Abstract
Dickeya and Pectobacterium spp. both cause blackleg and soft rot of potato, which can be a yield-reducing factor to potato production. The purpose of this study was to examine the interaction between these two bacterial genera causing potato infection, and subsequent disease development and yield responses under field conditions. Analysis of 883 potato samples collected in Northeastern USA using polymerase chain reaction determined that Dickeya dianthicola and P. parmentieri were found in 38.1% and 53.3% of all samples, respectively, and that 20.6% of samples contained both D. dianthicola and P. parmentieri. To further investigate the relationship between the two bacterial species and their interaction, field trials were established. Potato seed pieces of “Russet Burbank”, “Lamoka”, and “Atlantic” were inoculated with bacterial suspension of D. dianthicola at 107 colony-forming unite (CFU)/mL using a vacuum infiltration method, air dried, and then planted in the field. Two-year results showed that there was a high correlation (p < 0.01) between yield loss and percent of inoculated seed pieces. In a secondary field trial conducted in 2018 and 2019, seed pieces of potato “Shepody”, “Lamoka” and “Atlantic” were inoculated with D. dianthicola, P. parmentieri, or mixture of both species, and then planted. In 2019, disease severity index, as measured by the most sensitive variety “Lamoka”, was 16.2 with D. dianthicola inoculation, 10.4 with P. parmentieri, 25.4 with inoculation with both bacteria. Two-year data had a similar trend. Thus, D. dianthicola was more virulent than P. parmentieri, but the co-inoculation of the two species resulted in increased disease severity compared to single-species inoculation with either pathogen.
1. Introduction
Blackleg and soft rot (BSR) of potato (Solanum tuberosum) is caused by many bacterial species in the genera Dickeya and Pectobacterium. The predominant species of bacteria responsible for the disease varies depending on geographical locations. For example, Pectobacterium atrosepticum was dominant in Europe before 1970, but Dickeya dianthicola followed by D. solani, have become dominant in some European countries in recent decades [1,2]. An outbreak of BSR in Northeastern USA was caused by D. dianthicola in 2015 and the following years, while D. chrysanthemi was found in central USA, and Pectobacterium spp. was found to be widespread in the USA [3,4,5,6,7,8,9]. Symptoms of BSR were expressed as rot and blackened stems (blackleg), wilting plants and decayed tubers (soft rot), which have threatened potato production, resulting in millions of dollars in losses for the potato industry [3].
BSR caused by a pathogen complex adds an additional layer of difficulty in the development of effective control measures. One plant may be infected by more than one pathogen [10,11]. Such a complex disease structure is determined based on the interactions between pathogen species, host plant, biotic and abiotic environmental factors [10,12,13,14,15].
It is now generally believed that D. dianthicola is primarily a seed-borne pathogen, and most Pectobacterium spp. are soil inhabitants but can be seed-borne as well. Therefore, stored tubers are the most important source in harboring bacterial pathogens and initiating disease. Pathogens surviving in soil can colonize roots, then penetrate and move into the xylem [16]. Some studies indicate that P. parmentieri is less virulent than P. carotovorum, owing to the lack of Type III secretion system (T3SS) [17,18]. However, we have demonstrated that P. parmentieri isolates are highly pathogenic on both the tubers and stems of potato [19].
In the USA outbreak of BSR in 2015 and the following years, Dickeya dianthicola was determined to be the predominant pathogen involved, based on its primary and predominant detection and isolation from field samples. However, the detection frequency of this species progressively declined in the following years. In contrast, some Pectobacterium spp. were found on postharvest tubers and later identified in symptomatic potato plants in increasing amounts in subsequent years. Common species of BSR causal agents included P. parmentieri, P. polaris, P. carotovorum subsp. carotovorum, P. c. subsp. oderiferium, and other Pectobacterium spp. [5,7,19,20]. The above species were classified as one species, Erwinia carotovorum, before 1981, which were further differentiated into several subspecies soon afterwards [21,22,23]. In addition, P. parmentieri extended its distribution beyond the Northeastern USA, causing blackleg and soft rot of potato [24].
