) assigned to bacterial phylogenetic groups from the superficial sediment of Camarones river, Atacama Desert.

Table 2.
Dominant phylogenetic groups present in the sediment of Camarones river, Atacama Desert.
3.3. Isolation and Characterization of Bacterial Arsenic-Resistant
After culturing in the presence of As, a total of 11 different As-resistant strains were isolated from the sediment. All bacterial strains isolated were able to grow on agar containing either 7 mM As(III) or 20 mM As(V) at 25 °C, concentrations allowing to considered these strains as As-resistant [35].
One of the bacterial strains, labelled as VC-19, was able to grow at As concentrations exceeding 20 mM As(III) and 100 Mm As(V). Sequencing of PCR product (1200 bp) demonstrated the presence of the arsenite oxidase aio gene in this strain. The biochemical profile of this strain and the closest GenBank match to its 16S rDNA sequence revealed that VC-19 strain (GenBank Accession Number MG545624) possessed a high degree of similarity (99.8%) with Pseudomonas migulae. Moreover, the tree reconstruction affiliated VC-19 strain to Pseudomonas genus, ratifying that the analysed sequence of this strain can be ascribed to P. migulae (Figure 2).

Figure 2.
Phylogenetic tree reconstruction based on the rRNA 16s analyses of Pseudomonas migulae VC-19 strain. (GenBank N° Access MG545624)
3.4. Growth Kinetics
In order to determine the effect of As on the growth of P. migulae VC-19 strain, its growth kinetics was studied in the presence of 0.5 mM As(III) or As(V) or its absence by means of the Gompertz model. The model demonstrated that after of 48 h of incubation this bacterial strain cultured in the presence of 0.5 mM As(III) showed a better growth than the control, with a growth rate (µm) of 0,88 and 0,79 h−1, respectively (Figure 3). Student’s t test (with 95% confidence) demonstrated statistically significant differences between both conditions (p < 0.05). When cultured in the presence of 0.5 mM As (V), P. migulae VC-19 strain showed a growth rate (µm) of 0.78 h−1, significantly lower than in the presence of As (III) Figure 3).
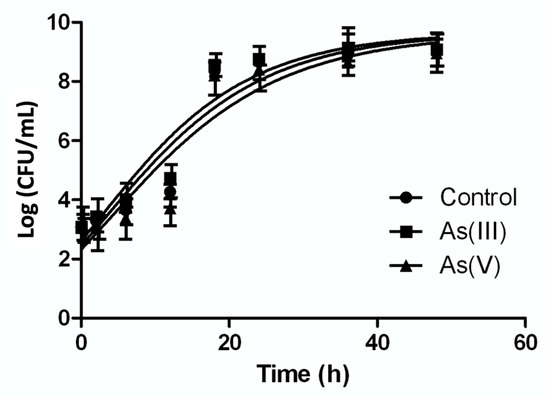
Figure 3.
Growth kinetics of Pseudomonas migulae VC-19 strain in the presence of 0.5 mM As(III) or 0.5 mM As(V). The same strain cultured in the absence of arsenic was used as control. Kinetics was adjusted with Gompertz growth.
3.5. Biofilm Formation in a Batch Experiment by P. migulae VC-19
The ability of 105 CFU mL−1 of P. migulae VC-19 strain growing in the exponential phase to adhere to activated carbon in the presence or absence of As(III) was monitored by fluorescence microscopy and SEM. The number of bacterial cells per gram of carbon was evaluated up to 72 h of culture by fluorescence microscopy. Both, with or without As(III), the bacterial strain formed a stable biofilm containing 109 and 108 cells g−1 of activated carbon, respectively (Figure 4). P. migulae VC-19 strain, cultured in the presence of As(III) showed similar growth rate (µm) than the control, 0,60 [CFU h−1] under both conditions (p > 0.05). In the presence of As(V), the biofilm presented a stable growth, similar to that of the control culture and the culture containing As (III).
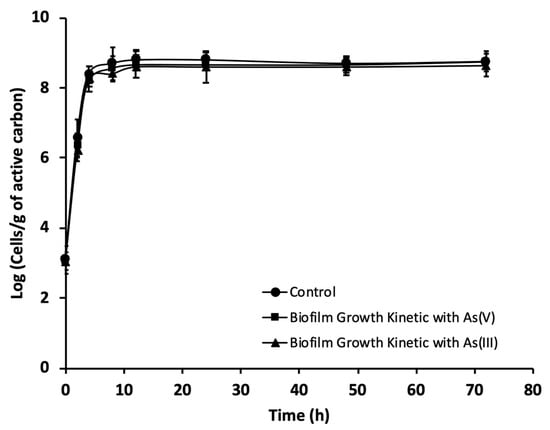
Figure 4.
Growth kinetics, during a 72 h period, of P. migulae VC-19 on activated carbon plus As(III) or As(V), monitored by means of DAPI staining. The control lacked As-species.
