Abstract
Salmonella enterica is a common cause of many enteric infections worldwide and is successfully engineered to deliver heterologous antigens to be used as vaccines. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs) RNA-guided Cas9 endonuclease is a promising genome editing tool. In the current study, a CRISPR-Cas9 system was used to target S. enterica sdiA that encodes signal molecule receptor SdiA and responds to the quorum sensing (QS) signaling compounds N-acylhomoserine lactones (AHLs). For this purpose, sdiA was targeted in both S. enterica wild type (WT) and the ΔssaV mutant strain, where SsaV has been reported to be an essential component of SPI2-T3SS. The impact of sdiA mutation on S. enterica virulence was evaluated at both early invasion and later intracellular replication in both the presence and absence of AHL. Additionally, the influence of sdiA mutation on the pathogenesis S. enterica WT and mutants was investigated in vivo, using mice infection model. Finally, the minimum inhibitory concentrations (MICs) of various antibiotics against S. enterica strains were determined. Present findings show that mutation in sdiA significantly affects S. enterica biofilm formation, cell adhesion and invasion. However, sdiA mutation did not affect bacterial intracellular survival. Moreover, in vivo bacterial pathogenesis was markedly lowered in S. enterica ΔsdiA in comparison with the wild-type strain. Significantly, double-mutant sdiA and ssaV attenuated the S. enterica virulence and in vivo pathogenesis. Moreover, mutations in selected genes increased Salmonella susceptibility to tested antibiotics, as revealed by determining the MICs and MBICs of these antibiotics. Altogether, current results clearly highlight the importance of the CRISPR-Cas9 system as a bacterial genome editing tool and the valuable role of SdiA in S. enterica virulence. The present findings extend the understanding of virulence regulation and host pathogenesis of Salmonella enterica.
1. Introduction
Salmonella enterica are facultative anaerobic intracellular Gram-negative non-lactose fermenting motile bacteria that belong to the family Enterobacteriaceae. S. enterica infections greatly vary from a mild gastroenteritis, caused mostly by S. enterica serovars Typhimurium (S. Typhimurium) and Enteritidis (S. Enteritidis), to serious systemic infections of typhoid fever caused by S. enterica serovar Typhi (S. Typhi) or Paratyphi (S. Paratyphi) [1]. The encoding genes for numerous significant virulence factors of S. enterica are arranged in specific loci called Salmonella Pathogenicity Islands (SPI) [2]. Salmonella deploys intricate virulence factors named type III secretion systems (TTSS), which mediate distinct functions [3]. Two major SPI encode TTSS to translocate Salmonella effectors in different phases of pathogenesis [4]. SPI1-TTSS translocates TTSS effector proteins into host cell cytoplasm during early stages of invasion, while SPI2-TTSS translocates TTSS effector proteins responsible for bacterial intracellular survival at later stages [2,5]. Previous studies described the role of the SPI2-T3SS machinery component SsaV and its importance for the secretion of most T3SS effectors [6,7,8]. Importantly, mutations in ssaV could lead to a significant reduction in S. enterica virulence through decreasing the translocation of SPI2-effector proteins, as this decrease affects the ability of S. enterica to survive intracellularly [7].
Quorum sensing (QS) is a way that bacteria use autoinducer (AI) molecules, such as N-acylhomoserine lactones (AHLs), for cell-to-cell communication, which plays a crucial role in bacterial virulence [9,10,11]. It has been shown that S. enterica contains at least two types of QS systems, one is induced by acylhomoserine lactone (AHL) and the other is induced by autoinducer-2 (AI-2) [12]. S. enterica employs QS to enhance bacterial virulence and pathogenesis through regulation of biofilm formation, virulence factors’ production and swarming motility [13,14]. S. enterica does not encode an AHL synthase, but it encodes SdiA, a LuxR homolog, which detects AHLs. A variety of AHL molecules with different acyl chain length and substituents at the C-3 position have been reported to mediate QS [15]. SdiA detects solely AHLs produced by other bacterial species and therefore plays a significant role in QS [16,17].
