Impact of Phage Therapy on Multidrug-Resistant Escherichia coli Intestinal Carriage in a Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Bacterial Strains and Gastric Gavage
2.3. Phages and Gastric-Rectal Administration
2.4. Murine Model of Intestinal Colonisation
2.5. Faecal Collection and Culture
2.6. In-Vivo Evaluation of Phage Therapy
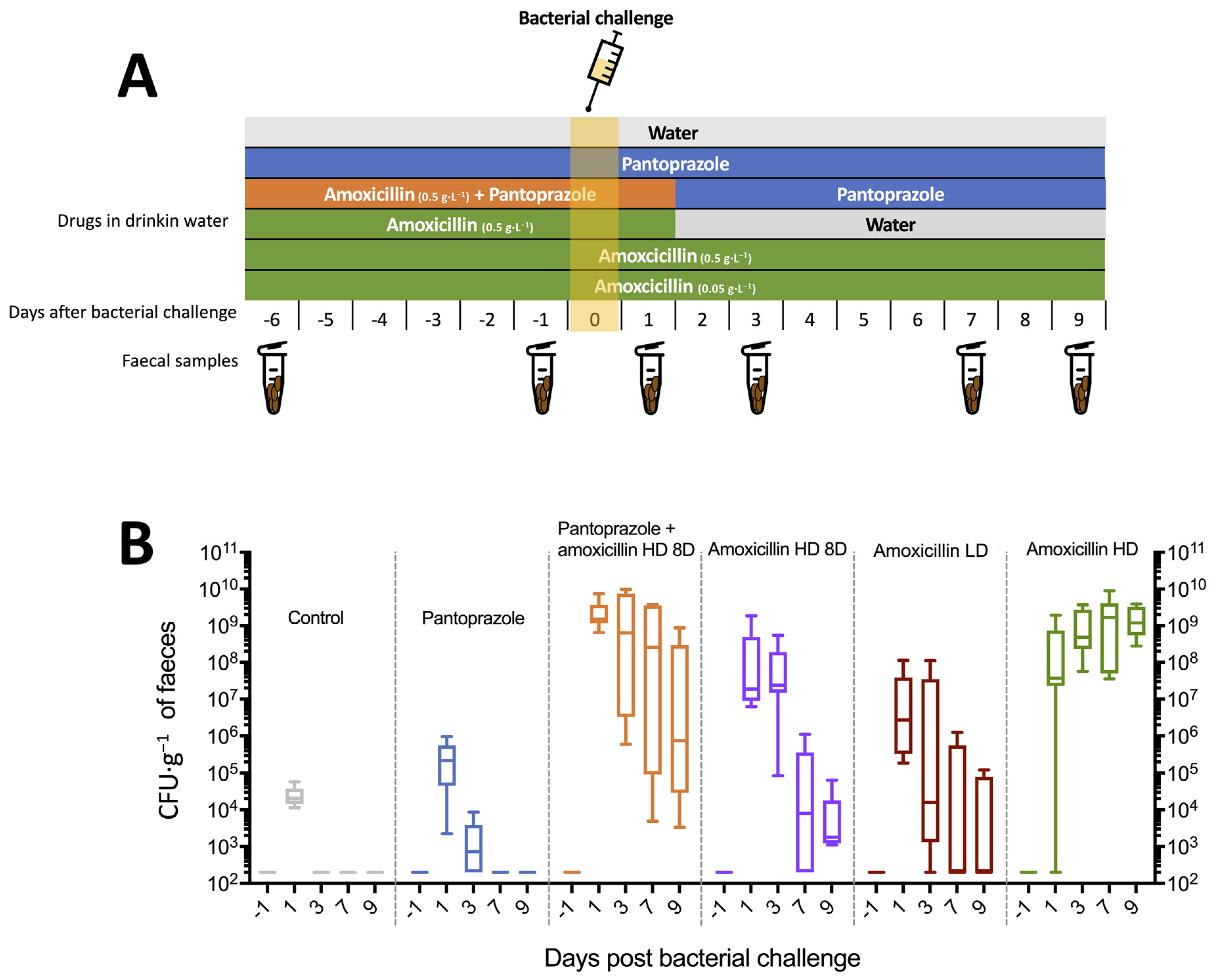
2.6.1. Experiment 1
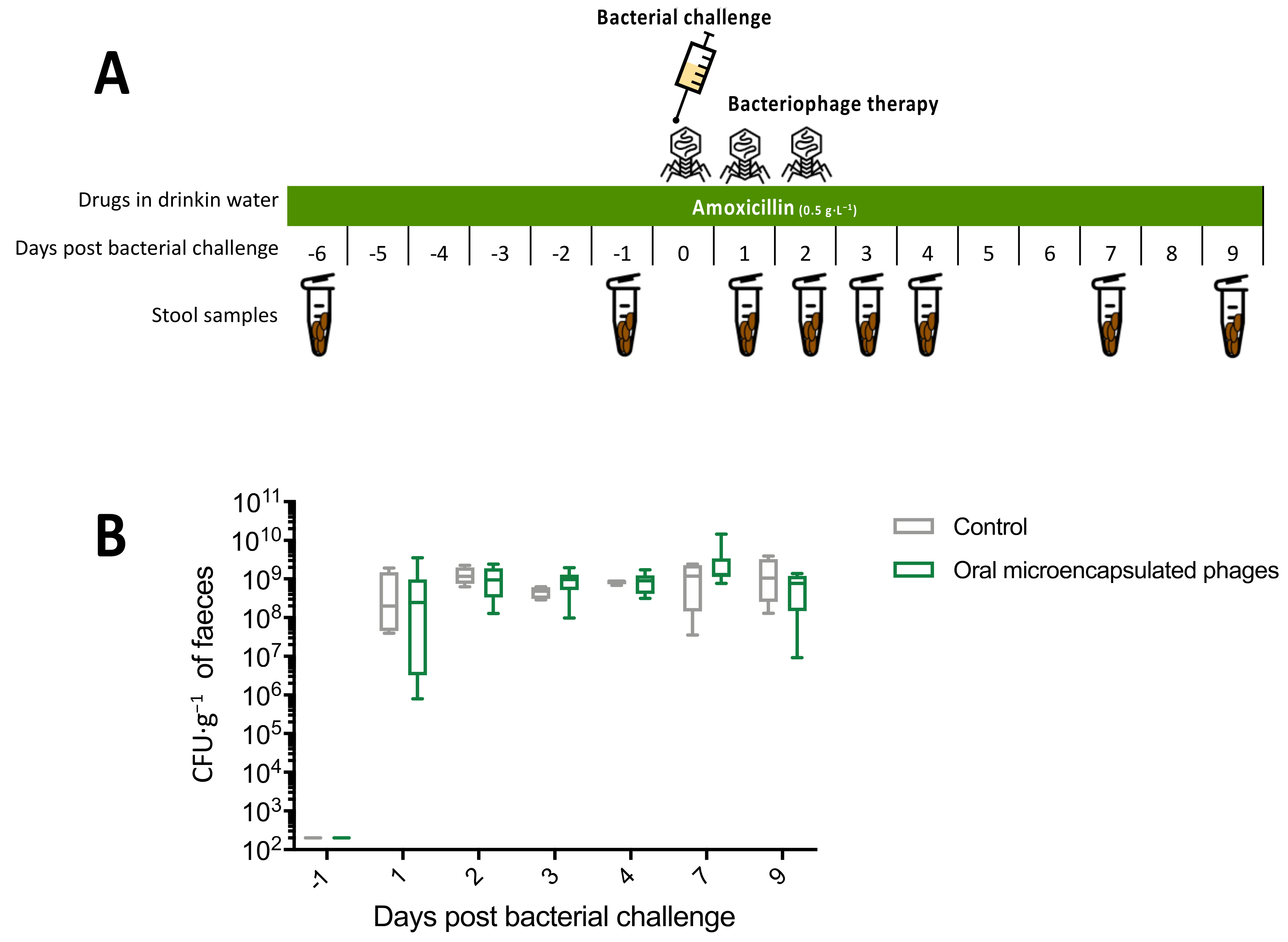
2.6.2. Experiment 2
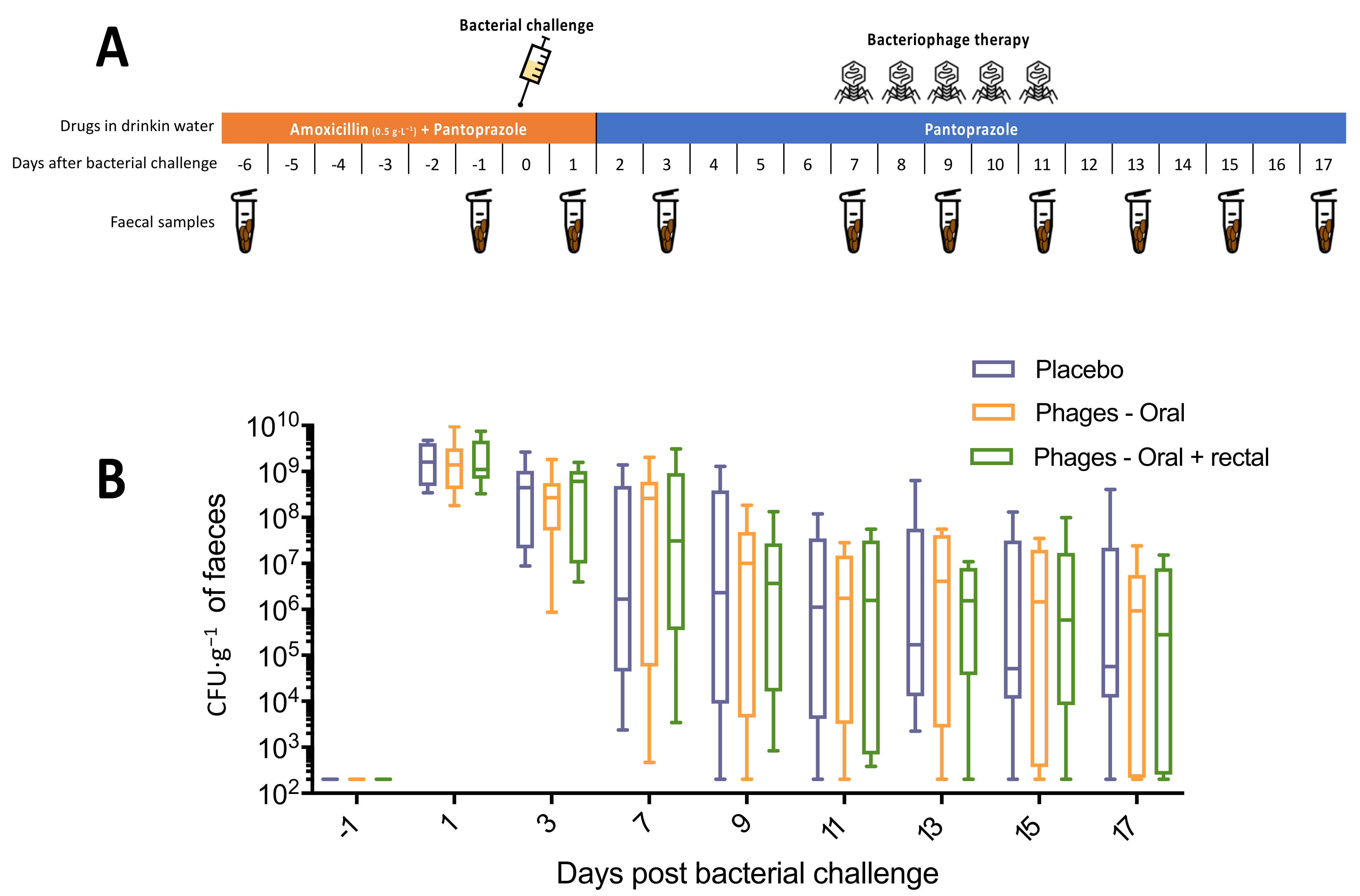
2.6.3. Experiment 3
2.6.4. Statistical Analysis
3. Results
3.1. Isolation and Selection of Phages
3.2. Development of the Murine Model of ESBL E. coli
3.3. In Vivo Evaluation of Phage Therapy
3.3.1. Experiment 1: Oral Cocktail of Phages
3.3.2. Experiment 2: Microencapsulated Phages
3.3.3. Experiment 3: Oral and Rectal Cocktail of Phages with Pantoprazole
4. Discussion
4.1. Summary of Results
4.2. Effects of PPI on Digestive Colonisation
4.3. Effectiveness and Limitations of Phages in Reducing Digestive Carriage
4.4. Limitations of the Study
4.5. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Researach, Discovery and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 7 November 2021).
- Anthony, W.E.; Burnham, C.-A.D.; Dantas, G.; Kwon, J.H. The gut microbiome as a reservoir for antimicrobial resistance. J. Infect. Dis. 2021, 223, S209–S213. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Stobberingh, E.E.; Savelkoul, P.H.M.; Wolffs, P.F.G. The human microbiome as a reservoir of antimicrobial resistance. Front. Microbiol. 2013, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Navon-Venezia, S.; Kaye, K.S.; Ben-Ami, R.; Schwartz, D.; Carmeli, Y. Clinical and economic impact of bacteremia with extended- spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2006, 50, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Denis, B.; Lafaurie, M.; Donay, J.L.; Fontaine, J.P.; Oksenhendler, E.; Raffoux, E.; Hennequin, C.; Allez, M.; Socie, G.; Maziers, N.; et al. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: A five-year study. Int. J. Infect. Dis. 2015, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.; Wolkewitz, M.; Davey, P.G.; Koller, W.; Berger, J.; Nagler, J.; Icket, C.; Kalenic, S.; Horvatic, J.; Seifert, H.; et al. Burden of antimicrobial resistance in European hospitals: Excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J. Antimicrob. Chemother. 2011, 66, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Sengupta, S.; Kukreja, A.; Ponnampalavanar, S.S.; Ng, O.T.; Marimuthu, K. The role of hospital environment in transmissions of multidrug-resistant gram-negative organisms. Antimicrob. Resist. Infect. Control. 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Mazzaferri, F.; de Smet, A.M.; Bragantini, D.; Eggimann, P.; Huttner, B.D.; Kuijper, E.J.; Lucet, J.C.; Mutters, N.T.; Sanguinetti, M.; et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 2019, 25, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Brives, C.; Pourraz, J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Sarker, S.A.; Berger, B.; Deng, Y.; Kieser, S.; Foata, F.; Moine, D.; Descombes, P.; Sultana, S.; Huq, S.; Bardhan, P.K.; et al. Oral application of Escherichia coli bacteriophage: Safety tests in healthy and diarrheal children from Bangladesh. Environ. Microbiol. 2017, 19, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Divya Ganeshan, S.; Hosseinidoust, Z. Phage therapy with a focus on the human microbiota. Antibiotics 2019, 8, E131. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Galtier, M.; De Sordi, L.; Maura, D.; Arachchi, H.; Volant, S.; Dillies, M.A.; Debarbieux, L. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol. 2016, 18, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Vinner, G.K.; Richards, K.; Leppanen, M.; Sagona, A.P.; Malik, D.J. Microencapsulation of enteric bacteriophages in a pH-Responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 2019, 11, E475. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, U.; Rao, A.; Pultz, M.J.; Jump, R.L.; Aron, D.C.; Donskey, C.J. Suppression of gastric acid production by proton pump inhibitor treatment facilitates colonization of the large intestine by vancomycin-resistant Enterococcus spp. and Klebsiella pneumoniae in clindamycin-treated mice. Antimicrob. Agents Chemother. 2006, 50, 3905–3907. [Google Scholar] [CrossRef] [PubMed]
- Denou, E.; Bruttin, A.; Barretto, C.; Ngom-Bru, C.; Brüssow, H.; Zuber, S. T4 phages against Escherichia coli diarrhea: Potential and problems. Virology 2009, 388, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, U.; Nerandzic, M.M.; Pultz, M.J.; Donskey, C.J. Gastrointestinal colonization with a cephalosporinase-producing bacteroides species preserves colonization resistance against vancomycin-resistant enterococcus and Clostridium difficile in cephalosporin-treated mice. Antimicrob. Agents Chemother. 2014, 58, 4535–4542. [Google Scholar] [CrossRef] [PubMed]
- Hertz, F.B.; Løbner-Olesen, A.; Frimodt-Møller, N. Antibiotic selection of Escherichia coli sequence type 131 in a mouse intestinal colonization model. Antimicrob. Agents Chemother. 2014, 58, 6139–6144. [Google Scholar] [CrossRef][Green Version]
- Willems, R.P.; van Dijk, K.J.; Ket, J.C.F.; Vandenbroucke-Grauls, C.M.J.E. Evaluation of the association between gastric acid suppression and risk of intestinal colonization with multidrug-resistant microorganisms: A systematic review and meta-analysis. JAMA Intern. Med. 2020, 180, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Al-Ghalith, G.A.; Grégoire, M.; Chapelet, G.; Javaudin, F.; Dailly, E.; Batard, E.; Knights, D.; Montassier, E. Systematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther. 2018, 47, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Maura, D.; Morello, E.; du Merle, L.; Bomme, P.; Le Bouguénec, C.; Debarbieux, L. Intestinal colonization by enteroaggregative Escherichia coli supports long-term bacteriophage replication in mice. Environ. Microbiol. 2012, 14, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Denou, E.; Bruttin, A.; Serra-Moreno, R.; Dillmann, M.-L.; Brüssow, H. In vivo replication of T4 and T7 bacteriophages in germ-free mice colonized with Escherichia coli. Virology 2009, 393, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Chibani-Chennoufi, S.; Sidoti, J.; Bruttin, A.; Kutter, E.; Sarker, S.; Brüssow, H. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: Implications for phage therapy. Antimicrob. Agents Chemother. 2004, 48, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Tanji, Y.; Shimada, T.; Fukudomi, H.; Miyanaga, K.; Nakai, Y.; Unno, H. Therapeutic use of phage cocktail for controlling Escherichia coli O157:H7 in gastrointestinal tract of mice. J. Biosci. Bioeng. 2005, 100, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages reduce pathogenic escherichia coli counts in mice without distorting gut microbiota. Front. Microbiol. 2019, 10, 1984. [Google Scholar] [CrossRef] [PubMed]
- Maura, D.; Galtier, M.; Le Bouguénec, C.; Debarbieux, L. Virulent bacteriophages can target O104: H4 enteroaggregative Escherichia coli in the mouse intestine. Antimicrob. Agents Chemother. 2012, 56, 6235–6242. [Google Scholar] [CrossRef]
- Galtier, M.; De Sordi, L.; Sivignon, A.; de Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages targeting adherent invasive escherichia coli strains as a promising new treatment for crohn’s disease. J. Crohns Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef]