Besides monitoring cell counts by fluorescence microscopy, biofilm formation by P. migulae VC-19 strain on activated carbon was also monitored by SEM. In the presence or absence of As(III), small cells were randomly distributed on the surface of activated carbon after 24 h of incubation (Figure 5A and Figure 6A). After 48 h of incubation, cultures with or without As(III) showed bacteria anchored to the activated carbon and to each other by means of fibrils, bridging the space between bacteria (Figure 5B and Figure 6B). After 72 h of incubation, both cultures in the presence or absence of As(III), a mature biofilm was observed with attached bacteria associated to extracellular polysaccharides covering the sessile cells and serving as a matrix for further biofilm formation and stabilizing it was observed in both cultures. (Figure 5C and Figure 6C).
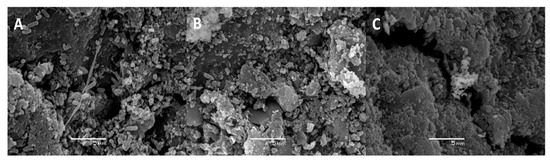
Figure 5.
Biofilm formation by P. migulae VC-19 in batch experiments containing activated carbon and no Arsenic after 24 h (A), 48 h (B) and 72 h (C) of incubation at 30 °C under aerobic conditions.

Figure 6.
Biofilm formation by P. migulae VC-19 strain in a batch experiments containing activated carbon and 0.5 mM As(III) (Na3AsO3) after 24 h (A), 48 h (B) and 72 h (C) of incubation at 30 °C under aerobic conditions.
3.6. Arsenic Oxidation in Batch Experiments
Arsenite oxidation by planktonic or immobilized P. migulae cells was evaluated by HPLC/HG/ASS. Immobilized P. migulae cells were able to oxidize 0.5 mM As(III), under aerobic conditions, leading to 100% As(III) conversion into As(V) after 36 h of incubation, a rate of 12.86 μg mL−1 h−1 (Figure 7).
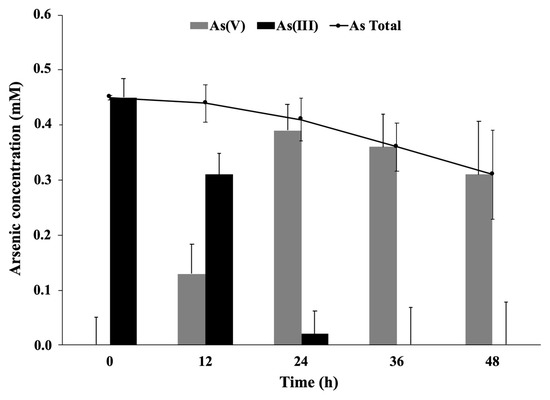
Figure 7.
Arsenite oxidation by P. migulae VC-19 strain immobilized on activated carbon, cultured at 30 °C during 48 h.
Planktonic P. migulae cells, cultured under similar conditions, were able to oxidize 100% As(III) to As(V) after 48 h, a rate of 10.07 μg mL−1 h−1 (Figure 8). A decrease of total-As present in the supernatant, concomitant with the decrease of As (V), was also observed in the batch experiments implemented with immobilized cells (Figure 7).
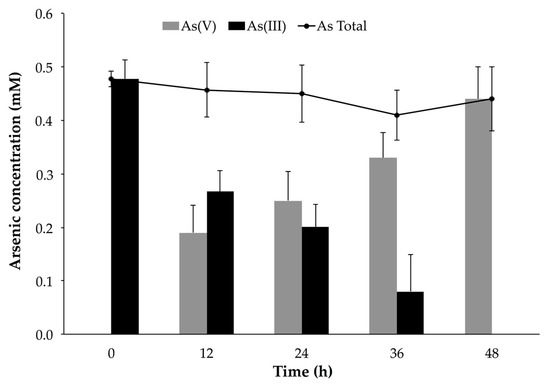
Figure 8.
Arsenite oxidation by P. migulae VC-19 strain in planktonic conditions at 30 °C, during 48 h.
4. Discussion
Certain aquatic environments are characterized by high concentrations of different minerals. Because of its geological characteristics, the Andean plateau of the Atacama Desert (Chile), including the Camarones river, naturally possesses high concentrations of As (1000 ug/L) [19,20]. In particular, the presence of As in the Camarones river turns it a potential source to isolate bacteria possessing strategies to survive in an As rich environments [41]. These strategies may include As bioaccumulation, biomineralization, biosorption, and biotransformation [42]. There are reports describing those bacterial communities in As-rich groundwater and surface waters are dominated by Firmicutes, Alpha-, Beta-, Gamma- and Epsilon-proteobacteria [43,44,45,46,47]. In our study, a massive parallel sequencing analysis (Illumina) revealed a significant shift in the bacterial communities of the As contaminated Camarones river sediment studied, where Proteobacteria and Firmicutes were dominating groups (≥1% of total classified sequences). Similar results were reported by Leon et al. [48], showing that the Camarones river, also part of the Atacama Desert, possessed a higher relative abundance of Proteobacteria (40.3%), Firmicutes (24.82%) and Acidobacteria (12.02%). The bacterial diversity reported by that report was also greater than the one found in our work. The greater diversity reported for the Camarones river may be the consequence of the “altiplanico winter” which causes very intense precipitations in this zone, resulting in a severe downstream flow of water of high turbidity which carries suspended solids [49].
