Spiroplasma Infection among Ixodid Ticks Exhibits Species Dependence and Suggests a Vertical Pattern of Transmission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Identification of Tick Species
2.3. DNA Extraction
2.4. Detection of Spiroplasma in Ticks
2.5. Molecular Characterization of Spiroplasma
2.6. Phylogenetic Analysis
2.7. Phylogenetic Analysis
3. Results
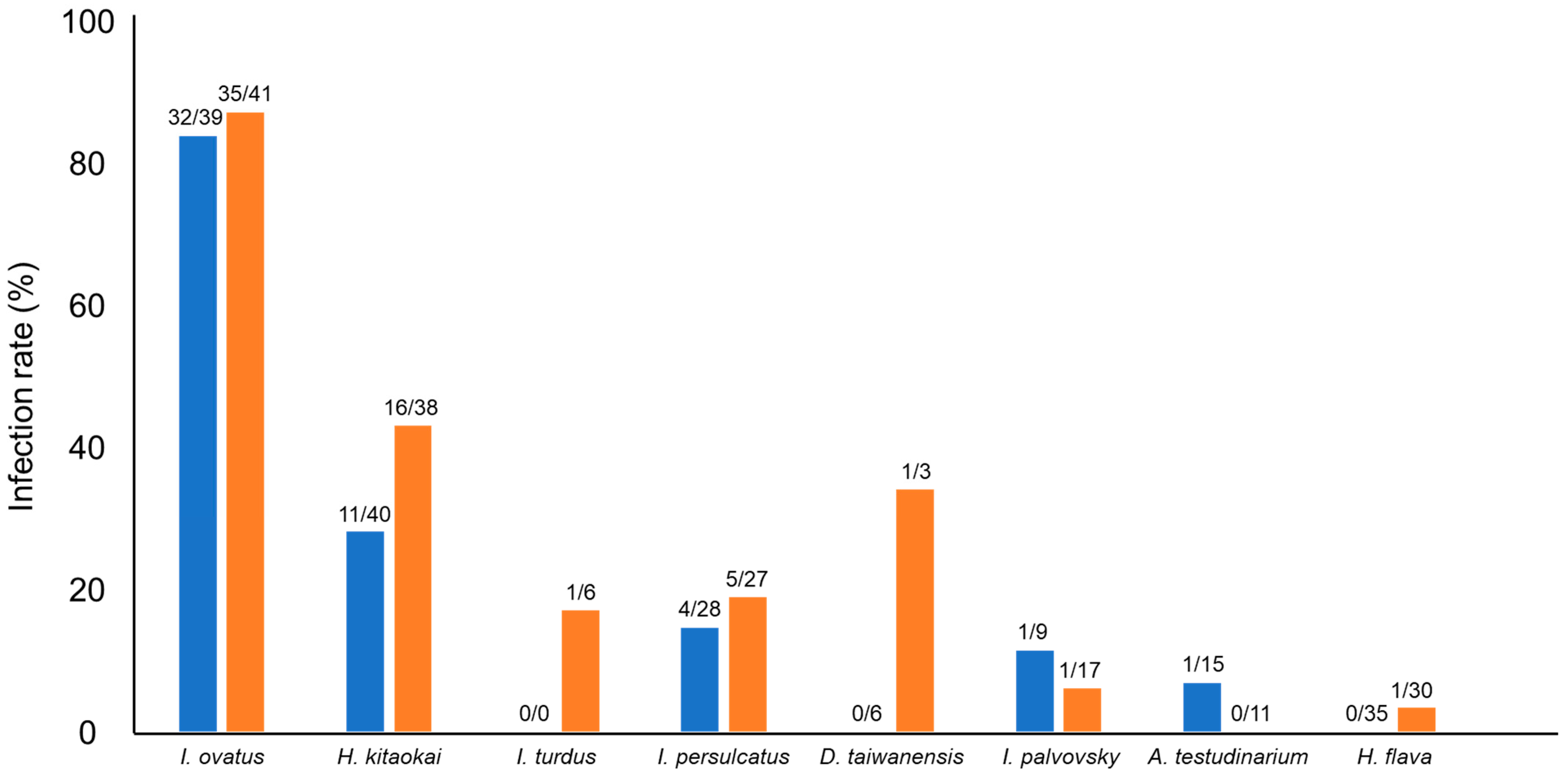
3.1. Infection Rate of Spiroplasma in Different Tick Species
3.2. 16S rDNA Genotyping of Spiroplasma in Ticks
3.3. Characterization of Spiroplasma Based on the Sequences of ITS Region, dnaA, and rpoB Genes
3.4. Effect of the Genetic Background on Spiroplasma Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haselkorn, T.S.; Markow, T.A.; Moran, N.A. Multiple introductions of the Spiroplasma bacterial endosymbiont into Drosophila. Mol. Ecol. 2009, 18, 1294–1305. [Google Scholar] [CrossRef]
- Goryacheva, I.; Blekhman, A.; Andrianov, B.; Romanov, D.; Zakharov, I. Spiroplasma infection in Harmonia axyridis—Diversity and multiple infection. PLoS ONE 2018, 13, e0198190. [Google Scholar] [CrossRef] [PubMed]
- Gasparich, G.E.; Whitcomb, R.F.; Dodge, D.; French, F.E.; Glass, J.; Williamson, D.L. The genus Spiroplasma and its non-helical descendants: Phylogenetic classification, correlation with phenotype and roots of the Mycoplasma mycoides clade. Int. J. Syst. Evol. Microbiol. 2004, 54, 893–918. [Google Scholar] [CrossRef] [Green Version]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, P.T.; Leong, J.S.; Koop, B.F.; Perlman, S.J. Transcriptional responses in a Drosophila defensive symbiosis. Mol. Ecol. 2014, 23, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Whitcomb, R.F.; Chen, T.A.; Williamson, D.L.; Liao, C.; Tully, J.G.; Bové, J.M.; Mouches, C.; Rose, D.L.; Coan, M.E.; Clark, T.B. Spiroplasma kunkelii sp. nov.: Characterization of the Etiological Agent of Corn Stunt Disease. Int. J. Syst. Bacteriol. 1986, 36, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Anbutsu, H.; Fukatsu, T. Tissue-specific infection dynamics of male-killing and nonmale-killing spiroplasmas in Drosophila melanogaster. FEMS Microbiol. Ecol. 2006, 57, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, H.; Solferini, V.N.; Klaczko, L.B.; Hurst, G.D.D. Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol. Biol. 2005, 14, 281–287. [Google Scholar] [CrossRef]
- Jiggins, F.M.; Hurst, G.D.D.; Jiggins, C.D.; Schulenburg, J.H.G.v.d.; Majerus, M.E.N. The butterfly Danaus chrysippus is infected by a male- killing Spiroplasma bacterium. Parasitology 2000, 120, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Majerus, T.M.O.; Graf Von Der Schulenburg, J.H.; Majerus, M.E.N.; Hurst, G.D.D. Molecular identification of a male-killing agent in the ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Insect Mol. Biol. 1999, 8, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Takada, A. Illustrations of Common Adult Ticks in the Mainland Japan. Bull. Hoshizaki Green Found 2015, 18, 287–305. (In Japanese) [Google Scholar]