Whether the co-existence of Dickeya and Pectobacterium spp. has advantages for pathogen infection is a key subject that impacts both biological study and disease management. Our questions were: Is the predominance of bacterial species influenced by environmental factors or bacterial virulence? Is there an enhanced response when both species co-occur in the same infected plant tissue? The aims of this study were to understand how bacterial infection affected potato growth and the corresponding yield response. Specifically, we wanted to determine how multiple species of pathogens interact, and how they affect disease development and yield response.
2. Materials and Methods
2.1. Frequency and Distribution of Dickeya dianthicola and Pectobacterium parmentieri in Naturally Infected Potato
From 2015 to 2020, symptomatic potato stems and tubers showing BSR were collected from fields in most of the Northeastern state by growers, consultants, or vegetable pathologists. All samples were processed and assayed at the University of Maine. Segments of diseased stem tissue or tuber peel were obtained from the samples. Bacteria were extracted from the tissue sap by incubating them in sterile distilled water [25]. Genomic DNA was extracted from the stem- or tuber-derived sap using the FastDNA® SPIN Kit (MP Biomedical, Santa Ana, CA, USA). Concentration of DNA was estimated using NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific Inc, Wilmington, DE, USA), and diluted to 10 ng/µL for polymerase chain reaction (PCR). A total of 883 DNA samples were selected and amplified by PCR with primer pairs of PW7011 specific for P. parmentieri, and DIA-A specific for D. dianthicola [26,27]. PCR amplifications were performed in a 25 µL total volume reaction system containing 17.9 µL of sterile water, 5 µL of 5× PCR buffer, with MgCl2 at a final concentration of 0.25 mM dNTPs, 0.1 µM of each pair of primers, 0.5 U GoTaq DNA Polymerase and 1 µL of DNA. All PCR reagents were from Promega Corporation (Madison, MI, USA). The thermal cycler for primer DIA-A was programmed for an initial denaturation of 5 min at 94 °C, followed by 35 cycles of 1 min at 94 °C, 30 s at 53 °C and 1 min at 72 °C, with a final elongation of 10 min at 72 °C, while the annealing temperature of primer PW7011 was set at 67 °C for 30 s.
2.2. Tuber Inoculation Using Vacuum Infiltration
Dickeya dianthicola strain ME30 and P. parmentieri strain ME175 were cultured on crystal violate pectate (CVP) plates at 28 °C for 48 h. The culture was transferred to tryptic soy broth (TSB) and incubated on a shaker overnight at 28 °C and 120 rpm. The chamber was disinfected with 10% bleach between treatments. At plant inoculation, the prepared bacterial culture was diluted with non-autoclaved tap water and adjusted to 107 CFU/mL as working inoculum. Well-suberized seed pieces were washed and submerged into the bacteria suspension in a vacuum chamber (BVV10GL, BEST VALUE VACS, Naperville, IL, USA) and tightly sealed. The chamber held between −60 and −80 kPa for 15 min [28,29]. A control was set up by treating seed pieces with tap water (pathogen free) as “non-inoculated” through the same procedure in the vacuum chamber. The treated tubers were air dried and planted one week after inoculation.
2.3. Effects of Bacterial Inoculation on Potato Growth
Field studies were conducted in 2019 and 2020 at Presque Isle, Maine. A randomized complete random block design (RCBD) was used with four replicate blocks. Potato varieties “Lamoka”, “Atlantic”, and “Russet Burbank” were inoculated with D. dianthicola strain ME30 using the vacuum infiltration method as described above. For each variety, 50 tubers were planted on 23 May 2019 and 27 May 2020, and each treatment contained a mixture of bacteria-inoculated and bacteria-free tubers at different percentages, including 0, 10%, 20%, 40% and 80%, which were arranged in RCBD. Fertilizer (N:P:K = 14:14:14) was applied at time of planting at a rate of 12.3 kg/a. Nuprid 2SC (a.i. 21.4% imidacloprid) was also applied at time of planting at 14.6 mL/a to control insects. Plant stand was evaluated on 1 July 2019 and 13 July 2020. Plants were visually inspected for the development of BSR symptoms. Disease severity was evaluated weekly or every other week after symptoms appeared and rated from 0 to 5, with 0 being healthy and 5 being dead plants. Potato vines were killed with two applications of Reglone at 17.5 mL/a. Potatoes were harvested on 5 September 2019, and 2 October 2020. Total yield was measured.
