In Vivo Endophytic, Rhizospheric and Epiphytic Colonization of Vitis vinifera by the Plant-Growth Promoting and Antifungal Strain Pseudomonas protegens MP12
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pseudomonas protegens MP12, Botrytis cinerea BC and Plant Materials
2.2. Transformation of P. protegens MP12 with the Fluorescent Marker Protein dsRed
2.3. Detection of Fluorescent dsRed-Tagged P. protegens MP12 Inside Root Tissue
2.4. Plant Inoculation
2.4.1. Endophytic Inoculation Treatments
2.4.2. Rhizospheric Inoculation Treatments
2.4.3. Epiphytic Inoculation Treatments
2.5. Preparation of Plant Samples and Bacterial Counts
2.5.1. Post Endophytic Inoculation Treatments
2.5.2. Post Epiphytic Inoculation Treatments
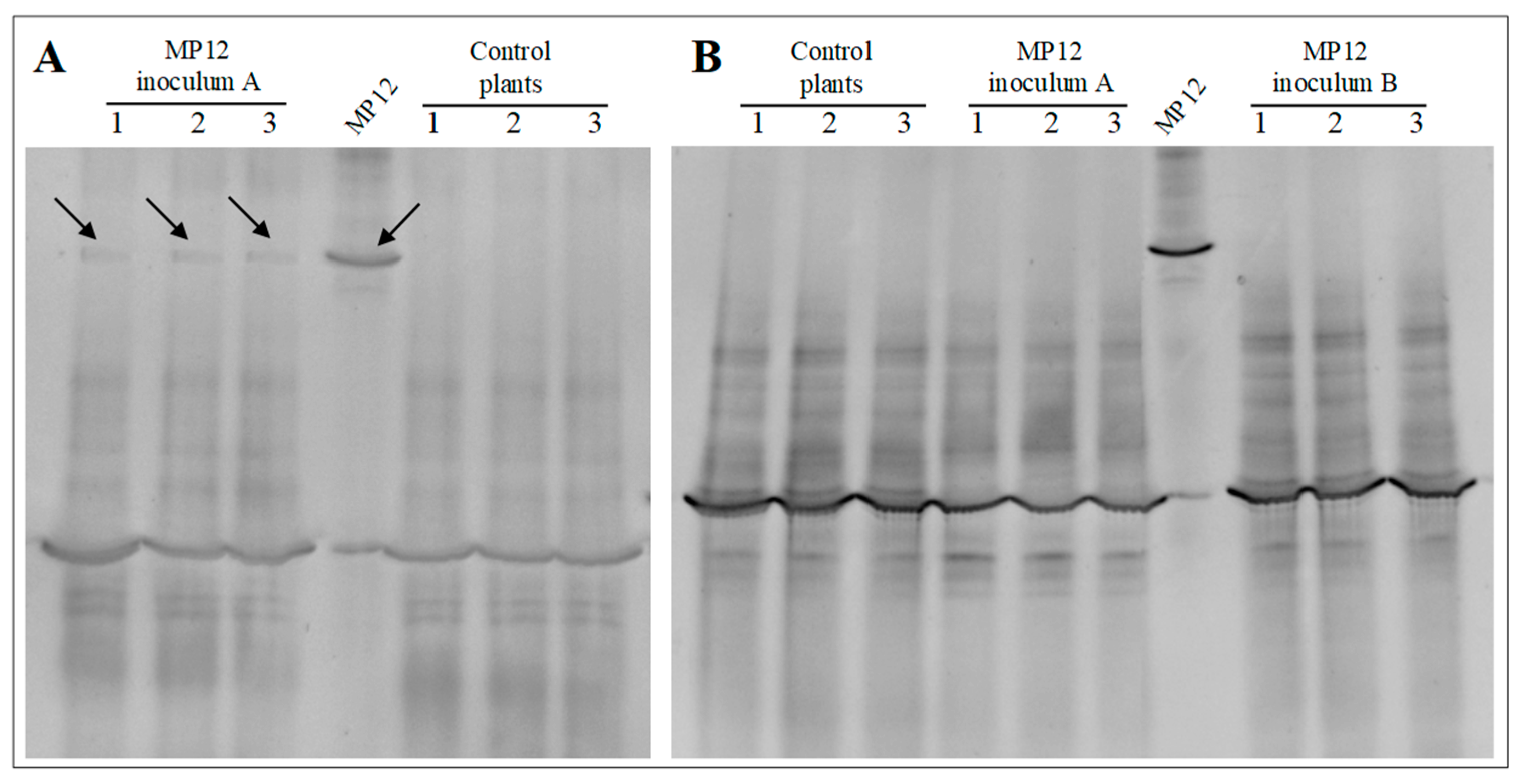
2.6. Recovery of Inoculated Bacteria through Molecular Analysis
2.7. Evaluation of Grafted Plant Quality
2.8. Evaluation of Fungal Infection on Grapevine Leaves Treated with P. protegens MP12
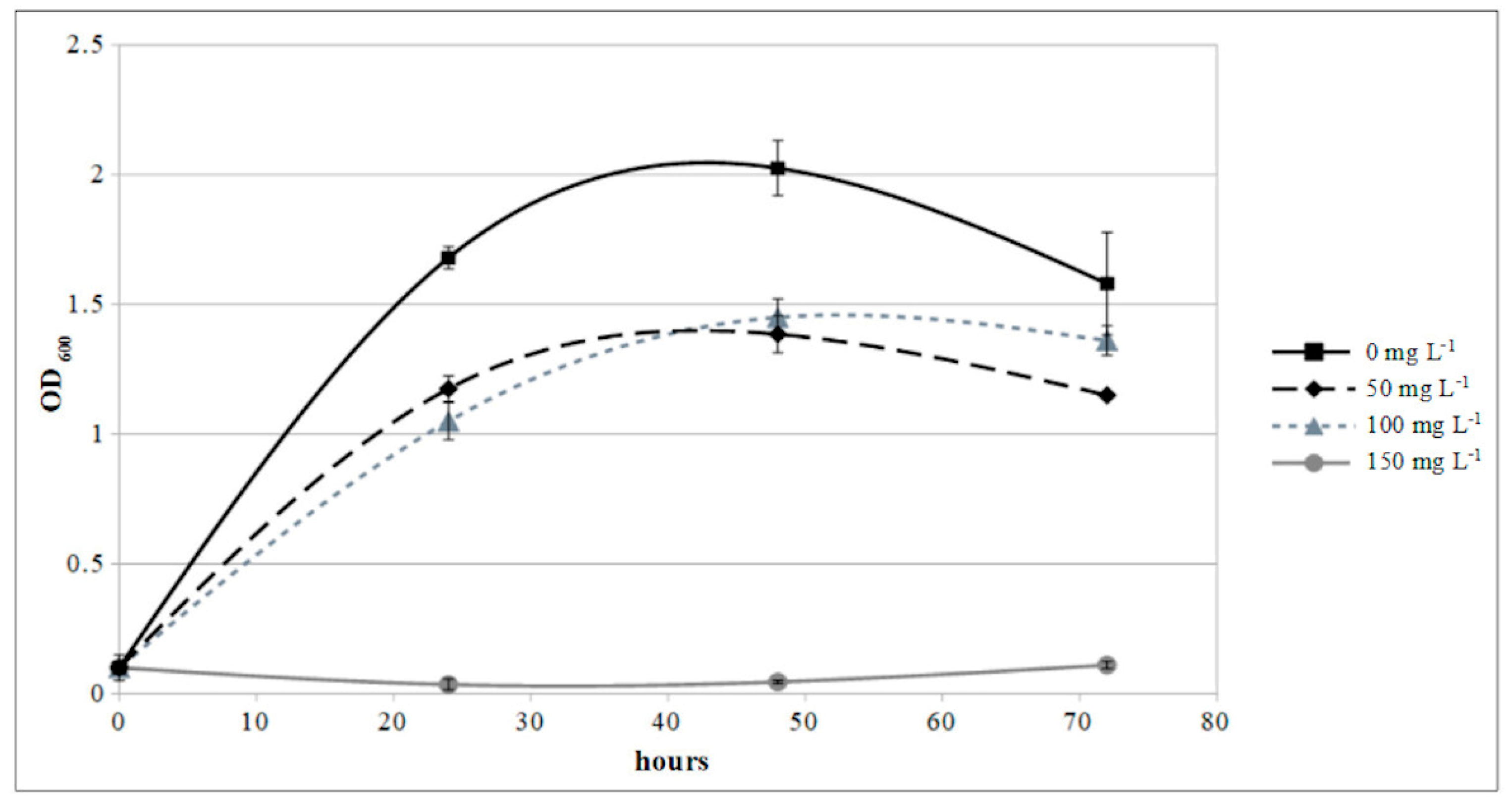
2.9. Copper Sensitivity Assay
2.10. Statistical Analysis
3. Results
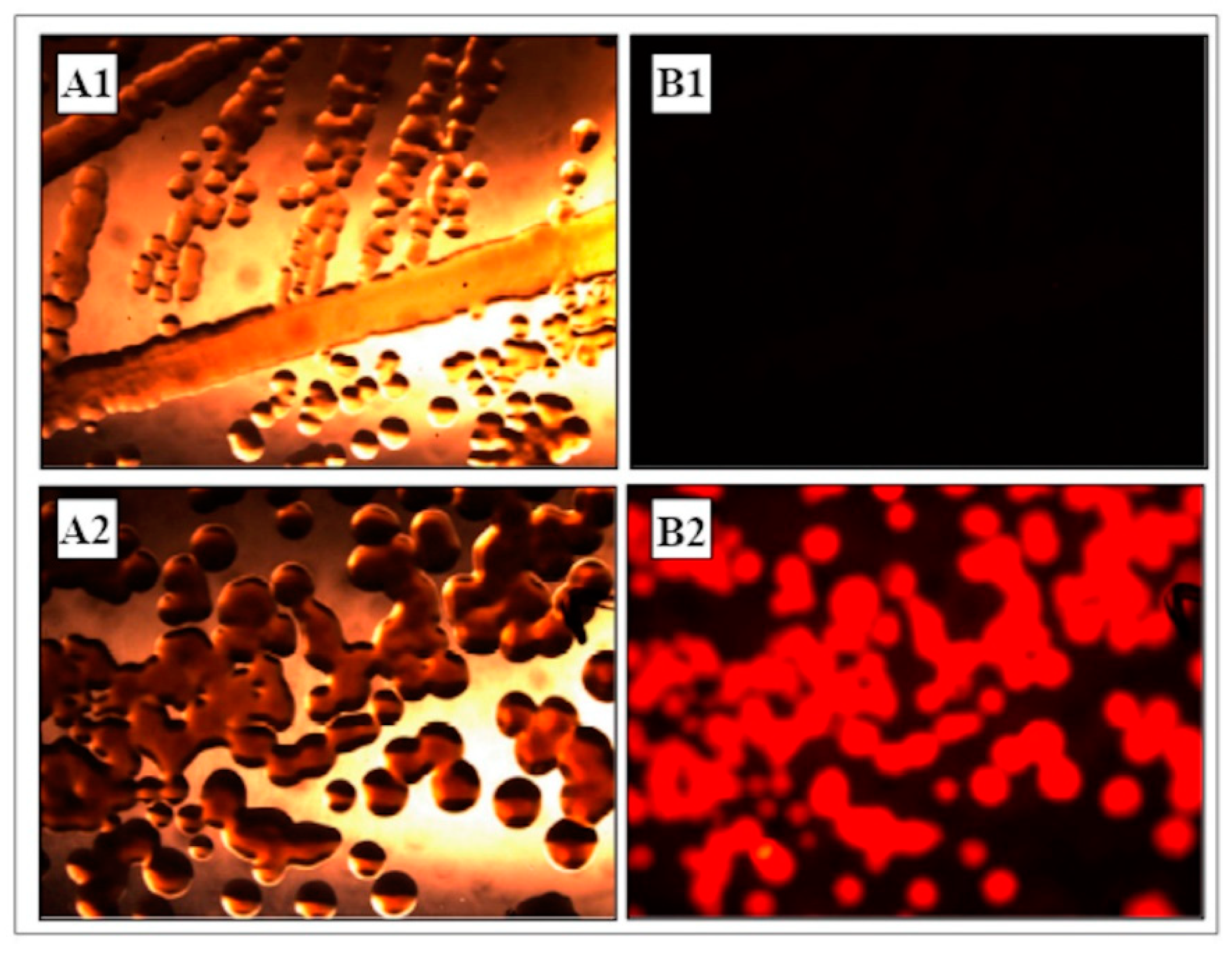
3.1. Construction and Analysis of dsRed-Tagged P. protegens MP12 Strain
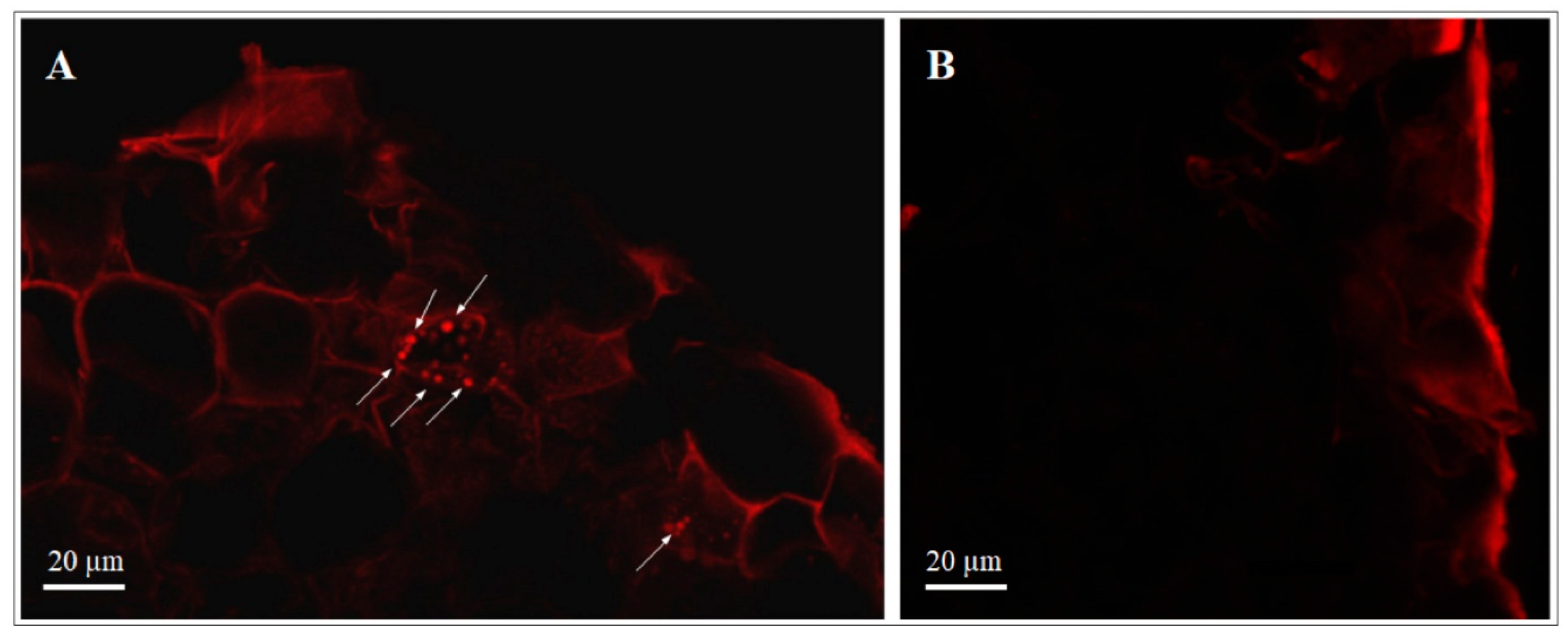
3.2. Endophytic Colonization
3.2.1. Transformed dsRed-Tagged P. protegens MP12 in Root Tissue
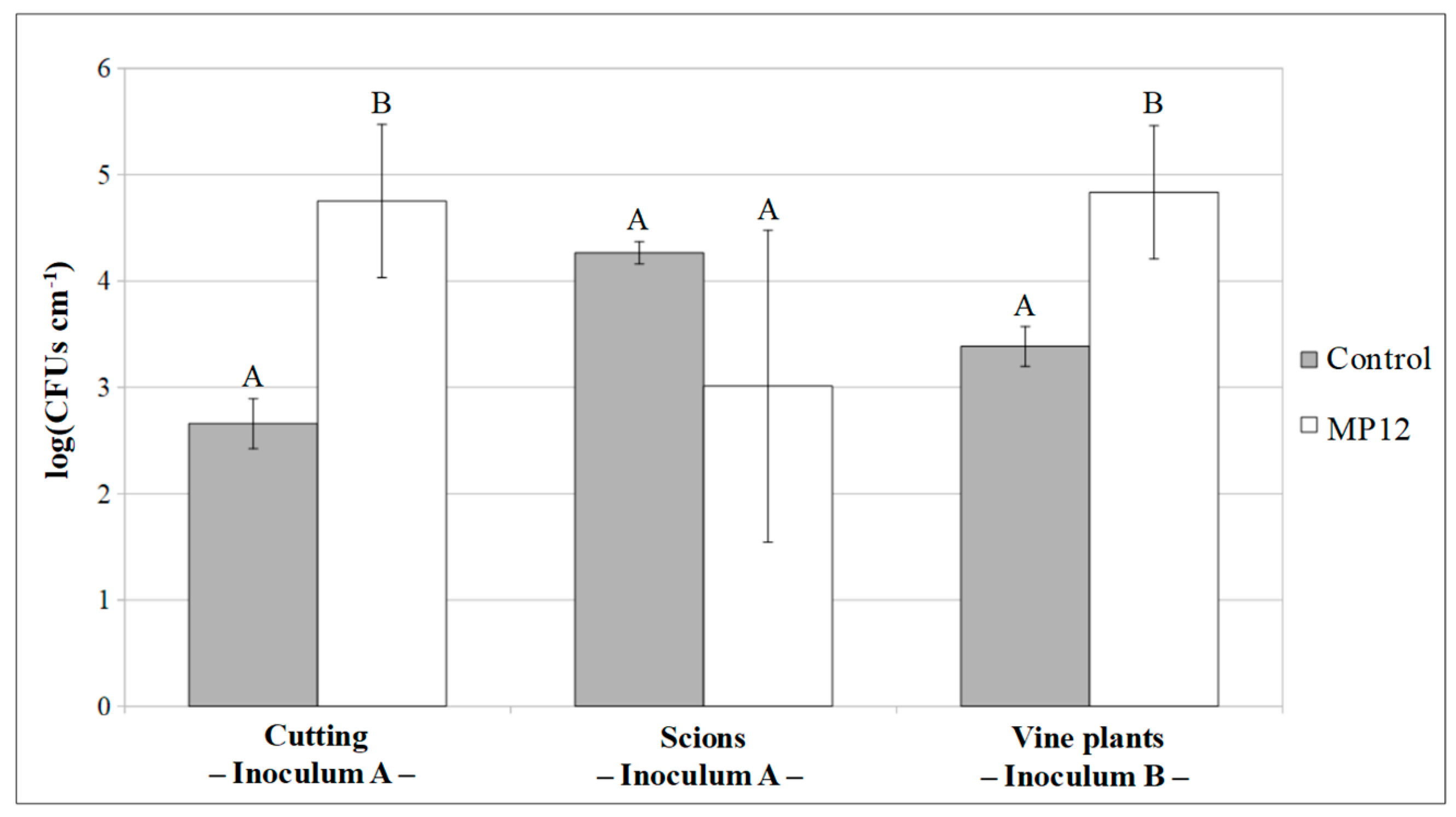
3.2.2. Colonization of Vine Plants by P. protegens MP12
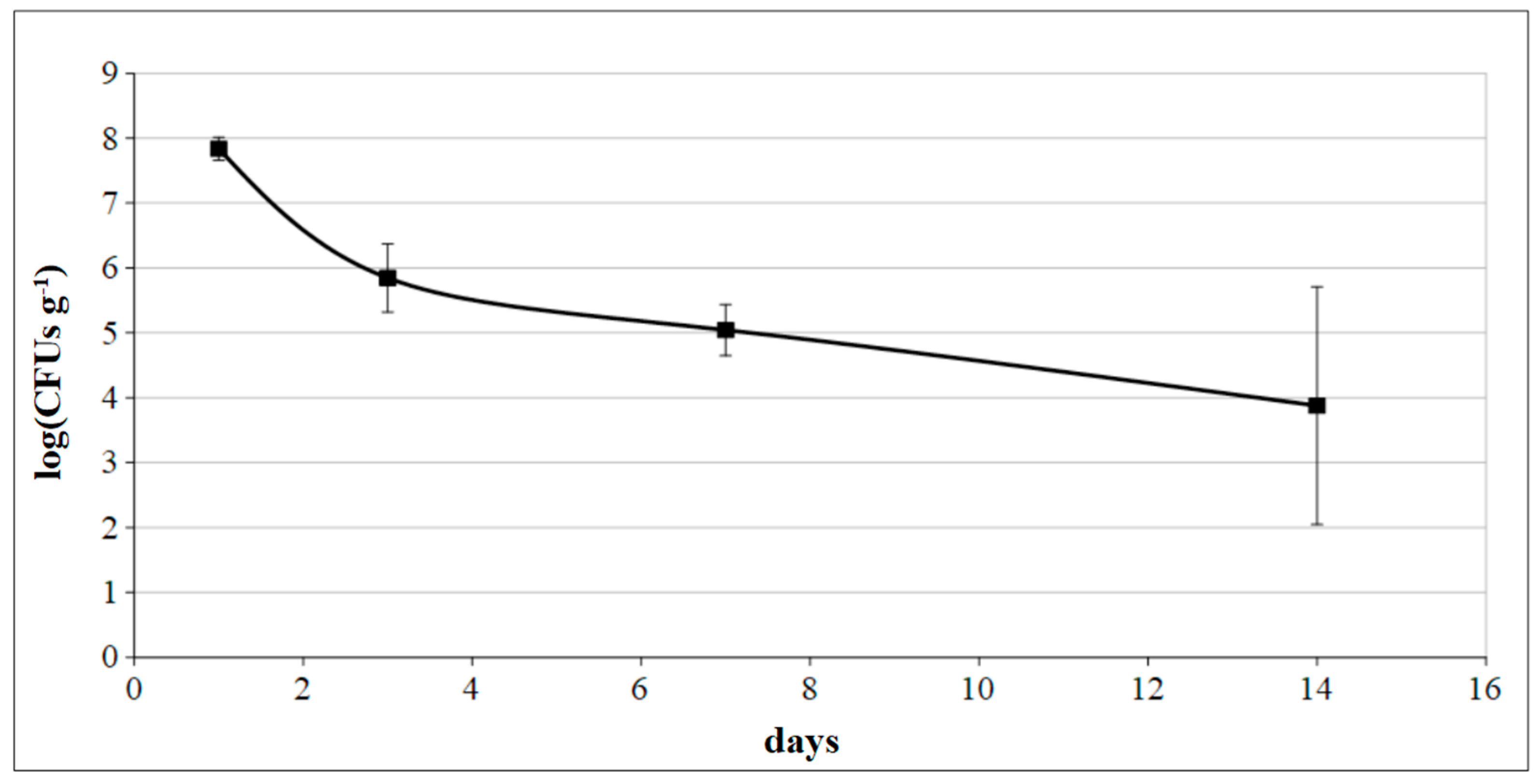
3.3. Rhizospheric Inoculum
3.4. Quality Evaluation of Grafted Plants
3.5. Epiphytic Inoculum, Fungal Infection Assay, and Copper Sensitivity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alston, J.M.; Sambucci, O. Grapes in the world economy. In The Grape Genome; Cantu, D., Walker, M.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar]