- Cepko, L.C.S.; Garling, E.E.; Dinsdale, M.J.; Scott, W.P.; Bandy, L.; Nice, T.; Faber-Hammond, J.; Mellies, J.L. Myoviridae phage PDX kills enteroaggregative Escherichia coli without human microbiome dysbiosis. J. Med. Microbiol. 2020, 69, 309–323. [Google Scholar] [CrossRef]
- Javaudin, F.; Latour, C.; Debarbieux, L.; Lamy-Besnier, Q. Intestinal bacteriophage therapy: Looking for optimal efficacy. Clin. Microbiol. Rev. 2021, 34, e0013621. [Google Scholar] [CrossRef] [PubMed]
- Labedan, B. Requirement for a fluid host cell membrane in injection of coliphage T5 DNA. J. Virol. 1984, 49, 273–275. [Google Scholar] [CrossRef]
- Ohshima, Y.; Schumacher-Perdreau, F.; Peters, G.; Pulverer, G. The role of capsule as a barrier to bacteriophage adsorption in an encapsulated Staphylococcus simulans strain. Med. Microbiol. Immunol. 1988, 177, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Binetti, A.G.; Quiberoni, A.; Reinheimer, J.A. Phage adsorption to Streptococcus thermophilus. Influence of environmental factors and characterization of cell-receptors. Food Res. Int. 2002, 35, 73–83. [Google Scholar] [CrossRef]
- Lourenço, M.; Chaffringeon, L.; Lamy-Besnier, Q.; Pédron, T.; Campagne, P.; Eberl, C.; Bérard, M.; Stecher, B.; Debarbieux, L.; De Sordi, L. The spatial heterogeneity of the gut limits predation and fosters coexistence of bacteria and bacteriophages. Cell Host Microbe 2020, 28, 390–401.e5. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.; García-Rodríguez, A.; Cano-Sarabia, M.; Maspoch, D.; Marcos, R.; Cortés, P.; Llagostera, M. Biodistribution of liposome-encapsulated bacteriophages and their transcytosis during oral phage therapy. Front. Microbiol. 2019, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.; McAllister, T.A.; Niu, Y.D.; Stephens, T.P.; Mazzocco, A.; Waddell, T.E.; Johnson, R.P. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 2010, 73, 1304–1312. [Google Scholar] [CrossRef]