2.4. Interaction between D. dianthicola and P. parmentieri in Potato Infection
Field studies were performed in 2018 and repeated in 2019 in Presque Isle, Maine. Dickeya dianthicola strain ME30 and P. parmentieri strain ME175 were used. Seed pieces of potato “Lamoka”, “Atlantic”, and “Shepody” were inoculated with either ME30, ME175, or mixture of both bacterial species using the vacuum infiltration method as described above. For the co-inoculation, cell suspension of strains ME30 and ME175 were mixed together at a 1:1 ratio with the final concentration of 107 CFU/mL, which was then used for vacuum infiltration. The treated tubers were air dried for at least one week before planting. A randomized complete block design (RCBD) was applied with four replications in the field. Thirty seed pieces were planted in each plot on 25 May 2018 and 28 May 2019. Field management was as described in the above trial. Emergence was assessed as the number of emerged plants per plot on 2 July 2018 and 1 July 2019. Plants were visually inspected for the development of blackleg symptoms with 0 to 5 disease severity scale, as described above. Disease incidence was calculated as DSD = number of symptomatic stems/total number of stems. Disease severity index (DSI) was calculated as DSI = (sum (disease scale frequency × score of disease scale))/((total number of stems) × (maximal disease severity index)) × 100. Potatoes were harvested on 5 September 2018 and 19 September 2019. Total yield was measured.
2.5. Statistical Analysis
Statistical analysis was conducted using the SAS statistical program (SAS university edition, Red Hat (64-bit) version, SAS Institute Inc., Cary, NC, USA). Categorical variables were analyzed using chi-square test. Numerical variables, including emergence, disease incidence, disease severity index and yield were analyzed using the ANOVA procedure with Tukey’s multiple range test at a significance level α = 0.05. Emergence loss and yield loss (Y) were regressed against percentage of seed infection (X) using linear regression equation Y = b0 + b1X. Emergence loss was expressed as (noninfected plot stand count − infected plot stand count)/noninfected plot stand count. Yield loss = (yield in noninfected plot − yield in infected plot/yield in noninfected plot).
3. Results
3.1. Frequency and Distribution of Dickeya dianthicola and Pectobacterium parmentieri in Naturally Infected Potato
Potato samples collected from fields were assayed using PCR to detect both Dickeya and Pectobacterium spp. Out of the 883 samples, 761 were from potato stems, and 122 were from potato tubers. Primers DIA-A, specific to D. dianthicola, and PW7011 specific to P. parmentieri were used in the detection. Through the six years, 38.1% of samples contained D. dianthicola and 53.3% of samples contained P. parmentieri. Furthermore, 20.6% of samples contained both D. dianthicola and P. parmentieri, and 29.2% of samples contained neither D. dianthicola nor P, parmentieri (Table 1). Chi-square test showed that the percentage of D. dianthicola in stems (40.3%) was significantly (p < 0.001) higher than in tubers (23.8%), whereas the percentage of P. parmentieri positive on stems (51.8%) was significantly (p < 0.01) lower than the one on tubers (63.1%) (Table 2).

Table 1.
Detection of Dickeya and Pectobacterium spp. in symptomatic potato stems or tubers using polymerase chain reaction (PCR).

Table 2.
Chi-square test on tissue distribution of Dickeya dianthicola (DDi) and Pectobacterium parmentieri (PPa) on potato tissues showing blackleg and soft rot symptoms.
3.2. Effects of Bacterial Inoculation on Potato Growth
An extremely dry growing season in 2020 resulted in low emergence, with the non-inoculated treatment having an emergence rate of 33%, 56% and 67% for “Lamoka”, “Atlantic”, and “Russet Burbank”, respectively (Table 3). Potato varieties showed different responses in yield and emergence to bacterial inoculation (Table 3). The responses were evaluated with regression models. Emergence loss and inoculation had a linear relationship with correlation coefficients of 0.66 on “Lamoka”, 0.79 on “Atlantic” and 0.60 on “Russet Burbank”, while yield loss and inoculation on “Lamoka” and “Atlantic” showed linear relationships with correlation coefficients of 0.66 and 0.74, respectively (Figure 1. The level of disease susceptibility was estimated by the disease incidence and the slope of the regression equation between emergence loss and inoculation. From high to low, disease incidence in 2019 was observed on “Lamoka” (31.7%), “Atlantic” (4.4%), and “Russet Burbank” (0) (Figure 2). Slope of the regression equation between emergence loss and inoculation was “Lamoka” (0.687), “Atlantic” (0.475), and “Russet Burbank” (0.332) (Figure 1). “Lamoka” was the most susceptible variety, with 100% inoculated seed resulted in 68.7% emergence loss and a corresponding 68.1% yield loss (Figure 1). For the tolerant variety “Russet Burbank”, yield was not significantly affected by bacterial inoculation because emergence was not significantly reduced (Figure 1).