Many reports describe a variety arsenic-resistant and transforming bacterial species from aquatic environments and soil, being Proteobacteria species predominant [50,51]. Some culturable bacterial species representative of these Phylum, such as those belonging to the Pseudomonas genus, have been previously isolated from several environments, including drinking water, and have demonstrated the ability to grow in higher As concentrations. In our study, P. migulae VC-19 was able in grow in the presence of 20 mM As (III) and 100 mM As (V). Dey et al. [52] reported the isolation of twelve colonies from ground-water resistant to 100–5000 ppm arsenate and 100–1000 ppm arsenite. In addition, other bacterial genera, such as Bacillus, klebsiella, Dienococcus, Acidthiobacillus and Desulfitobacterium have been reported as arsenic resistant [53].
Since the growth curve of P. migulae VC-19 strain cultured in the presence of As (III), when modelled by the Gompertz function, showed to grow better than the control. Numerous heterotrophic As(III)-oxidizing bacteria, such as Hydrogenophaga sp. NT-14 and A. tumefaciens GW4, have been reported to capable to obtain energy from the oxidation of arsenite, using the metalloid as the electron source and carbonate as unique carbon source [21,40,54,55,56,57,58,59,60,61,62,63]. However, As (III) oxidation by heterotrophic bacteria is generally considered to be a detoxification mechanism rather than one that can support growth [64]. Zhang et al. [65] reported a novel chemoautotrophic As(III)-oxidizing bacterium, isolated from an As-contaminated paddy soil, able to derive energy from the oxidation of As(III) to As(V), under both aerobic and anaerobic conditions using O2 or NO3− as electron acceptor.
When we studied the As (III) genetic resistance of P. migulae VC-19, by means of PCR and subsequent sequencing, the presence of the As oxidizing aio gene was detected. Structurally, the aio codified arsenite oxidase is composed of the minor AioB subunit and the main AioA subunit (90–100 kDa), considered a molybdoprotein. The minor subunit is considered to be the main responsible for the interaction with the electron transport proteins (cytochrome). Since the genes encoding these cytochromes are expressed together with the aioAB genes, both genes are generally in the same operon [25]. In addition, the oxidation of highly toxic arsenite to less toxic arsenate encoded by arsenite oxidase aio gene is a key step of the As-detoxification mechanism by microorganisms [66,67].
P. migulae VC-19 strain was able to produce a stable biofilm on active carbon in the presence or absence of As(III). After 72 h of incubation, SEM analyses showed both in presence or absence of As(III), micro-fibrillar structures and bacterial cells embedded by a matrix probably constituted by extracellular polysaccharides, which has been previously described for other microorganisms [68], and which are characteristic of a mature bacterial biofilm, in which extracellular polysaccharides are secreted by attached bacteria and serve as a matrix for the attachment of other cells [69]. In addition, Decho [70] describe that biofilm-mediated bioremediation presents advantage to bioremediation with planktonic microorganisms because cells in a biofilm have a better adaptation, survival and high microbial biomass. Zeng et al. [71] describe that the biofilm formation significantly promoted the bacterial resistance to arsenic.
Our study demonstrated that the immobilization of the P. migulae VC-19 strain on activated carbon allowed the development and maintenance of a biofilm, which showed increased oxidation rates than that of planktonic cells. Simeonova et al. [72], studying Herminiimonas arsenoxidans immobilized on calcium alginate, demonstrated that immobilized cells oxidized arsenite faster than planktonic cells. Valenzuela et al. [40] reported similar results studying the oxidation of arsenite to arsenate by Pseudomonas arsenicoxydans cells immobilized on zeolite. Ito et al. [73] reported in continuous experiments, conducted using a bioreactor with immobilised arsenite oxidising bacteria, an As(III) oxidation efficiency of 92%, with an As(III) oxidation rate of 1 × 10−9 μg/cell/min.