Recently, S. enterica was used as a carrier to deliver heterologous antigen fusions to stimulate both humoral and cellular immune responses [1,18]. S. enterica was engineered to be a candidate for bacteria-mediated tumor therapy [19,20]. The approved safety of S. enterica mutants, as well as other factors, makes these bacteria a promising carrier for vaccination against both bacterial, viral infections and cancer, as well [1]. Editing of S. enterica chromosome is essential in order to develop new mutant strains which can be used efficiently as carriers for vaccination purposes or used themselves as vaccines [1,19]. Interestingly, affordable and efficient genome editing tools have been developed recently in order to engineer both eukaryotic and prokaryotic organisms.
The CRISPR RNA guided endonuclease is a promising and efficient genome editing tool [21,22]. The CRISPR-Cas system was discovered as a naturally occurring adaptive microbial immune system against invading viruses and other mobile genetic elements [23,24]. Importantly, the CRISPR-Cas9 system was successfully used in targeting the genome of both bacterial [25,26,27,28] and eukaryotic cells [29,30,31]. Mutagenesis introduces a selection marker in the edited locus or requires a process of two steps that includes a counter system for selection [32]. Genome editing tools, such as zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALENs) and homing meganucleases, have been programmed to cut genomes in specific locations. However, these engineering techniques have been reported to be difficult to use and expensive [25,33].
The CRISPR loci consist of a repeated array of short sequences separated by short spacer sequences; these spacer sequences are complementary to genomes of invading viruses, as well as bacterial and archaeal plasmids [34,35,36,37]. The CRISPR-Cas immunity system occurs in three stages: First, Cas proteins integrate short sequences of invading DNA into CRISPR array as a new spacers [38]. Second, as a consequence, the CRISPR array will be transcribed and processed to produce small CRISPR RNAs (crRNAs) that contain a spacer sequence. Finally, crRNAs in association with Cas nucleases target the spacer sequence, leading to its cleavage resulting in destruction of invader’s DNA [23,24,39]. There are three major types of prokaryotic CRISPR immune that are grouped according to operon organization and cas gene conservation [39]: The type II CRISPR-Cas system is characterized by RNA-guided Cas9 endonuclease activity. It is the simplest of all Cas systems to be used to interfere or even edit both eukaryotic or prokaryotic genomes [31,40,41]. The Cas9 endonuclease activity requires guide sequence (crRNA) to guarantee precise targeting, as well as an immediate downstream motif sequence (PAM). In order to edit the bacterial genome, it is necessary to transfer a vector encoding Cas9 and its guide and recombination template containing the desired mutation [25]. The spacer or PAM sequences must be altered in order to prevent re-cleavage of Cas9 of target genome. This approach has been efficiently used to manipulate several bacterial species [25,26,42].
The current study investigated the effect of sdiA mutation on S. enterica pathogenesis. The virulence of both S. enterica wild type (WT) and ΔssaV mutant is evaluated herein. The S. enterica ΔssaV mutant has been studied as a carrier for vaccination [32,43,44]. In addition, S. enterica chromosome was edited by using an efficient CRISPR-Cas9 system. Moreover, sdiA, which plays an essential role in QS, was targeted in two sites, using Cas9 encoding plasmids in both S. enterica WT and ssaV mutants. This study aimed to elucidate how much the mutation in sdiA and ssaV separately, as well as double mutation, would affect Salmonella virulence. The influence of sdiA mutation on the pathogenesis of both S. enterica WT and ΔssaV mutants in early stages of invasion and intracellular survival are characterized. Finally, the effect of sdiA mutation on biofilm formation, susceptibility to antibiotics and in vivo pathogenesis are characterized.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids Enzymes, Media and Chemicals
S. enterica serovar Typhimurium NCTC 12023, and the S. Typhimurium ΔssaV mutant were kindly provided by Hensel’s lab (Germany). Plasmids pCRISPR and pCas9 were obtained from Addgene (http://www.addgene.org/, accessed on 12 May 2021) with No. 42875 and 42876, respectively [25]. Plasmids were introduced into S. enterica strains by electroporation, and recombinant strains were cultured in medium containing kanamycin (50 µg/mL), or chloramphenicol (25 µg/mL). All enzymes used to clone CRISPR plasmids and restriction endonuclease were provided from New England Biolabs, USA. Tryptone soy broth (TSB), Tryptic Soy Agar (TSA), Mueller Hinton (MH) broth and agar and Luria–Bertani (LB) broth and agar were purchased from Oxoid (Hampshire, UK). Dulbecco′s Modified Eagle′s Medium (DMEM) medium was obtained from Sigma-Aldrich (St. Louis, MO, USA). The used N-acylhomoserine lactones is N-hexanoyl-DL-homoserine lactone (CAS Number: 106983-28-2) was ordered from Sigma-Aldrich (St. Louis, MO, USA). All used chemicals were of pharmaceutical grade.