- Kwak, M.L. A checklist of the ticks (Acari: Argasidae, Ixodidae) of Japan. Exp. Appl. Acarol. 2018, 75, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sanada-Morimura, S.; Matsumura, M.; Noda, H. Male killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus. J. Hered. 2013, 104, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, M.J.; Perlman, S.J. Generality of toxins in defensive symbiosis: Ribosome-inactivating proteins and defense against parasitic wasps in Drosophila. PLoS Pathog. 2017, 13, e1006431. [Google Scholar] [CrossRef] [Green Version]
- Lukasik, P.; Guo, H.; van Asch, M.; Ferrari, J.; Godfray, H.C.J. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 2013, 26, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Vilchez, I.; Mateos, M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 2010, 5, e12149. [Google Scholar] [CrossRef]
- Bastian, F.O.; Elzer, P.H.; Wu, X. Spiroplasma spp. biofilm formation is instrumental for their role in the pathogenesis of plant, insect and animal diseases. Exp. Mol. Pathol. 2012, 93, 116–128. [Google Scholar] [CrossRef]
- Mouches, C.; Bové, J.M.; Albisetti, J.; Clark, T.B.; Tully, J.G. A Spiroplasma of serogroup IV causes a May-disease-like disorder of honeybees in Southwestern France. Microb. Ecol. 1982, 8, 387–399. [Google Scholar] [CrossRef]
- Cockburn, S.N.; Haselkorn, T.S.; Hamilton, P.T.; Landzberg, E.; Jaenike, J.; Perlman, S.J. Dynamics of the continent-wide spread of a Drosophila defensive symbiont. Ecol. Lett. 2013, 16, 609–616. [Google Scholar] [CrossRef]
- Jaenike, J.; Brekke, T.D. Defensive endosymbionts: A cryptic trophic level in community ecology. Ecol. Lett. 2011, 14, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.G.; Whitcomb, R.F.; Rose, D.L.; Bove, J.M. Spiroplasma mirum, a new species from the rabbit tick (Haemaphysalis leporispalustris). Int. J. Syst. Bacteriol. 1982, 32, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Tully, J.G.; Rose, D.L.; Yunker, C.E.; Cory, J.; Whitcomb, R.F.; Williamson, D.L. Helical Mycoplasmas (Spiroplasmas) from Ixodes Ticks. Science 1981, 212, 1043–1045. [Google Scholar] [CrossRef]