Table 3.
Effects of infection level of seed pieces with Dickeya dianthicola on yield and emergence of potato in 2020.
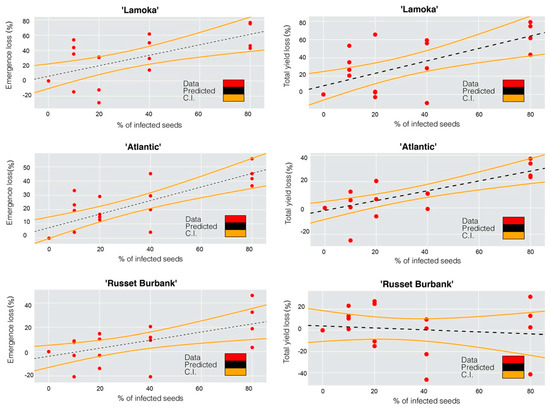
Figure 1.
Correlation between (1) (left panels) emergence loss and infection percent of potato “Lamoka” (r = 0.66, p = 0.0017), “Atlantic” (r = 0.79, p < 0.0001), “Russet Burbank” (r = 0.60, p = 0.0056) seed pieces inoculated with Dickeya dianthicola; (2) (right panels) yield loss and infection percent of potato “Lamoka” (r = 0.66, p = 0.0014), “Atlantic” (r = 0.74, p = 0.0002), “Russet Burbank” (r = 0.13, p = 0.5850) seed pieces inoculated with Dickeya dianthicola. Predicted data and confident intervals (CI) were shown as the dash lines and orange curves, respectively, 2020.
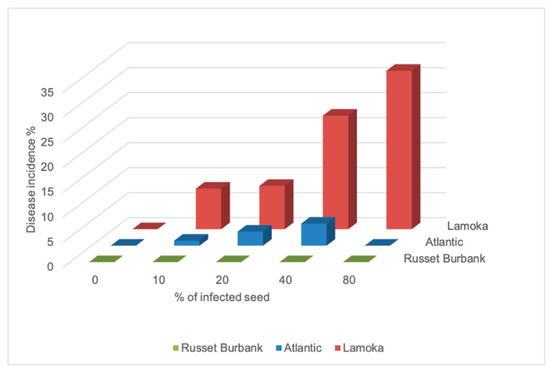
Figure 2.
Disease incidence affected by infection of potato “Lamoka”, “Russet Burbank”, and “Atlantic” seed pieces inoculated with Dickeya dianthicola in 2019.
3.3. Interaction between D. dianthicola and P. parmentieri in Potato Infection
Three potato varieties had different responses when inoculated with either D. dianthicola P. parmentieri or mixture of both bacterial species, with “Lamoka” being the most susceptible variety, “Atlantic” and “Shepody” being the less susceptible varieties. This result was consistent in both years. For example, in 2019, disease symptoms with inoculation of the bacterial mixture appeared on “Lamoka” at 49 days post planting with the disease severity index of 5.7, while the disease severity indexes of “Atlantic” and “Shepody” at 49 days post planting were 0.75 and 1.6, respectively (Figure 3). At the end of the growing season, the disease severity index of “Lamoka” was 25.4, while “Atlantic” and “Shepody” showed a relatively lower disease severity index of 7.0 and 7.1, respectively (Figure 3).

Figure 3.
Disease progress of blackleg in potato “Lamoka”, “Atlantic” and “Shepody” grown from seed pieces inoculated with either Dickeya dianthicola (DDi), Pectobacterium parmentieri (PPa), or DDi + PPa under field conditions in 2018 (upper panels) and 2019 (bottom panels). Non-inoculated (NI) plants were used for control. Disease incidence or disease severity index was analyzed using the ANOVA procedure with Tukey’s multiple range test at a significance level α = 0.05. Mean values at same day point with different letters were significantly different (p < 0.05).