- Armijo, G.; Espinoza, C.; Loyola, R.; Restovic, F.; Santibáñez, C.; Schlechter, R.; Agurto, M.; Arce-Johnson, P. Grapevine biotechnology: Molecular approaches underlying abiotic and biotic stress responses. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 3–42. [Google Scholar]
- Sambucci, O.; Alston, J.M.; Fuller, K.B.; Lusk, J. The pecuniary and nonpecuniary costs of powdery mildew and the potential value of resistant grape varieties in California. Am. J. Enol. Vitic. 2019, 70, 177–187. [Google Scholar] [CrossRef]
- Sabatier, P.; Poulenard, J.; Fanget, B.; Reyss, J.L.; Develle, A.L.; Wilhelm, B.; Ployon, E.; Pignol, C.; Naffrechoux, E.; Dorioz, J.M.; et al. Long-term relationships among pesticide applications, mobility, and soil erosion in a vineyard watershed. Proc. Natl. Acad. Sci. USA 2014, 111, 15647–15652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreolli, M.; Lampis, S.; Vallini, G. Diversity, distribution and functional role of bacterial endophytes in Vitis vinifera. In Endophytes: Biology and Biotechnology; Maheshwari, D.K., Ed.; Springer: Cham, Switzerland, 2017; pp. 233–266. [Google Scholar]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Aziz, A.; Verhagen, B.; Magnin-Robert, M.; Couderchet, M.; Clément, C.; Jeandet, P.; Trotel-Aziz, P. Effectiveness of beneficial bacteria to promote systemic resistance of grapevine to gray mold as related to phytoalexin production in vineyards. Plant Soil 2016, 405, 141–153. [Google Scholar] [CrossRef]