- Qiu, Y.; Nakao, R.; Ohnuma, A.; Kawamori, F.; Sugimoto, C. Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLoS ONE 2014, 9, e103961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taroura, S.; Shimada, Y.; SAKATA, Y.; Miyama, T.; Hiraoka, H.; Watanabe, M.; Itamoto, K.; Okuda, M.; Inokuma, H. Detection of DNA of ‘Candidatus Mycoplasma haemominutum’ and Spiroplasma sp. in Unfed Ticks Collected from Vegetation in Japan. J. Vet. Med. Sci. 2006, 67, 1277–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, K.; Greiner-Fischer, S.; Hotzel, H.; Ebsen, M.; Theegarten, D. Isolation of Spiroplasma sp. from an Ixodes tick. Int. J. Med. Microbiol. 2006, 296, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Meli, M.L.; Perreten, A.; Farkas, R.; Willi, B.; Beugnet, F.; Lutz, H.; Hofmann-Lehmann, R. Molecular investigation of hard ticks (Acari: Ixodidae) and fleas (Siphonaptera: Pulicidae) as potential vectors of rickettsial and mycoplasmal agents. Vet. Microbiol. 2010, 140, 98–104. [Google Scholar] [CrossRef] [Green Version]
- van Oosten, A.R.; Duron, O.; Heylen, D.J.A. Ticks and Tick-borne Diseases Sex ratios of the tick Ixodes arboricola are strongly female-biased, but there are no indications of sex-distorting bacteria. Ticks Tick Borne Dis. 2018, 9, 307–313. [Google Scholar] [CrossRef]
- Duron, O.; Binetruy, F.; Noël, V.; Cremaschi, J.; McCoy, K.D.; Arnathau, C.; Plantard, O.; Goolsby, J.; Pérez de León, A.A.; Heylen, D.J.A.; et al. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 2017, 26, 2905–2921. [Google Scholar] [CrossRef] [Green Version]
- Bell-Sakyi, L.; Palomar, A.M.; Kazimirova, M. Isolation and propagation of a Spiroplasma sp. from Slovakian Ixodes ricinus ticks in Ixodes spp. cell lines. Ticks Tick Borne Dis. 2015, 6, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Binetruy, F.; Bailly, X.; Chevillon, C.; Martin, O.Y.; Bernasconi, M.V.; Duron, O. Phylogenetics of the Spiroplasma ixodetis endosymbiont reveals past transfers between ticks and other arthropods. Ticks Tick Borne Dis. 2019, 10, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Aonuma, H.; Kanuka, H. Distribution of tick-borne diseases in Japan: Past patterns and implications for the future. J. Infect. Chemother. 2018, 24, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Thu, M.J.; Qiu, Y.; Kataoka-Nakamura, C.; Sugimoto, C.; Katakura, K.; Isoda, N.; Nakao, R. Isolation of Rickettsia, Rickettsiella, and Spiroplasma from Questing Ticks in Japan Using Arthropod Cells. Vector Borne Zoonotic Dis. 2019, 19, 474–485. [Google Scholar] [CrossRef]
- Yamaguti, N.; Tipton, V.J.; Keegan, H.L.; Toshioka, S. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ. Sci. Bull. Biol. Ser. 1971, 15, 1–226. [Google Scholar]