2.2. Targeting sdiA by CRISPR/Cas9
Two plasmids were employed: the first plasmid, pCas9, encodes the Cas9, trcrRNA and crRNA to target guide sequence number 1. The other plasmid, pCRISPR, encodes crRNA for guide sequence number 2 to be targeted by Cas9. It was shown that mutation induction can be facilitated by the co-selection of transformable cells and use of dual-RNA:Cas9 cleavage to induce a small induction of recombination at the target locus. Both plasmids pCas9 and pCRISPR were transformed to competent cells, followed by selection on kanamycin and chloramphenicol-containing LB [25].
The guide sequences shown in Figure 1 were chosen for targeting sdiA, using a CRISPER/Cas system. Plasmids pCas9 and pCRISPR were digested by BsaI restriction endonuclease, and digested plasmids were gel-purified. The protocol provided by Addgene was followed to clone a spacer sequence into pCas9 and pCRISPR. Briefly, a spacer sequence of 20 bp was chosen upstream to NGG to be targeted by Cas9 nuclease and was designed with BsaI restriction cut site ends to be ligated directly to BsaI-digested pCas9 and pCRISPR. Oligonucleotides used for plasmids construction are listed in (Table 1). Oligo nucleotides I and II were designed to target the first site, and Oligos III and IV were designed to target the second site (Table 1). The Oligo nucleotides ordered to by synthesized from Sigma Custom DNA Oligos (St. Louis, MO, USA). Oligos I, II, III and IV were phosphorylated using T4 PNK enzyme. Phosphorylated oligo I was annealed to oligo II, and oligo III was annealed to oligo IV in 1M NaCl at 95 °C for 5 min and slowly cooled down to room temperature. Diluted annealed oligos I and II were ligated to BsaI-digested pCas9 plasmid, and the diluted annealed oligos III and IV were ligated to BsaI-digested pCRISPR plasmid. The ligated plasmids to spacer sequences were electroporated sequentially to S. enterica WT and ∆ssaV mutant competent cells. The transformed cells were grown at 37 °C for 1 h in LB broth containing kanamycin (50 µg/mL) and chloramphenicol (25 µg/mL). Then 100 µL was spread over LB agar containing kanamycin (50 µg/mL) and chloramphenicol (25 µg/mL) and incubated overnight at 37 °C to select the proper clones that harbor the plasmids carrying resistant genes to these antibiotics. For confirmation of proper cloning, the negative colony PCR clones, using oligo I or oligo III, and sdiA-Rev primer were chosen. PCR products were visualized by electrophoresis on agarose gel (0.7%), using 1X TAE (Tris-acetate-EDTA) running buffer at 80–120V, and visualized by 0.5 g/mL ethidium bromide.
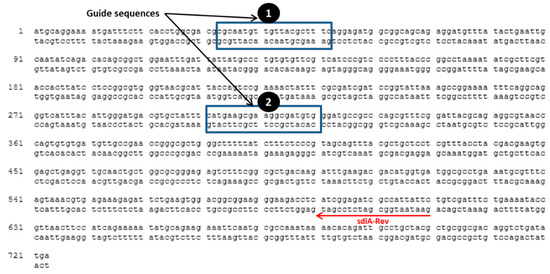
Figure 1.