- Pertot, I.; Prodorutti, D.; Colombini, A.; Pasini, L. Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. BioControl 2016, 61, 257–267. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Li, Y.; Fu, X.; Wang, Q. Screening and characterization of endophytic Bacillus for biocontrol of grapevine downy mildew. Crop. Prot. 2017, 96, 173–179. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Bacillus species as the most promising bacterial biocontrol agents in rhizosphere and endorhiza of plants grown in rotation with each other. Eur. J. Plant Pathol. 2018, 150, 497–506. [Google Scholar] [CrossRef]
- Bruisson, S.; Zufferey, M.; L’Haridon, F.; Trutmann, E.; Anand, A.; Dutartre, A.; De Vrieze, M.; Weisskopf, L. Endophytes and epiphytes from the grapevine leaf microbiome as potential biocontrol agents against phytopathogens. Front. Microbiol. 2019, 10, 2726. [Google Scholar] [CrossRef] [Green Version]
- Zeriouh, H.; de Vicente, A.; Pérez-García, A.; Romero, D. Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ. Microbiol. 2014, 16, 2196–2211. [Google Scholar] [CrossRef]
- Abdallah, D.B.; Frikha-Gargouri, O.; Tounsi, S. Rizhospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol. Control 2018, 124, 61–67. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compant, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Ait Barka, E.; Clement, C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: From the rhizosphere to inflorescence tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreolli, M.; Zapparoli, G.; Angelini, E.; Lucchetta, G.; Lampis, S.; Vallini, G. Pseudomonas protegens MP12: A plant growth-promoting endophytic bacterium with broad-spectrum antifungal activity against grapevine phytopathogens. Microbiol. Res. 2019, 219, 123–131. [Google