Dickeya dianthicola was more aggressive than P. parmentieri in the field. For example, in the season of 2019 and on the most susceptible variety “Lamoka”, symptoms were first observed on potato plants inoculation with D. dianthicola than P. parmentieri. Disease severity index was 6.7 by D. dianthicola inoculation but 0.7 by P. parmentieri inoculation (Figure 3). Furthermore, by the end of the season, the disease severity index of “Lamoka” caused by D. dianthicola was 16.2 higher than that caused by P. parmentieri (10.4) (Figure 3). However, the mixture of both bacteria showed the highest disease level, with disease severity index being 25.4 (Figure 3). Thus, the mixture of two bacterial species was aggravated in plant infection compared to either of the two species. The responses of “Shepody” and “Atlantic” were similar to “Lamoka” with infection by either a single or a mixture of bacterial species, although the difference among treatments was not significant (p > 0.05), because both varieties were less susceptible to blackleg (Figure 3). At early growing stage of potato, a higher disease incidence was observed with D. dianthicola inoculation compared to the treatment with P. parmentieri inoculation. Although inoculation with the mixture of bacterial species initially showed disease level slightly lower than that of D. dianthicola, the disease severity index of the mixture inoculation soon surpassed D. dianthicola alone. Although not statistically significant, the disease severity index of treatments in 2018 showed a trend of D. dianthicola inoculation resulting in a numerically higher disease severity index than P. parmentieri inoculation, regardless of potato varieties (Figure 3).
All bacterial inoculations significantly reduced potato yield compared to the non-inoculated treatment in 2019; yield differences in the 2018 trial were not significant (Table 4). The treatment with a mixture of bacterial species did not show significant difference compared to only D. dianthicola inoculation (Table 3). Thus, D. dianthicola was a key to causing higher yield loss when potatoes were either infected solely with D. dianthicola or coinfected with D. dianthicola and P. parmentieri.

Table 4.
Effects of seed tuber inoculation with Dickeya dianthicola (DDi), Pectobacterium parmentieri (PPa) and non-inoculated (NI) on emergence and yield of potato varieties in years 2018 and 2019.
4. Discussion
Dickeya dianthicola was found to be the predominant species causing BSR in potato during the 2015 outbreak in the Northeastern USA. There was a trend where the number of samples received which were infected by P. parmentieri increased during subsequent years, but the percentage of total samples infected by D. dianthicola declined through the six years of the study. Pectobacterium parmentieri developed into a separate problem along with D. dianthicola, which was persistently detected as the predominant pathogen in subsequent years. Furthermore, D. dianthicola was more likely a pathogen associated with stems, and P. parmentieri was highly associated with potato tubers. It is possible that P. parmentieri is a better tuber-rotter than D. dianthicola.
We identified 20.6% infected potato plants contained both D. dianthicola and P. parmentieri. In addition, several other Pectobacterium spp. have also frequently been found in naturally infected seed potatoes (unpublished). It seemed the coexistence of multiple bacterial species is common in blackleg, forming a pathogen complex. Since D. dianthicola was consistently isolated as the predominant species, it is believed that D. dianthicola was the primary pathogen that aggressively caused disease in the field. Many Pectobacterium spp. can survive well in soil or they are soil inhabitants. Since they are weak pathogens, they may not cause infection. However, their availability in field allows them to follow the initiation of infection by Dickeya spp. and thus increase disease severity. However, come of Pectobacterium spp. such as P. parmentieri can be carried over to storage, where they seem to be highly active in causing tuber rot. A portion of the bacteria will move with seed pieces and may infect potato independently or collaboratively with D. dianthicola. From this point, BSR is a seedborne disease regardless of the pathogen taxon.
It is possible that D. dianthicola, compared to P. parmentieri, has more virulence-related factors in plant infection, such as enzymes to degrade pectin and cellulose [30], secretion systems to transfer virulence effectors [17,31]. Regarding pathogenicity, D. dianthicola might have advantages over P. parmentieri owing to the lack of T3SS in P. parmentieri [17,18], as T3SS contributes to the virulence of bacteria during the early stages of infection [32,33]. Studies have reported that D. dadantii exhibited a high number of pectinase-related enzymes that would enhance the degradation of pectin [34], and Dickeya spp. possess complex regulatory networks in order to express virulence genes [35]. However, detailed research about virulence-related mechanisms has not been established for D. dianthicola.