Guide sequences in S. enterica serovar Typhimurium NCTC 12023 sdiA gene (Gene ID, 1253471; NCBI Reference Sequence, NC_003197.2 (2039655..2040395). Oligo I and II were designed and annealed to target the first guide sequence; oligo III and IV were designed and annealed to target the second sequence site. For confirmation of the mutation in the selected sites, colony PCR was performed by using sdiA-Rev and oligo I and III as reverse and forward primers to confirm mutation in first site and second site, respectively.

Table 1.
Oligonucleotides used in this study.
2.3. Adhesion Assay
Overnight cultures of S. enterica serovar Typhimurium (S. Typhimurium) WT and ∆ssaV mutant with or without sdiA targeted CRISPR-Cas9 (ΔsdiA) strains were prepared, diluted with fresh TSB and adjusted to a cell density of 1 × 106 CFU/mL (OD600 = 0.4) for adhesion assay, as previously described [45].
2.3.1. Adhesion to Epithelial Cells
Monolayers of HeLa cells were cultured in 24-well plates in DMEM medium [46,47]. HeLa cells were passaged with 70% confluent and washed with sterile PBS before adhesion assay. Bacterial cultures S. Typhimurium WT, ∆ssaV, ΔsdiA and ∆ssaVΔsdiA (1 × 106 CFU/mL) and DMEM with or without N-hexanoyl-DL-homoserine lactone (AHL) in final concentration 0.001 µM were added to wells. Incubation was continued for 1 h at 37 °C. Next, epithelial cells were washed 3 times with PBS and lysed at room temperature for 20 min in Triton X-100 (1%). The bacterial suspensions were serially diluted, plated on TSA and incubated overnight at 37 °C for colony counting. The bacterial counts were used to evaluate adhesion rate. Experiment was performed in triplicate, and the means and standard deviations were calculated.
2.3.2. Adhesion to Abiotic Surface and Biofilm Formation
S. Typhimurium strains; WT, ∆ssaV, ΔsdiA and ∆ssaVΔsdiA were cultured with or without N-hexanoyl-DL-homoserine lactone AHL (0.001 µM) in polystyrene microtiter plate and incubated at 37 °C either for 1 h (for evaluation of adhesion) or for 24 h (for evaluation of biofilm formation) [45,47,48,49]. Incubated plates were washed gently 3 times with phosphate buffer saline (PBS), fixed at 60 °C for 25 min, stained with crystal violet (0.1%) for 15 min and finally washed with PBS. The adhered crystal violet was extracted with ethanol, and optical densities were measured at 590 nm. The assay was repeated in triplicate, and results were expressed as the means ± standard deviations.
2.4. Invasion Assay and Intracellular Replication
Internalization of S. Typhimurium strains within different cell lines was evaluated by using the gentamicin protection assay, as formerly described [50]. Briefly, 24-wells polystyrene plates were seeded with HeLa cells and/or RAW264.7 at cell density of 5 × 105 and 2 × 105 cells/well, respectively. Tested strains WT, ∆ssaV, ΔsdiA and ∆ssaVΔsdiA were subcultured from overnight cultures and incubated at 37 °C for 4 h to induce SPI1 conditions. A master-mix of the inoculum (1 × 105 bacteria/well) multiplicity of infection (MOI 1) for HeLa cell infection or raw macrophage was prepared in DMEM, and 300 μL was added to each well. The bacterial infections were performed in either in the absence or presence of N-hexanoyl-DL-homoserine lactone AHL (0.001 µM). Non-internalized bacteria were washed out with pre-warmed PBS after 30 min, and the adhered extracellular bacteria were killed by incubation in media containing gentamicin (100 µg/mL) for 1 h. For invasion assays, HeLa cells were lysed with 0.1% Triton X-100 for 10 min at 25 °C. To determine intracellular bacteria, the inoculum and the lysates were serially diluted and plated onto Mueller Hinton (MH) plates. The percentage of invading Salmonella (1 h against inoculum × 100) was calculated. To assay the intracellular replication, the infected cells were washed with PBS and lysed with TritonX-100 (0.1%) for 10 min in 25 °C at 2 and 16 h post-infection. The inoculum and the lysates were serially diluted and plated onto MH plates. The phagocytosed cells numbers/relative untaken cells (2 h against inoculum × 100) and x-fold intracellular replication (16 h against 2 h) were evaluated.