Scholar] [CrossRef] [PubMed]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Lorenzini, M.; Azzolini, M.; Tosi, E.; Zapparoli, G. Postharvest grape infection of Botrytis cinerea and its interactions with other moulds under withering conditions to produce noble-rotten grapes. J. Appl. Microbiol. 2013, 114, 762–770. [Google Scholar] [CrossRef]
- Choi, K.H.; Gaynor, J.B.; White, K.G.; Lopez, C.; Bosio, C.M.; Karkhoff-Schweizer, R.R.; Schweizer, H.P. A Tn 7-based broad-range bacterial cloning and expression system. Nat. Methods 2005, 2, 443–448. [Google Scholar] [CrossRef]
- Choi, K.H.; Schweizer, H.P. mini-Tn 7 insertion in bacteria with single att Tn 7 sites: Example Pseudomonas aeruginosa. Nat. Protoc. 2006, 1, 153–161. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; University of California Berkeley: Berkeley, CA, USA, 1950; pp. 30–32. [Google Scholar]
- Mavrodi, O.V.; McSpadden Gardener, B.B.; Mavrodi, D.V.; Bonsall, R.F.; Weller, D.M.; Thomashow, L.S. Genetic diversity of phlD from 2,4-diacetylphloroglucinol producing fluorescent Pseudomonas spp. Biol. Control 2001, 91, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Andreolli, M.; Lampis, S.; Brignoli, P.; Vallini, G. Trichoderma longibrachiatum Evx1 is a fungal biocatalyst suitable for the remediation of soils contaminated with diesel fuel and polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2016, 23, 9134–9143. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Albertarelli, N.; Lampis, S.; Brignoli, P.; Khoei, N.S.; Vallini, G. Bioremediation of diesel contamination at an underground storage tank site: A spatial analysis of the microbial community. World J. Microbiol. Biotechnol. 2016, 32, 6. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzini, M.; Zapparoli, G. Characterization and pathogenicity of Alternaria spp. strains associated with grape bunch rot during post-harvest withering. Int. J. Food Microbiol. 2014, 186, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Iasur-Kruh, L.; Zahavi, T.; Barkai, R.; Freilich, S.; Zchori-Fein, E.; Naor, V. Dyella-like bacterium isolated from an insect as a potential biocontrol agent against grapevine yellows. Phytopathology 2018, 108, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Germaine, K.; Keogh, E.; Garcia-Cabellos, G.; Borremans, B.; van der Lelie, D.; Barac, T.; Oeyen, L.; Vangronsveld, J.; Moore, F.P.; Moore, E.R.B.; et al. Colonisation of poplar trees by GFP expressing bacterial endophytes. FEMS Microbiol. Ecol. 2004, 48, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Wicaksono, W.A.; Jones, E.E.; Monk, J.; Ridgway, H.J. Using bacterial endophytes from a New Zealand native medicinal plant for control of grapevine trunk diseases. Biol. Control 2017, 114, 65–72. [Google Scholar] [CrossRef]
- Thorne, E.T.; Young, B.M.; Young, G.M.; Stevenson, J.F.; Labavitch, J.M.; Matthews, M.A.; Rost, T.L. The structure of xylem vessels in grapevine (Vitaceae) and a possible passive mechanism for the systemic spread of bacterial disease. Am. J. Bot. 2006, 93, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Chatelet, D.S.; Matthews, M.A.; Rost, T.L. Xylem structure and connectivity in grapevine (Vitis vinifera) shoots provides a passive mechanism for the spread of bacteria in grape plants. Ann. Bot. 2006, 98, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.Á.