- Nakao, M.; Miyamoto, K.; Kitaoka, S. A new record of Ixodes pavlovskyi Pomerantzev from Hokkaido, Japan (Acari: Ixodidae). Med. Entomol. Zool. 1992, 43, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Thu, M.J.; Qiu, Y.; Matsuno, K.; Kajihara, M.; Mori-Kajihara, A.; Omori, R.; Monma, N.; Chiba, K.; Seto, J.; Gokuden, M.; et al. Diversity of spotted fever group rickettsiae and their association with host ticks in Japan. Sci. Rep. 2019, 9, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doudoumis, V.; Blow, F.; Saridaki, A.; Augustinos, A.; Dyer, N.A.; Goodhead, I.; Solano, P.; Rayaisse, J.B.; Takac, P.; Mekonnen, S.; et al. Challenging the Wigglesworthia, Sodalis, Wolbachia symbiosis dogma in tsetse flies: Spiroplasma is present in both laboratory and natural populations. Sci. Rep. 2017, 7, 4699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.H.; Singh, K.S.; Gordon, I.J.; Omufwoko, K.S.; Collins, S.; Warren, I.A.; Munby, H.; Brattstrom, O.; Traut, W.; Dino, J.; et al. Whole-chromosome hitchhiking driven by a male-killing endosymbiont. PLoS Biol. 2020, 18, e3000610. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE: Thousando Oaks, CA, USA, 2019. [Google Scholar]
- Schneider, D.I.; Saarman, N.; Onyango, M.G.; Hyseni, C.; Opiro, R.; Echodu, R.; O’Neill, M.; Bloch, D.; Vigneron, A.; Johnson, T.J.; et al. Spatio-temporal distribution of Spiroplasma infections in the tsetse fly (Glossina fuscipes fuscipes) in northern Uganda. PLoS Negl. Trop. Dis. 2019, 13, e0007340. [Google Scholar] [CrossRef] [Green Version]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 82599. [Google Scholar] [CrossRef]
- Klubal, R.; Kopecky, J.; Nesvorna, M.; Sparagano, O.A.; Thomayerova, J.; Hubert, J. Prevalence of pathogenic bacteria in Ixodes ricinus ticks in Central Bohemia. Exp. Appl. Acarol. 2016, 68, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Watts, T.; Haselkorn, T.S.; Moran, N.A.; Markow, T.A. Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS ONE 2009, 4, e5703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.J.; Mihaljevic, J.R.; Des Marteaux, L.; Hrček, J. Metacommunity theory for transmission of heritable symbionts within insect communities. Ecol. Evol. 2019, 10, 1703–1721. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Sekeyova, Z.; Raoult, D.; Mediannikov, O. Multiple tick-associated bacteria in Ixodes ricinus from Slovakia. Ticks Tick Borne Dis. 2012, 3, 406–410. [Google Scholar] [CrossRef]
- Clark, H.F. The suckling mouse cataract agent (SMCA). A slow mycoplasma-like agent. Prog. Med. Virol. 1974, 18, 307–322. [Google Scholar]
- Bastian, F.O.; Purnell, D.M.; Tully, J.G. Neuropathology of Spiroplasma infection in the rat brain. Am. J. Pathol. 1984, 114, 496–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tully, J.G.; Bastian, F.O.; Rose, D.L. Localization and persistence of spiroplasmas in an experimental brain infection in suckling rats. Ann. Microbiol. 1984, 135, 111–117. [Google Scholar] [CrossRef]