2.5. The Intracellular Behavior of Salmonella Mutants
2.5.1. Construction of SPI2 Expressing Plasmid
For testing the effectiveness of SPI2-T3SS-dependent translocation, pWSK9 PsseJsseJ::hSurvivin::HA plasmid was generated as previously described [18]. The hSurvivin gene was PCR-amplified by employing primers hSurvivin-HA-Rev-XbaI and hSurv-For-EcoRV, and template plasmid pWSK29 PsseAsscBsseF::hSurvivin::HA (provided kindly by Prof. Hensel, University of Osanabrueck, Germany). The obtained hSurvivin and pWSK29 plasmid were digested with XbaI and EcoRV and ligated together. The sseJ gene was PCR-amplified by using SseJ-Rev-EcoRV and SseJ-Pro-For-KpnI primers prior to its digestion with KpnI and EcoRV. The sseJ gene and pWSK29::hSurvivin were digested with KpnI and EcoRV and ligated to obtain plasmids pWSK29 PsseJsseJ::hSurvivin::HA. Constructed plasmid was electroporated in S. Typhimurium strains WT, ∆ssaV, ΔsdiA and ∆ssaVΔsdiA component cells. Positive clones were selected on LB containing carbenicillin (50 µg/mL). Obtained plasmid was confirmed by colony PCR and diagnostic digestion, and they were sequenced by using T7-Seq and T3-Seq primers [18].
2.5.2. Evaluation of SPI2 Effectors Expression
Plasmid pWsk29 PsseJsseJ::hSurvivin was transferred to WT, ∆ssaV, ΔsdiA and ∆ssaVΔsdiA strains. Tested mutants and expression rates were analyzed as described before [18]. Briefly, tested strains harboring plasmid expressing SPI2 effector protein SseJ-hSurvivin tagged with HA regulated by PsseJ promoter were cultured in SPI2-inducing minimal media (PCN-P, pH 5.8). Bacterial cells were collected by centrifugation after 6 h. Equal amounts of bacterial cells were lysed and exposed to SDS-PAGE. Western blots were used to detect HA epitope tag, using fluorescent-labeled secondary antibodies. The signal intensities were measured by using the Odyssey system (Li-Cor) in comparison to control DnaK (cytosolic heat shock protein). The experiment was performed in triplicate, and ratios of HA/DnaK signals were calculated and expressed as means ± standard deviation.
2.5.3. Evaluation of Translocation Efficiency
S. enterica WT, ∆ssaV, ΔsdiA and ∆ssaVΔsdiA provided with constructed plasmid for the expression of HA tagged SPI2 effector were used to infect raw macrophage or HeLa cells in absence or presence of AHL at MOI of 100, as described previously [18]. Briefly, cells were fixed at 16 h after infection; Salmonella LPS (rabbit anti-Salmonella O1,4,5, Difco, BD) and the HA epitope tag (Roche, Basel, Switzerland) were immuno-stained. The cells were analyzed by microscopy, using a Leica laser-scanning confocal microscope. The fluorescence intensities of tagged protein were detected by J-image program in HeLa cells and macrophages. Infected cells harboring similar number of intracellular bacteria were chosen, and the signal of fluorescence intensities for HA tagged proteins were measured. The mean signal intensities and standard deviations were calculated for at least 30 infected cells per tested strains.