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R. Use of endophytic and rhizosphere actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; He, S. A prominent role of the flagellin receptor flagellin-sensing2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol. 2010, 153, 1188–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trdá, L.; Fernandez, O.; Boutrot, F.; Héloir, M.C.; Kelloniemi, J.; Daire, X.; Adrian, M.; Clément, C.; Zipfel, C.; Dorey, S.; et al. The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol. 2014, 201, 1371–1384. [Google Scholar] [CrossRef]
- Newman, M.A.; Sundelin, T.; Nielsen, J.T.; Erbs, G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vallet, A.; Mesters, J.R.; Thomma, B.P. The battle for chitin recognition in plant-microbe interactions. FEMS Microbiol. Rev. 2015, 39, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, D.; Zahringer, U.; Gerber, I.; Dubery, I.; Hartung, T.; Bors, W.; Hutzler, P.; Durner, J. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA. 2004, 101, 15811–15816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alquéres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol. Plant Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Cui, Q.; Li, X.; Yin, J.; Ravichandran, V.; Pan, D.; Fu, J.; Tu, Q.; Wang, H.; Bian, X.; et al. Engineering Pseudomonas protegens Pf-5 to improve its antifungal activity and nitrogen fixation. Microbiol. Biotechnol. 2020, 13, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Blanco, J.; Bakker, P.A. Interactions between plants and beneficial Pseudomonas spp.: Exploiting bacterial traits for crop protection. Antonie van Leeuwenhoek 2007, 92, 367–389. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Halleen, F.; Crous, P.W.; Petrini, O. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Aust. Plant Pathol. 2003, 32, 47–52. [Google Scholar] [CrossRef]