Wan et al. [74] investigating the combined processes of biological As (III) oxidation and removal of metalloid by zero-valent iron, reported an incomplete As removal, associated to kinetic limitation of As removal or by a continuous formation of iron corrosion products, enable to trap As. In our study, the total arsenic decreases over time, due to a possible adsorption of the metalloid on the activated carbon used as support for the P. migulae VC-19 biofilm. Adsorption onto activated carbon has been described as one of the most effective methods for heavy metal removal [75,76,77]. In general, microbial oxidation of As (III) in bioreactors with immobilized biomass, followed by adsorption is a promising and environment-friendly system for the treatment of As(III)-contaminated waters [78,79,80,81].
5. Conclusions
The results of the present study demonstrated that heterotrophic arsenite oxidising bacteria, such as P. migulae, are good potential candidates for biological As-detoxification systems. The reason is that As abatement requires, as an initial step, the oxidation of the metalloid for its subsequent precipitation and removal. Therefore, making use of bacteria for the biological oxidation of As may circumvent the requirement of strong chemical oxidants, compounds which are usually even more toxic than arsenic itself.
Author Contributions
Conceptualization, V.L.C.; methodology, V.L.C., R.M., P.A., V.G.-F., J.Y. and B.B.; software, C.V. (Claudia Vilo), C.V. (Cristian Valenzuela), V.G.-F. and V.L.C.; validation, V.L.C., R.M., J.Y., C.T.S.; formal analysis, V.L.C., C.V. (Claudia Vilo), C.T.S.; investigation, C.H., V.G.-F., J.Y. and V.L.C.; resources, V.L.C.; data curation, C.V. (Claudia Vilo), C.V. (Cristian Valenzuela); writing—original draft preparation, C.H., B.B., P.A., C.T.S., and V.L.C.; writing—review and editing, V.L.C., C.T.S., P.A., J.Y.; visualization, R.M., M.R., V.G.-F., P.A., C.T.S., V.L.C.; supervision, V.L.C.; project administration, V.L.C.; funding acquisition, V.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Grant FONDECYT N°11130383 ANID-Chile and Grant 216.036.043.1.0, VRID, University of Concepcion, Chile.
Acknowledgments
The authors acknowledge the assistance of the staff of the Electron Microscopy Laboratory, University of Concepcion, Chile.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, X.-H.; Zhang, P.P.; Chen, X.-G.; Wu, D.D.; Ye, Y. Natural and anthropogenic influences on the arsenic geochemistry of lacustrine sediment from a typical fault-controlled highland lake: Yangzonghai Lake, Yunnan, China. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Huq, M.E.; Fahad, S.; Shao, Z.; Sarven, M.; Khan, B.; Alam, M.; Saeed, M.; Ullah, H.; Adnan, M.; Saud, S.; et al. Arsenic in a groundwater environment in Bangladesh: Occurrence and mobilization. Journal of Environmental Management 2020, 262, 110318. [Google Scholar] [CrossRef]
- Jebelli, M.; Maleki, A.; Amoozegar, M.; Kalantar, E.; Shahmoradi, B.; Gharibi, F. Isolation and identification of indigenous prokaryotic bacteria from arsenic-contaminated water resources and their impact on arsenic transformation. Ecotoxicol. Environ. Saf. 2017, 140, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Stolz, J.; Oremland, R. Microbial Arsenic Metabolism: New Twists on an Old Poison. Microbe Mag. 2010, 5, 53–59. [Google Scholar] [CrossRef]
- Cui, J.; Jing, C. A review of arsenic interfacial geochemistry in groundwater and the role of organic matter. Ecotoxicol. Environ. Saf. 2019, 183. [Google Scholar] [CrossRef]
- Rosen, B.P. Biochemistry of arsenic detoxification. FEBS Lett. 2002, 529, 86–92. [Google Scholar] [CrossRef]
- Abid, M.; Niazi, N.; Bibi, I.; Farooqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.; Arshad, M. Arsenic(V) biosorption by charred orange peel in aqueous environments. Int. J. Phytoremed. 2016, 18, 442–449. [Google Scholar] [CrossRef]
- Kruger, M.C.; Bertin, P.N.; Heipieper, H.J.; Arsène-Ploetze, F. Bacterial metabolism of environmental arsenic--mechanisms and biotechnological applications. Appl. Microbiol. Biotechnol. 2013, 97, 3827–3841. [Google Scholar] [CrossRef]