2.6. The Effect on Mutation on Bacterial Susceptibility to Antibiotics
The effect of mutation on susceptibility of tested strains to different antibiotics was characterized by determining both the minimum inhibitory concentrations (MICs) and minimum biofilm inhibitory concentrations (MBICs) of tested antibiotics. These antibiotics include ampicillin, ampicillin/sulbactam, amoxicillin/clavulanic acid, piperacillin, azetronam, imipenem, cephardine, ceftazidime, cefotaxime, cefepime, ciprofloxacin, levofloxacin, gatifloxacin, tobramycin, gentamycin, tetracycline, chloramphenicol and trimethoprim/sulfamethoxazole. The broth microdilution method was employed according to Clinical Laboratory and Standards Institute Guidelines (CLSI, 2015) to determine the MICs of tested strains to different antibiotics [51,52]. MBICs are determined by broth dilution method as described earlier [47,53]. Briefly, the optical densities of overnight cultures from tested strains were adjusted equivalent to 0.5 McFarland standard. Aliquots (100 μL) of the cultures were transferred to the wells of microtiter plates and incubated overnight at 37 °C. The plates were washed with PBS and dried, and serial dilutions of antibiotics in MH broth were added to wells containing adhered biofilms. After overnight incubation at 37 °C, MBICs were considered as the lowest concentrations of antibiotics that showed no visible growth in the wells. Both positive control (inoculated bacteria in broth without addition of antibiotics) and negative control (sterile broth without bacteria) were included in the experiment. The antibiotics susceptibility experiment was repeated in triplicate.
2.7. In Vivo Assessment of the Pathogenesis of Tested Mutants
The influence of sdiA mutation on S. enterica pathogenesis was characterized in vivo in mice by using the protective assay, as described previously [10,54,55]. Briefly, the cell densities of tested strains overnight cultures were adjusted to approximately 1 × 108 CFU/mL in LB broth. Six groups of female albino mice with similar weights were included in the assay, each containing ten mice. The first and second groups were used as negative controls, where mice were intraperitoneally injected with 100 μL PBS or kept uninoculated. Mice in the third group were injected intraperitoneally with 100 µL of S. Typhimurium WT strain. Mice in the fourth, fifth and sixth groups were injected with 100 µL of S. Typhimurium ∆ssaV, ΔsdiA or ∆ssaVΔsdiA strains, respectively. Mice in all groups were kept at room temperature, with normal feeding and aeration. Mice survival in each group was recorded daily over 5 successive days and plotted by using the Kaplan–Meier method, and significance (* p < 0.05) was calculated by using Log-rank test, GraphPad Prism 5.
2.8. Statistical Analysis
Assays were performed in triplicate, and data are presented as median and range, unless otherwise specified. The differences between S. enterica WT and mutant strains were analyzed by a t-test, using the GraphPad Prism 5 software. A two-tailed p-value < 0.05 was considered statistically significant.
3. Results
3.1. CRISPR/Cas9 System Targets sdiA
S. enterica sdiA was targeted by a CRISPR/Cas9 system. Two guide sequences were chosen carefully to be targeted in order to achieve more efficient interference with S. enterica sdiA. Positive clones were selected on LB containing kanamycin and chloramphenicol. In spite of large number of escapers, colony PCR using oligo I or oligo III and sdiA-Rev primers was performed (Figure 2), and negative clones were selected and further tested.
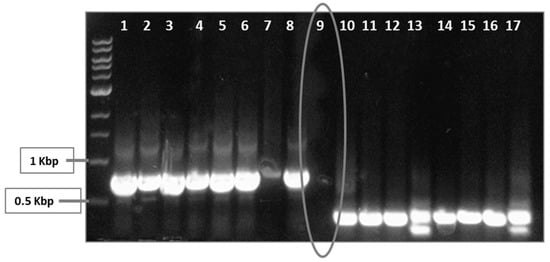
Figure 2.