- Ganeshan, G.; Manoj Kumar, A. Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J. Plant Interact. 2005, 1, 123–134. [Google Scholar] [CrossRef]
- Hammer, P.E.; Hill, S.; Ligon, J. Characterization of genes from Pseudomonas fluorescens involved in the synthesis of pyrrolnitrin. Phytopathology 1995, 69, 480–482. [Google Scholar]
- Ramette, A.; Frapolli, M.; Fischer-Le Saux, M.; Gruffaz, C.; Meyer, J.-M.; Defago, G.; Sutra, L.; Moenne-Loccoz, Y. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 2011, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, A.; Singh, U.P.; Sarnia, B.K.; Singh, D.P.; Singh, K.P.; Singh, A. Foliar application of plant growth-promoting rhizobacteria increases antifungal compounds in pea (Pisum sativum) against Erysiphe pisi. Microbiology 2007, 35, 129–134. [Google Scholar]
- Özyilmaz, Ü.; Benlioglu, K. Enhanced biological control of phytophthora blight of pepper by biosurfactant-producing Pseudomonas. Plant Pathol. J. 2013, 29, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.Z.; Marian, M.; Enomoto, T.; Hieno, A.; Ina, H.; Suga, H.; Shimizu, M. Biocontrol of tomato bacterial wilt by foliar spray application of a novel strain of endophytic Bacillus sp. Microbes Environ. 2020, 35, ME20078. [Google Scholar] [CrossRef]
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Li, J.; Meng, Z.; Zhang, S.; Dong, B.; Qiao, K. Synergistic effect of combined application of a new fungicide fluopimomide with a biocontrol agent Bacillus methylotrophicus TA-1 for management of gray mold in tomato. Plant Dis. 2019, 103, 1991–1997. [Google Scholar] [CrossRef]