- Niazi, N.K.; Burton, E.D. Arsenic sorption to nanoparticulate mackinawite (FeS): An examination of phosphate competition. Environ. Pollut. 2016, 218, 111–117. [Google Scholar] [CrossRef]
- LeMonte, J.J.; Stuckey, J.W.; Sanchez, J.Z.; Tappero, R.; Rinklebe, J.; Sparks, D.L. Sea Level Rise Induced Arsenic Release from Historically Contaminated Coastal Soils. Environ. Sci. Technol. 2017, 51, 5913–5922. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Sharif, F.; Bashir, S.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; et al. Arsenic removal by natural and chemically modified water melon rind in aqueous solutions and groundwater. Sci. Total Environ. 2018, 645, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef]
- Rafiei, G.; Shirkoohi, R.; Saffari, M.; Salehipour, P.; Modarressi, M.H. The Impact of Long-term Exposure to Low Levels of Inorganic Arsenic on the Hypomethylation of SEPT9 Promoter in Epithelial-Mesenchymal Transformed Colorectal Cancer Cell Lines. Int. J. Mol. Cell Med. 2019, 8, 130–138. [Google Scholar] [CrossRef]
- Waqas, H.; Shan, A.; Khan, Y.; Nawaz, R.; Rizwan, M.; Saif Ur Rehman, M.; Shakoor, M.; Khan, W.; Jabeen, M. Human health risk assessment of arsenic in groundwater aquifers of Lahore, Pakistan. Hum. Ecol. Risk Assess. 2017, 23. [Google Scholar] [CrossRef]
- Khan, S.; Shahnaz, M.; Jehan, N.; Rehman, S.; Shah, M.; Din, I. Drinking water quality and human health risk in Charsadda district, Pakistan. J. Clean. Prod. 2013, 60, 93–101. [Google Scholar] [CrossRef]
- Shakoor, M.; Bibi, I.; Niazi, N.; Shahid, M.; Nawaz, M.; Farooqi, A.; Naidu, R.; Rahman, M.; Murtaza, G.; Luttge, A. The evaluation of arsenic contamination potential, speciation and hydrogeochemical behaviour in aquifers of Punjab, Pakistan. Chemosphere 2018, 199, 737–746. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, M.; Dumat, C.; Khalid, S.; Niazi, N.; Imran, M.; Bibi, I.; Ahmad, I.; Hammad, H.; Tabassum, R. Arsenic Level and Risk Assessment of Groundwater in Vehari, Punjab Province, Pakistan. Expo. Health 2017, in press. [Google Scholar] [CrossRef]
- Arriaza, B.; Amarasiriwardena, D.; Standen, V.; Yáñez, J.; Van Hoesen, J.; Figueroa, L. Living in poisoning environments: Invisible risks and human adaptation. Evol. Anthropol. 2018, 27, 188–196. [Google Scholar] [CrossRef]
- Yáñez, J.; Mansilla, H.D.; Santander, I.P.; Fierro, V.; Cornejo, L.; Barnes, R.M.; Amarasiriwardena, D. Urinary arsenic speciation profile in ethnic group of the Atacama desert (Chile) exposed to variable arsenic levels in drinking water. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2015, 50, 1–8. [Google Scholar] [CrossRef]
- Yáñez, J.; Fierro, V.; Mansilla, H.; Figueroa, L.; Cornejo, L.; Barnes, R.M. Arsenic speciation in human hair: A new perspective for epidemiological assessment in chronic arsenicism. J. Environ. Monit. 2005, 7, 1335–1341. [Google Scholar] [CrossRef]
- Valenzuela, C.; Campos, V.L.; Yañez, J.; Zaror, C.A.; Mondaca, M.A. Isolation of arsenite-oxidizing bacteria from arsenic-enriched sediments from Camarones river, Northern Chile. Bull. Environ. Contam. Toxicol. 2009, 82, 593–596. [Google Scholar] [CrossRef]
- Minatel, B.C.; Sage, A.P.; Anderson, C.; Hubaux, R.; Marshall, E.A.; Lam, W.L.; Martinez, V.D. Environmental arsenic exposure: From genetic susceptibility to pathogenesis. Environ. Int. 2018, 112, 183–197. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Singh, T.S.A. Arsenic removal by electrocoagulation process: Recent trends and removal mechanism. Chemosphere 2017, 181, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- Karn, S.K.; Pan, X.; Jenkinson, I.R. Bio-transformation and stabilization of arsenic (As) in contaminated soil using arsenic oxidizing bacteria and FeCl(3) amendment. 3 Biotech 2017, 7, 50. [Google Scholar] [CrossRef]
- Luong, V.T.; Kurz, E.E.C.; Hellriegel, U.; Luu, T.L.; Hoinkis, J.; Bundschuh, J. Iron-based subsurface arsenic removal technologies by aeration: A review of the current state and future prospects. Water Res. 2018, 133, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-S.; Bothner, B.; Rensing, C.; McDermott, T. Involvement of RpoN in Regulating Bacterial Arsenite Oxidation. Appl. Environ. Microbiol. 2012, 78, 5638–5645. [Google Scholar] [CrossRef] [PubMed]