- Bastian, F.O.; Sanders, D.E.; Forbes, W.A.; Hagius, S.D.; Walker, J.V.; Henk, W.G.; Enright, F.M.; Elzer, P.H. Spiroplasma spp. from transmissible spongiform encephalopathy brains or ticks induce spongiform encephalopathy in ruminants. J. Med. Microbiol. 2007, 56, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isogai, E.; Isogai, H.; Masuzawa, T.; Postic, D.; Baranton, G.; Kamewaka, Y.; Kimura, K.; Nishikawa, T.; Fuji, N.; Ishii, N.; et al. Borrelia burgdorferi sensu lato in an endemic environment: Wild sika deer (Cervus nippon yesoensis) with infected ticks and antibodies. Microbiol. Immunol. 1996, 40, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Nakao, R.; Shinjo, K.; Sakiyama, T.; Ogata, S.; Kusakisako, K.; Kinoshita, G.; Naguib, D.; Chatanga, E.; Mohamed, W.M.A.; Moustafa, M.A.M.; et al. Amblyomma testudinarium infestation on a brown bear (Ursus arctos yesoensis) captured in Hokkaido, a northern island of Japan. Parasitol. Int. 2021, 80, 102209. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Yamagishi, T.; Shimada, T.; Matsui, T.; Shimojima, M.; Saijo, M.; Oishi, K. Epidemiological and clinical features of severe fever with thrombocytopenia syndrome in Japan, 2013-2014. PLoS ONE 2016, 11, e0165207. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Toyomane, K.; Konnai, S.; Ohashi, K.; Nakao, M.; Ito, T.; Andoh, M.; Maeda, K.; Watarai, M.; Sato, K.; et al. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS ONE 2014, 9, e104532. [Google Scholar] [CrossRef]
- Mathé-Hubert, H.; Kaech, H.; Ganesanandamoorthy, P.; Vorburger, C. Evolutionary costs and benefits of infection with diverse strains of Spiroplasma in pea aphids. Evolution 2019, 73, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Harumoto, T.; Fukatsu, T.; Lemaitre, B. Common and unique strategies of male killing evolved in two distinct Drosophila symbionts. Proc. Biol. Sci. 2018, 285, 20172167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, P.G. Spiroplasmas in Leafhoppers: A review. Yale J. Biol. Med. 1983, 56, 745–751. [Google Scholar]
- Killiny, N.; Batailler, B.; Foissac, X.; Saillard, C. Identification of a Spiroplasma citri hydrophilic protein associated with insect transmissibility. Microbiology 2006, 152, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.X.; Song, Y.L.; Huang, H.J.; Zhao, D.S.; Xia, X.; Yang, K.; Lu, Y.J.; Hong, X.Y. Comparative analyses of salivary proteins from the facultative symbiont-infected and uninfected Tetranychus truncatus. Syst. Appl. Acarol. 2018, 23, 1027–1042. [Google Scholar] [CrossRef]
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef]



| Primer | Sequence (5’-3’) | Target Gene | Annealing Temperature (°C) | Purpose | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|---|
| spi_f1 | GGGTGAGTAACACGTATCT | 16S rDNA | 60 | PCR | 1028 | [13] |
| spi_r3 | CCTTCCTCTAGCTTACACTA | |||||
| 16S_s1 | ACCTTACCAGAAAGCCACGG | 16S rDNA | NA | Sequencing | NA | This study |