- Yerkovich, N.; Cantoro, R.; Palazzini, J.M.; Torres, A.; Chulze, S.N. Fusarium head blight in Argentina: Pathogen aggressiveness, triazole tolerance and biocontrol-cultivar combined strategy to reduce disease and deoxynivalenol in wheat. Crop. Prot. 2020, 137, 105300. [Google Scholar] [CrossRef]
- Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. OJ L 1986, 181, 6–12.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreolli, M.; Zapparoli, G.; Lampis, S.; Santi, C.; Angelini, E.; Bertazzon, N. In Vivo Endophytic, Rhizospheric and Epiphytic Colonization of Vitis vinifera by the Plant-Growth Promoting and Antifungal Strain Pseudomonas protegens MP12. Microorganisms 2021, 9, 234. https://doi.org/10.3390/microorganisms9020234
Andreolli M, Zapparoli G, Lampis S, Santi C, Angelini E, Bertazzon N. In Vivo Endophytic, Rhizospheric and Epiphytic Colonization of Vitis vinifera by the Plant-Growth Promoting and Antifungal Strain Pseudomonas protegens MP12. Microorganisms. 2021; 9(2):234. https://doi.org/10.3390/microorganisms9020234
Chicago/Turabian StyleAndreolli, Marco, Giacomo Zapparoli, Silvia Lampis, Chiara Santi, Elisa Angelini, and Nadia Bertazzon. 2021. "In Vivo Endophytic, Rhizospheric and Epiphytic Colonization of Vitis vinifera by the Plant-Growth Promoting and Antifungal Strain Pseudomonas protegens MP12" Microorganisms 9, no. 2: 234. https://doi.org/10.3390/microorganisms9020234
APA StyleAndreolli, M., Zapparoli, G., Lampis, S., Santi, C., Angelini, E., & Bertazzon, N. (2021). In Vivo Endophytic, Rhizospheric and Epiphytic Colonization of Vitis vinifera by the Plant-Growth Promoting and Antifungal Strain Pseudomonas protegens MP12. Microorganisms, 9(2), 234. https://doi.org/10.3390/microorganisms9020234