- Lieutaud, A.; van Lis, R.; Duval, S.; Capowiez, L.; Muller, D.; Lebrun, R.; Lignon, S.; Fardeau, M.L.; Lett, M.C.; Nitschke, W.; et al. Arsenite oxidase from Ralstonia sp. 22: Characterization of the enzyme and its interaction with soluble cytochromes. J. Biol. Chem. 2010, 285, 20433–20441. [Google Scholar] [CrossRef]
- Koechler, S.; Cleiss-arnold, J.; Proux, C.; Sismeiro, O.; Dillies, M.A.; Goulhen-Chollet, F.; Hommais, F.; Lièvremont, D.; Arsène-Ploetze, F.; Coppée, J.-Y.; et al. Multiple controls affect arsenite oxidase gene expression in Herminiimonas arsenicoxydans. BMC Microbiology 2010, 10, 53. [Google Scholar] [CrossRef]
- Campos, V.L.; Valenzuela, C.; Yarza, P.; Kampfer, P.; Vidal, R.; Zaror, C.; Mondaca, M.A.; Lopez-Lopez, A.; Rossello-Mora, R. Pseudomonas arsenicoxydans sp. nov., an arsenite-oxidizing strain isolated from the Atacama desert. Syst. Appl. Microbiol. 2010, 33, 193–197. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.; Ding, S.; Hao, C.; Xiu, W.; Hou, W. Arsenate reduction and mobilization in the presence of indigenous aerobic bacteria obtained from high arsenic aquifers of the Hetao basin, Inner Mongolia. Environ. Pollut. 2015, 203, 50–59. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Volume 1, p. 409. [Google Scholar]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.L.; Escalante, G.; Yañez, J.; Zaror, C.A.; Mondaca, M.A. Isolation of arsenite-oxidizing bacteria from a natural biofilm associated to volcanic rocks of Atacama Desert, Chile. J. Basic Microbiol. 2009, 49 (Suppl. 1), S93–S97. [Google Scholar] [CrossRef] [PubMed]
- Rokbani, A.; Bauda, P.; Billard, P. Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res. Microbiol. 2007, 158, 128–137. [Google Scholar] [CrossRef]
- Urdiain, M.; López-López, A.; Gonzalo, C.; Busse, H.J.; Langer, S.; Kämpfer, P.; Rosselló-Móra, R. Reclassification of Rhodobium marinum and Rhodobium pfennigii as Afifella marina gen. nov. comb. nov. and Afifella pfennigii comb. nov., a new genus of photoheterotrophic Alphaproteobacteria and emended descriptions of Rhodobium, Rhodobium orientis and Rhodobium gokarnense. Syst. Appl. Microbiol. 2008, 31, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Cuevas, J.-P.; Moraga, R.; Sánchez-Alonzo, K.; Valenzuela, C.; Aguayo, P.; Smith, C.T.; García, A.; Fernandez, Í.; Campos, V.L. Characterization of the Bacterial Biofilm Communities Present in Reverse-Osmosis Water Systems for Haemodialysis. Microorganisms 2020, 8, 1418. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.L.; León, C.; Mondaca, M.A.; Yañez, J.; Zaror, C. Arsenic mobilization by epilithic bacterial communities associated with volcanic rocks from Camarones River, Atacama Desert, northern Chile. Arch. Environ. Contam. Toxicol. 2011, 61, 185–192. [Google Scholar] [CrossRef]
- Valenzuela, C.; Moraga, R.; Leon, C.; Smith, C.; Mondaca, M.-A.; Campos, V. Arsenite Oxidation by Pseudomonas arsenicoxydans Immobilized on Zeolite and Its Potential Biotechnological Application. Bull. Environ. Contam. Toxicol. 2015, 94. [Google Scholar] [CrossRef]
- Escudero, L.V.; Casamayor, E.O.; Chong, G.; Pedrós-Alió, C.; Demergasso, C. (Distribution of microbial arsenic reduction, oxidation and extrusion genes along a wide range of environmental arsenic concentrations. PLoS ONE 2013, 8, e78890. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Liao, V.H.; Chu, Y.J.; Su, Y.C.; Hsiao, S.Y.; Wei, C.C.; Liu, C.W.; Liao, C.M.; Shen, W.C.; Chang, F.J. Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J. Contam. Hydrol. 2011, 123, 20–29. [Google Scholar] [CrossRef]
- Lin, X.; McKinley, J.; Resch, C.T.; Kaluzny, R.; Lauber, C.L.; Fredrickson, J.; Knight, R.; Konopka, A. Spatial and temporal dynamics of the microbial community in the Hanford unconfined aquifer. ISME J. 2012, 6, 1665–1676. [Google Scholar] [CrossRef]