| 16S_s2 | AGACCTTCATCAGTCACGCG | 16S rDNA | NA | Sequencing | NA | This study |
| 16S_s3 | GTAATATGTGCCAGCAGCCG | 16S rDNA | NA | Sequencing | NA | This study |
| 16S_s4 | ACCGCATTCTCCATCAGCTT | 16S rDNA | NA | Sequencing | NA | This study |
| SP-ITS-JO4 | GCCAGAAGTCAGTGTCCTAACCG | ITS1 | 56 | PCR | 301 | [13] |
| SP-ITS-N55 | ATTCCAAGCCATCCACCATACG | |||||
| SRdnaAF1 | GGAGAYTCTGGAYTAGGAAA | dnaA | 52 | PCR | 515 | [36] |
| SRdnaAR1 | CCYTCTAWYTTTCTRACATCA | |||||
| RpoBF1 | ATGGATCAAACAAATCCATTAGCAGA | rpoB | 60 | PCR | 1703 | [36] |
| RpoBR2 | GCATGTAATTTATCATCAACCATGTGTG | |||||
| RpoB_s1 | TGACCATTACTACGAGCAATAACA | rpoB | NA | Sequencing | NA | This study |
| RpoB_s2 | CCCCTGTTTTTGATGGTGCA | rpoB | NA | Sequencing | NA | This study |
| 16S rDNA Allele | Tick Species | No. of Positive/No. of Tested (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hokkaido | Tohoku | Kanto | Chubu | Kinki | Chugoku | Sikoku | Kyushu | Okinawa | ||
| G1 | I. ovatus | 21/44 (48) | 2/32 (6) | - | 0/4 (0) | - | - | - | - | - |
| G1 | I. persulcatus | 1/39 (3) | 0/8 (0) | - | 0/4 (0) | 0/4 (0) | - | - | - | - |
| G2 | H. kitaokai | - | 0/5 (0) | - | - | 2/12 (17) | - | 0/36 (0) | 0/45 (0) | - |
| G2 | I. ovatus | 1/44 (2) | 0/32 (0) | - | 0/4 (0) | - | - | - | - | - |
| G2 | I. persulcatus | 3/39 (7) | 0/8 (0) | - | 0/4 (0) | 0/4 (0) | - | - | - | - |
| G3 | I. ovatus | 3/44 (7) | 1/32 (3) | - | 3/4 (75) | - | - | - | - | - |
| G4 | I. ovatus | 1/44 (2) | 0/32 (0) | - | 0/4 (0) | - | - | - | - | - |
| G5 | I. ovatus | 1/44 (2) | 0/32 (0) | - | 0/4 (0) | - | - | - | - | - |
| G6 | I. ovatus | 3/44 (7) | 1/32 (3) | - | 1/4 (25) | - | - | - | - | - |
| G7 | I. ovatus | 1/44 (2) | 1/32 (3) | - | 0/4 (0) | - | - | - | - | - |
| G8 | I. ovatus | 1/44 (2) | 0/32 (0) | - | 0/4 (0) | - | - | - | - | - |
| G9 | D. taiwanensis | - | 0/1 (0) | - | ˗ | 1/4 (25) | - | - | 0/4 (0) | - |
| G9 | H. kitaokai | ˗ | 0/5 (0) | - | ˗ | 3/12 (25) | - | 2/36 (6) | 18/45 (40) | - |
| G9 | I. persulcatus | 0/39 (0) | 0/8 (0) | - | 0/4 (0) | 4/4 (100) | - | - | - | - |
| G9 | I. turdus | - | - | - | - | 0/2 (0) | - | 0/2 (0) | 1/2 (50) | - |
| G10 | A. testudinarium | - | ˗ | - | ˗ | - | 1/9 (11) | 0/15 (0) | 0/2 (0) | - |
| G10 | I. persulcatus | 0/39 (0) | 1/8 (13) | - | 0/4 (0) | 0/4 (0) | - | - | - | - |
| G11 | I. ovatus | 0/44 (0) | 16/32 (50) | - | 0/4 (0) | - | - | - | - | - |
| G12 | I. ovatus | 0/44 (0) | 2/32 (6) | - | 0/4 (0) | - | - | - | - | - |
| G13 | I. pavlovsky | 1/26 (4) | - | - | - | - | - | - | - | - |
| G14 | H. kitaokai | - | 0/5 (0) | - | - | 0/12 (0) | - | 0/36 (0) | 1/45 (2) | - |
| G15 | H. kitaokai | - | 1/5 (20) | - | - | 0/12 (0) | - | 0/36 (0) | 0/45 (0) | - |
| G16 | I. ovatus | 0/44 (0) | 1/32 (3) | - | 0/4 (0) | - | - | - | - | - |