- Conner, D.E.; Kotrola, J.S. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl. Environ. Microbiol. 1995, 61, 382–385. [Google Scholar] [CrossRef]
- Saez, A.C.; Zhang, J.; Rostagno, M.H.; Ebner, P.D. Direct feeding of microencapsulated bacteriophages to reduce Salmonella colonization in pigs. Foodborne Pathog. Dis. 2011, 8, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.K.; Zhang, J.; Rostagno, M.H.; Ebner, P.D. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl. Environ. Microbiol. 2010, 76, 48–53. [Google Scholar] [CrossRef]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: A randomized trial in children from bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javaudin, F.; Bémer, P.; Batard, E.; Montassier, E. Impact of Phage Therapy on Multidrug-Resistant Escherichia coli Intestinal Carriage in a Murine Model. Microorganisms 2021, 9, 2580. https://doi.org/10.3390/microorganisms9122580
Javaudin F, Bémer P, Batard E, Montassier E. Impact of Phage Therapy on Multidrug-Resistant Escherichia coli Intestinal Carriage in a Murine Model. Microorganisms. 2021; 9(12):2580. https://doi.org/10.3390/microorganisms9122580
Chicago/Turabian StyleJavaudin, François, Pascale Bémer, Eric Batard, and Emmanuel Montassier. 2021. "Impact of Phage Therapy on Multidrug-Resistant Escherichia coli Intestinal Carriage in a Murine Model" Microorganisms 9, no. 12: 2580. https://doi.org/10.3390/microorganisms9122580
APA StyleJavaudin, F., Bémer, P., Batard, E., & Montassier, E. (2021). Impact of Phage Therapy on Multidrug-Resistant Escherichia coli Intestinal Carriage in a Murine Model. Microorganisms, 9(12), 2580. https://doi.org/10.3390/microorganisms9122580