- Gnanaprakasam, E.T.; Lloyd, J.R.; Boothman, C.; Ahmed, K.M.; Choudhury, I.; Bostick, B.C.; van Geen, A.; Mailloux, B.J. Microbial Community Structure and Arsenic Biogeochemistry in Two Arsenic-Impacted Aquifers in Bangladesh. mBio. 2017, 28, e01326-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Jiang, Z.; Sinkkonen, A.; Wang, S.; Tu, J.; Wei, D.; Dong, H.; Wang, Y. Microbial Community of High Arsenic Groundwater in Agricultural Irrigation Area of Hetao Plain, Inner Mongolia. Front. Microbiol. 2016, 7, 1917. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; Zecchin, S.; Amalfitano, S.; Fazi, S.; Casentini, B.; Corsini, A.; Cavalca, L.; Rossetti, S. Phylogenetic Structure and Metabolic Properties of Microbial Communities in Arsenic-Rich Waters of Geothermal Origin. Front. Microbiol. 2017, 8, 2468. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.G.; Moraga, R.; Valenzuela, C.; Gugliandolo, C.; Lo Giudice, A.; Papale, M.; Vilo, C.; Dong, Q.; Smith, C.T.; Rossello-Mora, R.; et al. Effect of the natural arsenic gradient on the diversity and arsenic resistance of bacterial communities of the sediments of Camarones River (Atacama Desert, Chile). PLoS ONE 2018, 13, e0195080. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kazy, S.K.; Sar, P. Characterization of arsenic resistant bacteria from arsenic rich groundwater of West Bengal, India. Ecotoxicology 2013, 22, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Kazy, S.K.; Banerjee, T.D.; Gupta, A.K.; Pal, T.; Sar, P. Arsenic biotransformation and release by bacteria indigenous to arsenic contaminated groundwater. Bioresour. Technol. 2015, 188, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.; Mallavarapu, M.; Naidu, R. Oxidation of arsenite to arsenate in growth medium and groundwater using a novel arsenite-oxidizing diazotrophic bacterium isolated from soil. Int. Biodeterior. Biodegrad. 2016, 106, 178–182. [Google Scholar] [CrossRef]
- Dey, U.; Chatterjee, S.; Mondal, N. Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol. Rep. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Reddy, G.S.N.; Sengupta, S.; Shivaji, S. Deinococcus indicus sp. nov., an arsenic-resistant bacterium from an aquifer in West Bengal, India. Int. J. Syst. Evol. Microbiol. 2004, 54, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Wu, J.; Cheng, L.; Liu, D.F.; Lau, T.C.; Yu, H.Q. Dependence of arsenic resistance and reduction capacity of Aeromonas hydrophila on carbon substrate. J. Hazard. Mater. 2021, 403, 123611. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Walker, D.J.F.; Vautour, K.E.; Dixon, S.; Holmes, D.E. Arsenic Detoxification by Geobacter Species. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Drewniak, L.; Rajpert, L.; Aleksandra, M.; Sklodowska, A. Dissolution of Arsenic Minerals Mediated by Dissimilatory Arsenate Reducing Bacteria: Estimation of the Physiological Potential for Arsenic Mobilization. BioMed Res. Int. 2014, 841892. [Google Scholar] [CrossRef]
- Nandre, V.; Bachate, S.; Salunkhe, R.; Bagade, A.; Shouche, Y.; Kodam, K. Enhanced Detoxification of Arsenic Under Carbon Starvation: A New Insight into Microbial Arsenic Physiology. Curr. Microbiol. 2017, 74. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.T.; Li, X.; Hu, M.; Li, F.; Young, L.Y.; Sun, W.; Huang, W.; Cui, J. Transcriptional Activity of Arsenic-Reducing Bacteria and Genes Regulated by Lactate and Biochar during Arsenic Transformation in Flooded Paddy Soil. Environ. Sci. Technol. 2017, 52. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, I. Arsenite Oxidation by Bacillus sp. Strain SeaH-As22w Isolated from Coastal Seawater in Yeosu Bay. Environ. Eng. Res. 2010, 15. [Google Scholar] [CrossRef]
- Zeeshanur, R.; Lebin, T.; Ved, P. Study of As-resistant bacteria from Nadia, India and a survey of two As resistance-related proteins. J. Basic Microbiol. 2020, 60, 47–57. [Google Scholar] [CrossRef]
- Lièvremont, D.; N’Negue, M.A.; Behra, P.; Lett, M.C. Biological oxidation of arsenite: Batch reactor experiments in presence of kutnahorite and chabazite. Chemosphere 2003, 51, 419–428. [Google Scholar] [CrossRef]
- Vanden Hoven, R.N.; Santini, J.M. Arsenite oxidation by the heterotroph Hydrogenophaga sp. str. NT-14: The arsenite oxidase and its physiological electron acceptor. Biochim. Biophys. Acta 2004, 1656, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qin, D.; Zhang, S.; Wang, L.; Li, J.; Rensing, C.; McDermott, T.R.; Wang, G. Fate of arsenate following arsenite oxidation in Agrobacterium tumefaciens GW4. Environ. Microbiol. 2015, 17, 1926–1940. [Google Scholar] [CrossRef]