| G17 | I. pavlovsky | 1/26 (4) | - | - | - | - | - | - | - | - |
| Spiroplasma Haplotype | 16S rDNA | ITS | dnaA | rpoB | Tick Species |
|---|---|---|---|---|---|
| SP1 | G1 | T3 | A1 | B1 | I. ovatus |
| SP2 | G1 | T1 | - | - | I. persulcatus |
| SP3 | G2 | T1 | A1 | B4 | H. kitaokai |
| SP4 | G2 | T1 | A1 | - | H. kitaokai |
| SP5 | G2 | T2 | - | - | I. ovatus |
| SP6 | G2 | T1 | A2 | B1 | I. persulcatus |
| SP7 | G2 | T1 | A2 | B7 | I. persulcatus |
| SP8 | G2 | T1 | - | - | I. persulcatus |
| SP9 | G3 | T2 | - | - | I. ovatus |
| SP10 | G4 | T1 | A2 | B3 | I. ovatus |
| SP11 | G5 | T3 | A2 | B3 | I. ovatus |
| SP12 | G6 | T2 | - | - | I. ovatus |
| SP13 | G7 | T2 | A1 | - | I. ovatus |
| SP14 | G8 | T1 | A2 | B3 | I. ovatus |
| SP15 | G9 | T1 | A2 | B2 | D. taiwanensis |
| SP16 | G9 | T1 | A2 | B4 | H. kitaokai |
| SP17 | G9 | T1 | A2 | B7 | H. kitaokai |
| SP18 | G9 | - | A2 | B7 | H. kitaokai |
| SP19 | G9 | T1 | - | - | I. persulcatus |
| SP20 | G9 | T1 | A1 | - | I. persulcatus |
| SP21 | G9 | T1 | A1 | B7 | I. persulcatus |
| SP22 | G9 | T5 | - | B6 | I. persulcatus |
| SP23 | G9 | T1 | - | B5 | I. turdus |
| SP24 | G10 | T1 | - | - | A. testudinarium |
| G10 | T1 | - | - | I. persulcatus | |
| SP25 | G11 | T2 | - | - | I. ovatus |
| SP26 | G12 | T1 | - | - | I. ovatus |
| SP27 | G13 | T1 | - | - | I. pavlovsky |
| SP28 | G14 | T1 | - | - | H. kitaokai |
| SP29 | G15 | T1 | - | - | H. kitaokai |
| SP30 | G16 | T2 | - | - | I. ovatus |
| SP31 | G17 | T4 | - | - | I. pavlovsky |
| Model | Predictor Variable | Random Variable | AIC | BIC | logLik | Dev | Chisq | Df | Pr (>Chisq) | |
|---|---|---|---|---|---|---|---|---|---|---|
| M1-1 | Species | No | 99.33 | 195.26 | −28.67 | 57.33 | NA | NA | NA | |
| M1-2 | Species | District | 74.43 | 174.93 | −15.22 | 30.43 | 26.90 | 1 | 2.14 × 10−7 | *** |
| M2-1 | Year | No | 467.30 | 481.00 | −230.65 | 461.30 | NA | NA | NA | |
| M2-2 | Year | District | 459.39 | 477.67 | −225.70 | 451.39 | 9.90 | 1 | 0.00164998 | *** |
| M3-1 | Sex | No | 495.23 | 513.50 | −243.61 | 487.23 | NA | NA | NA | |
| M3-2 | Sex | District | 451.24 | 474.08 | −220.62 | 441.24 | 45.99 | 1 | 1.19 × 10−11 | *** |
| M4-1 | Season | No | 538.56 | 556.83 | −265.28 | 530.56 | NA | NA | NA | |
| M4-2 | Season | District | 465.98 | 488.82 | −227.99 | 455.98 | 74.58 | 1 | 5.83 × 10−18 | *** |
| Model | Predictor Variable | Random Variable | AIC | BIC | LogLik | Deviance | Chisq | Df | Pr (>Chisq) | |
|---|---|---|---|---|---|---|---|---|---|---|
| M5 | NO | District | 464.22 | 477.92 | −229.11 | 458.22 | NA | NA | NA | |
| M7 | Year | District | 459.39 | 477.67 | −225.70 | 451.39 | 6.82 | 1 | 0.00899482 | ** |
| M8 | Season | District | 451.24 | 474.08 | −220.62 | 441.24 | 10.16 | 1 | 0.00143586 | ** |