Our two-year data indicated that D. dianthicola causes a higher level of BSR severity than P. parmentieri, and inoculation with both bacterial species did increases BSR severity. This suggested that D. dianthicola should be considered the primary pathogen in disease control. Meanwhile, due to the wide distribution of P. parmentieri, it is possible that both bacterial species co-existed in the environment and co-infected or -damaged potato plants. This could be a consequence of synergy between the two bacterial species. In production, if D. dianthicola is eliminated, the less-aggressive P. parmentieri would likely have a lower impact on potato growth and yield. More importantly, it would remove the complex of co-infection with two or more bacteria. Therefore, it is necessary to further investigate how Dickeya spp. and Pectobacterium spp. interact with each other inside potato plants in increasing disease severity.
Competition, cooperation, and coexistence are major means of interactions of bacteria that co-exist in a same niche [36]. In this study, there was no antagonistic effect between D. dianthicola and P. parmentieri when they coexisted, but they aggravated the disease epidemic at a higher level. Surprisingly, the highest disease severity caused by the mixture of D. dianthicola and P. parmentieri did not transfer into the highest yield loss. We interpreted that yield is constrained by early infection and stand loss, and compensation by the potato plant, and less affected by late-season disease progress. Furthermore, among the three bacterial treatments, one with P. parmentieri had a lesser impact on yield compared to the other two. This implied that D. dianthicola was more aggressive or virulent than P. parmentieri.
Yield losses of potato caused by artificial inoculation of D. dianthicola were evaluated in this study. There was a high correlation of percentage of seed inoculation/contamination with lack of emergence, as well as with yield reduction. This was because yield per area of field was highly correlated with plant emergence or the total number of plants. BSR showed up in the growing season at a low level and most of the plants survived. Therefore, a low percentage of in-season disease did not significantly affect potato yield. In addition to disease severity, yield is also dependent on weather conditions, such as precipitation and soil temperature, soil properties, pathogen aggressiveness, and the ability of the crop to compensate for stand loss. Based on our results, “Lamoka” was the most susceptible variety to BSR, while “Russet Burbank” was more tolerant, “Atlantic” and “Shepody” were moderate. Such information may be incorporated in breeding program for BRS resistance.
In conclusion, tuber contamination with either D. dianthicola or P. parmentieri caused direct stand loss that transferred to yield loss, and the percent of tuber infection was negatively correlated with emergence when a single species of pathogen-infected potato, D. dianthicola, caused more symptoms than P. parmentieri. However, co-infection of the two species showed even higher disease severity of BSR, and a synergistic effect of bacterial species in plant infection.
Author Contributions
T.G. carried out the field trial of pathogens interaction and field trial of yield evaluation in 2020, data analysis, and manuscript drafting; F.E. carried out one-year field trial of yield evaluation in 2019; S.B.J. and R.P.L. helped in advising for field work, data analysis and manuscript revision; J.H. supervised the whole project. All authors have read and agreed to the published version of the manuscript.
Funding
This project was partially supported by USDA-NIFA-SCRI (2017-5118-26827), USDA-ARS (58-8030-6-001), USDA-NIFA-Hatch (ME022010, ME03138 and ME031906), and Maine Potato Board.