- Wang, Q.; Han, Y.; Shi, K.; Wang, L.; Li, M.; Wang, G. An Oxidoreductase AioE is Responsible for Bacterial Arsenite Oxidation and Resistance. Sci. Rep. 2017, 7, 41536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, W.; Liu, B.; He, J.; Shen, Q.; Zhao, F.-J. Anaerobic Arsenite Oxidation by an Autotrophic Arsenite-Oxidizing Bacterium from an Arsenic-Contaminated Paddy Soil. Environ. Sci. Technol. 2015, 49, 5956–5964. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, V.; Zanchi, R.; Cavalca, L.; Corsini, A.; Romagnoli, C.; Canzi, E. Arsenite Oxidation in Ancylobacter dichloromethanicus As3-1b Strain: Detection of Genes Involved in Arsenite Oxidation and CO2 Fixation. Curr. Microbiol. 2012, 65, 212–218. [Google Scholar] [CrossRef]
- Rauschenbach, I.; Bini, E.; Häggblom, M.M.; Yee, N. Physiological response of Desulfurispirillum indicum S5 to arsenate and nitrate as terminal electron acceptors. FEMS Microbiol. Ecol. 2012, 81, 156–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lerner, A.; Castro-Sowinski, S.; Lerner, H.; Okon, Y.; Burdman, S. Glycogen phosphorylase is involved in stress endurance and biofilm formation in Azospirillum brasilense Sp7. FEMS Microbiol. Lett. 2009, 300, 75–82. [Google Scholar] [CrossRef]
- Gualdi, L.; Tagliabue, L.; Bertagnoli, S.; Ieranò, T.; De Castro, C.; Landini, P. Cellulose modulates biofilm formation by counteracting curli-mediated colonization of solid surfaces in Escherichia coli. Microbiology 2008, 154, 2017–2024. [Google Scholar] [CrossRef]
- Decho, A.W. Microbial biofilms in intertidal systems: An overview. Cont. Shelf Res. 2000, 20, 1257–1273. [Google Scholar] [CrossRef]
- Zeng, X.C.; He, Z.; Chen, X.; Cao, Q.A.D.; Li, H.; Wang, Y. Effects of arsenic on the biofilm formations of arsenite-oxidizing bacteria. Ecotoxicol. Environ. Saf. 2018, 165, 1–10. [Google Scholar] [CrossRef]
- Simeonova, D.D.; Lièvremont, D.; Lagarde, F.; Muller, D.A.; Groudeva, V.I.; Lett, M.C. Microplate screening assay for the detection of arsenite-oxidizing and arsenate-reducing bacteria. FEMS Microbiol. Lett. 2004, 237, 249–253. [Google Scholar] [CrossRef]
- Ito, A.; Miura, J.; Ishikawa, N.; Umita, T. Biological oxidation of arsenite in synthetic groundwater using immobilised bacteria. Water Res. 2012, 46, 4825–4831. [Google Scholar] [CrossRef]
- Wan, J.; Klein, J.; Simon, S.; Joulian, C.; Dictor, M.C.; Deluchat, V.; Dagot, C. AsIII oxidation by Thiomonas arsenivorans in up-flow fixed-bed reactors coupled to As sequestration onto zero-valent iron-coated sand. Water Res. 2010, 44, 5098–5108. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Bhowal, A.; Datta, S. Continuous biosorption in rotating packed-bed contactor. Ind. Eng. Chem. Res. 2008, 47, 4230–4235. [Google Scholar] [CrossRef]
- Panda, M.; Bhowal, A.; Datta, S. Removal of hexavalent chromium by biosorption process in rotating packed bed Environ. Sci. Technol. 2011, 45, 8460–8466. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.A.; Kundu, A.; Mukherjee, S.; Ng, Y.; Mukhopadhyay, S.; Redzwan, G.; Gupta, B.S. Arsenic removal by adsorption on activated carbon in a rotating packed bed. J. Water Process Eng. 2019, 30, 2214–7144. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristóvão, R.O.; Boaventura, R.A.; Vilar, V.J. As(III) and Cr(VI) oxyanion removal from water by advanced oxidation/reduction processes a review. Environ. Sci. Pollut. Res. Int. 2019, 26, 2203–2227. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, A.; Wang, Y.T. Modeling arsenite oxidation by chemoautotrophic Thiomonas arsenivorans strain b6 in a packed-bed bioreactor. Sci. Total. Environ. 2012, 432, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Battaglia-Brunet, F.; Crouzet, C.; Burnol, A.; Coulon, S.; Morin, D.; Joulian, C. Precipitation of arsenic sulphide from acidic water in a fixed-film bioreactor. Water Res. 2012, 46, 3923–3933. [Google Scholar] [CrossRef]
- Michon, J.; Dagot, C.; Deluchat, V.; Dictor, M.C.; Battaglia-Brunet, F.; Baudu, M. As(III) biological oxidation by CAsO1 consortium in fixed-bed reactor. Process Biochem. 2010, 45, 171–178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).