| M9 | Sex | District | 465.98 | 488.82 | −227.99 | 455.98 | 0.00 | 0 | NA | |
| M6 | Species | District | 74.43 | 174.93 | −15.22 | 30.43 | 425.55 | 17 | 8.34 × 10−80 | *** |
| M10 | Season + Species | District | 71.83 | 181.47 | −11.92 | 23.83 | 6.60 | 2 | 0.03694614 | * |
| M11 | Species + Season | District | 71.83 | 181.47 | −11.92 | 23.83 | 0.00 | 0 | NA | |
| M12 | Species + Season + Sex | District | 69.87 | 188.64 | −8.93 | 17.87 | 5.97 | 2 | 0.05065574 | . |
| 16S rDNA Allele | Tick Species (No. of Positive Samples) | Significance |
|---|---|---|
| G1 | I. ovatus (n = 23), I. persulcatus (n = 1) | I. ovatus |
| G2 | H. kitaokai (n = 2), I. ovatus (n = 1), I. persulcatus (n = 3) | Not significant |
| G3 | I. ovatus (n = 7) | Not significant |
| G4 | I. ovatus (n = 1) | NA |
| G5 | I. ovatus (n = 1) | NA |
| G6 | I. ovatus (n = 5) | Not significant |
| G7 | I. ovatus (n = 2) | NA |
| G8 | I. ovatus (n = 1) | NA |
| G9 | D. taiwanensis (n = 1), H. kitaokai (n = 23), I. turdus (n = 1), I. persulcatus (n = 4) | H. kitaokai |
| G10 | A. testudinarium (n = 1), I. persulcatus (n = 1) | NA |
| G11 | I. ovatus (n = 16) | I. ovatus |
| G12 | I. ovatus (n = 2) | NA |
| G13 | I. pavlovsky (n = 1) | NA |
| G14 | H. kitaokai (n = 1) | NA |
| G15 | H. kitaokai (n = 1) | NA |
| G16 | I. ovatus (n = 1) | NA |
| G17 | I. pavlovsky (n = 1) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, S.; Mohamed, W.M.A.; Kusakisako, K.; Thu, M.J.; Qiu, Y.; Moustafa, M.A.M.; Matsuno, K.; Katakura, K.; Nonaka, N.; Nakao, R. Spiroplasma Infection among Ixodid Ticks Exhibits Species Dependence and Suggests a Vertical Pattern of Transmission. Microorganisms 2021, 9, 333. https://doi.org/10.3390/microorganisms9020333
Ogata S, Mohamed WMA, Kusakisako K, Thu MJ, Qiu Y, Moustafa MAM, Matsuno K, Katakura K, Nonaka N, Nakao R. Spiroplasma Infection among Ixodid Ticks Exhibits Species Dependence and Suggests a Vertical Pattern of Transmission. Microorganisms. 2021; 9(2):333. https://doi.org/10.3390/microorganisms9020333
Chicago/Turabian StyleOgata, Shohei, Wessam Mohamed Ahmed Mohamed, Kodai Kusakisako, May June Thu, Yongjin Qiu, Mohamed Abdallah Mohamed Moustafa, Keita Matsuno, Ken Katakura, Nariaki Nonaka, and Ryo Nakao. 2021. "Spiroplasma Infection among Ixodid Ticks Exhibits Species Dependence and Suggests a Vertical Pattern of Transmission" Microorganisms 9, no. 2: 333. https://doi.org/10.3390/microorganisms9020333
APA StyleOgata, S., Mohamed, W. M. A., Kusakisako, K., Thu, M. J., Qiu, Y., Moustafa, M. A. M., Matsuno, K., Katakura, K., Nonaka, N., & Nakao, R. (2021). Spiroplasma Infection among Ixodid Ticks Exhibits Species Dependence and Suggests a Vertical Pattern of Transmission. Microorganisms, 9(2), 333. https://doi.org/10.3390/microorganisms9020333