Acknowledgments
Noah Rosenzweig and Gary Secor provided some ideas in one field trial. We thank Elbridge Giggie for field operations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van der Wolf, J.M.; Nijhuis, E.H.; Kowalewska, M.J.; Saddler, G.S.; Parkinson, N.; Elphinstone, J.G.; Pritchard, L.; Toth, I.K.; Lojkowska, E.; Potrykus, M.; et al. Dickeya solani sp nov., a pectinolytic plant-pathogenic bacterium isolated from potato (Solanum tuberosum). Int. J. Syst. Evol. Microbiol. 2014, 64, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Helias, V.; Pirhonen, M.; Tsror, L.; Elphinstone, J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Johnson, S. Dickeya, a new potato pathogen in Maine and elsewhere. Phytopathology 2016, 106, 3. [Google Scholar]
- Hao, J.; Jiang, H.; Johnson, S. Detection and characterization of Dickeya species in the outbreak of blackleg disease of potato in Maine. Phytopathology 2016, 106, 2. [Google Scholar]
- Ma, X.; Schloop, A.; Swingle, B.; Perry, K.L. Pectobacterium and Dickeya responsible for potato blackleg disease in New York State in 2016. Plant Dis. 2018, 102, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Baldwin, A.C.; Patel, R.D.; Kobayashi, D.Y.; Wyenandt, C.A. First report of Dickeya dianthicola causing blackleg and soft rot on potato (Solanum tuberosum) in New Jersey, USA. Plant Dis. 2019, 103, 146. [Google Scholar] [CrossRef]
- Jiang, H.H.; Hao, J.J.; Johnson, S.B.; Brueggeman, R.S.; Secor, G. First report of Dickeya dianthicola causing blackleg and bacterial soft rot on potato in Maine. Plant Dis. 2016, 100, 2320. [Google Scholar] [CrossRef]
- McNally, R.R.; Curland, R.D.; Webster, B.T.; Robinson, A.P.; Ishimaru, C.A. First report of stem rot on potato caused by Dickeya chrysanthemi in Minnesota. Plant Dis. 2018, 102, 238. [Google Scholar] [CrossRef]
- McNally, R.R.; Curland, R.D.; Webster, B.T.; Robinson, A.P.; Ishimaru, C.A. First report of blackleg and tuber soft rot of potato caused by Pectobacterium parmentieri in Minnesota and North Dakota. Plant Dis. 2017, 101, 2144. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Huang, Y.-J.; van den Bosch, F.; West, J.S. Coexistence of related pathogen species on arable crops in space and time. Annu. Rev. Phytopathol. 2006, 44, 163–182. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef] [PubMed]
- Short, F.L.; Murdoch, S.L.; Ryan, R.P. Polybacterial human disease: The ills of social networking. Trends Microbiol. 2014, 22, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Dung, J.K.S.; Johnson, D.A.; Schroeder, B.K. Role of co-infection by Pectobacterium and Verticillium dahliae in the development of early dying and aerial stem rot of Russet Burbank potato. Plant Pathol. 2014, 63, 299–307. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Studholme, D.J.; Taratufolo, M.C.; Cai, R.; Almeida, N.F.; Goodman, T.; Guttman, D.S.; Vinatzer, B.A.; Balestra, G.M. Pseudomonas syringae pv. actinidiae (PSA) isolates from recent bacterial canker of kiwifruit outbreaks belong to the same genetic lineage. PLoS ONE 2012, 7, e36518. [Google Scholar]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Grabe, G.J.; van der Wolf, J.M. Distribution of Dickeya spp. and Pectobacterium carotovorum subsp. carotovorum in naturally infected seed potatoes. Eur. J. Plant Pathol. 2009, 125, 263–275. [Google Scholar] [CrossRef]
- Kim, H.-S.; Ma, B.; Perna, N.T.; Charkowski, A.O. Phylogeny and virulence of naturally occurring type III secretion system-deficient Pectobacterium strains. Appl. Environ. Microbiol. 2009, 75, 4539–4549. [Google Scholar] [CrossRef]
- Nykyri, J.; Niemi, O.; Koskinen, P.; Nokso-Koivisto, J.; Pasanen, M.; Broberg, M.; Plyusnin, I.; Törönen, P.; Holm, L.; Pirhonen, M. Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathog. 2012, 8, e1003013. [Google Scholar] [CrossRef]
- Ge, T.; Marangoni, N.F.; Hao, J.; Johnson, S.; Larkin, R.P. Isolation and identification of bacteria causing blackleg and soft rot of potato in Northeastern US. Phytopathology 2018, 108, 62. [Google Scholar]
- Ge, T.L.; Jiang, H.H.; Hao, J.J.; Johnson, S.B. First report of Pectobacterium parmentieri causing bacterial soft rot and blackleg on potato in Maine. Plant Dis. 2018, 102, 437. [Google Scholar] [CrossRef]
- Gardan, L.; Gouy, C.; Christen, R.; Samson, R. Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 381–391. [Google Scholar] [CrossRef]
- Samson, R.; Legendre, J.B.; Christen, R.; Fischer-Le Saux, M.; Achouak, W.; Gardan, L. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov as Dickeya chrysanthemi comb. nov and Dickeya paradisiaca comb. nov and delineation of four novel species, Dickeya dadantii sp nov., Dickeya dianthicola sp nov., Dickeya dieffenbachiae sp nov and Dickeya zeae sp nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 1415–1427. [Google Scholar] [CrossRef]
- Perombelon, M.C.M.; Kelman, A. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–387. [Google Scholar] [CrossRef]
- Dung, J.K.S.; Johnson, D.A.; Schroeder, B.K. First report of Pectobacterium wasabiae causing aerial stem rot of potato in Washington State. Plant Dis. 2012, 96, 1819. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Jiang, H.; Johnson, S.B.; Larkin, R.; Charkowski, A.O.; Secor, G.; Hao, J. Genotyping Dickeya dianthicola causing potato blackleg and soft rot outbreak associated with inoculum geography in the United States. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Pritchard, L.; Humphris, S.; Saddler, G.S.; Parkinson, N.M.; Bertrand, V.; Elphinstone, J.G.; Toth, I.K. Detection of phytopathogens of the genus Dickeya using a PCR primer prediction pipeline for draft bacterial genome sequences. Plant Pathol. 2013, 62, 587–596. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, M.S.; Kim, B.K.; Choi, H.J.; Hahn, J.H.; Kim, C.; Kang, M.J.; Kim, S.H.; Park, D.S. Quantitative real-time polymerase chain reaction assay for detection of Pectobacterium wasabiae using YD repeat protein gene-based primers. Plant Dis. 2012, 96, 253–257. [Google Scholar] [CrossRef][Green Version]
- Czajkowski, R.; de Boer, W.J.; Velvis, H.; van der Wolf, J.M. Systemic colonization of potato plants by a soilborne, green fluorescent protein-tagged strain of Dickeya sp. biovar 3. Phytopathology 2010, 100, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Johnson, S.; Larkin, R.P.; Charkowski, A.O.; Hao, J. Pathogen synergism of blackleg disease on potato. Plant Health 2020 Online, 13 August 2020. Available online: https://apsnet.confex.com/apsnet/2020/meetingapp.cgi/Paper/15048 (accessed on 13 August 2020).
- Barras, F.; van Gijsegem, F.; Chatterjee, A.K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 1994, 32, 201–234. [Google Scholar] [CrossRef]
- Charkowski, A.; Blanco, C.; Condemine, G.; Expert, D.; Franza, T.; Hayes, C.; Hugouvieux-Cotte-Pattat, N.; Solanilla, E.L.; Low, D.; Moleleki, L. The role of secretion systems and small molecules in soft-rot Enterobacteriaceae pathogenicity. Annu. Rev. Phytopathol. 2012, 50, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.H.; Cui, Y.; Alfano, J.R.; Rodríguez-Palenzuela, P.; Rojas, C.M.; Chatterjee, A.K.; Collmer, A. Analysis of Erwinia chrysanthemi EC16 pelE∷ uidA, pelL∷ uidA, and hrpN∷ uidA mutants reveals strain-specific atypical regulation of the Hrp type III secretion system. Mol. Plant-Microbe Interact. 2004, 17, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Rantakari, A.; Virtaharju, O.; Vähämiko, S.; Taira, S.; Palva, E.T.; Saarilahti, H.T.; Romantschuk, M. Type III secretion contributes to the pathogenesis of the soft-rot pathogen Erwinia carotovora: Partial characterization of the hrp gene cluster. Mol. Plant-Microbe Interact. 2001, 14, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Dittoe, D.K.; Barabote, R.D.; Rothrock, M.J.; Ricke, S.C. Assessment of a potential role of Dickeya dadantii DSM 18020 as a pectinase producer for utilization in poultry diets based on in silico analyses. Front. Microbiol. 2020, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, S.; Muskhelisvili, G.; Nasser, W. Virulence program of a bacterial plant pathogen: The Dickeya model. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 142, pp. 51–92. [Google Scholar]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host–multi-pathogen warfare: Pathogen interactions in co-infected plants. Front. Plant Sci. 2017, 8, 1806. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).