Screening for positive and negative clones, using PCR. Transformed S. enterica cells were selected on LB-kanamycin/chloramphenicol plates. Two plasmids were employed herein: The first plasmid, pCas9, encodes the Cas9, trcrRNA and crRNA to target guide sequence number 1. The other plasmid, pCRISPR, encodes crRNA for guide sequence number 2 to be targeted by Cas9. Both plasmids pCas9 and pCRISPR were transformed followed by selection on kanamycin and chloramphenicol-containing LB. However, there were background cells that lack the desired mutation. Colony PCR was performed by targeting both guide sequence 1, using oligo I and sdiA-Rev (wells 1–8), and guide sequence 2, using oligo III and sdiA-Rev (wells 10–17). Positive PCR clones were omitted, while negative ones (encircled well no. 9) were selected.
3.2. Functional Testing of S. enterica ∆sdiA
It has been shown that S. enterica SdiA detects and responds to AHL signals produced by other microbial species [56,57]. The role of SdiA in adhesion [58] and biofilm formation [59] was further characterized. To test the success of targeting S. enterica sdiA by CRISPR/Cas9 system, both the adhesion and biofilm-formation capabilities of Salmonella ΔsdiA were evaluated in comparison with both S. enterica WT and ∆ssaV strains. Bacterial adhesion to epithelial HeLa cells was performed in both the presence and absence of AHL (Figure 3). S. enterica WT, ∆ssaV and ΔsdiA strains did not exhibit adherence capability to epithelial cells in the absence of AHL. However, the adherence capacity of tested strains significantly increased in the presence of AHL (p < 0.0001). In the presence of AHL, the number of adhering S. enterica ΔsdiA cells was significantly lower than S. enterica WT and ΔssaV (p < 0.0001). Bacterial adhesion to epithelial cells was not affected by ssaV mutation, and the number of adhering cells was not affected in presence of AHL (p = 0.085).
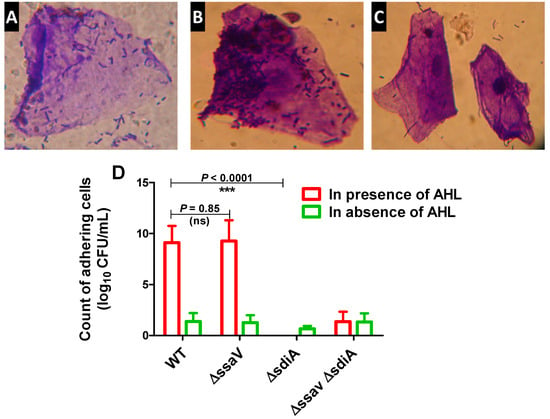
Figure 3.
Adhesion of S. enterica strains to HeLa cells. HeLa cells were co-cultured with S. enterica WT, ΔssaV, ΔsdiA or ΔsdiA ΔssaV both in the presence and absence of AHL for 1 h at 37 °C. Microscopic examination of crystal violet-stained adhering S. enterica to HeLa cells either in presence or absence of AHL. (A) S. enterica WT adhesion in absence of AHL, (B) Salmonella enterica WT adhesion in presence of AHL and (C) S. enterica ΔsdiA mutant adhesion in presence of AHL. (D) AHL significantly increases adhesion of S. enterica WT and ΔssaV but not ΔsdiA and ΔsdiA ΔssaV mutants to Hela cells. Epithelial cells were lysed with Triton X-100 (1%). Adhering bacteria were serially diluted and counted on agar plates. Experiment was performed in triplicate, and results are represented as means ± standard deviations; p-value < 0.05 was considered statistically significant, using a Student’s t-test (*** = p < 0.001).
Moreover, adhesion to abiotic surface and biofilm formation of S. enterica WT, ΔssaV and ΔsdiA strains were tested both in the presence and absence of AHL (Figure 4). S. enterica WT, ΔssaV, ΔsdiA and ΔssaVΔsdiA strains were cultured with or without AHL in polystyrene microtiter plate and incubated either for 1 h (for evaluation of adhesion) or for 24 h (for evaluation of biofilm formation). Importantly, AHL significantly increased the adhesion and biofilm formation of both S. enterica WT and ΔssaV. Moreover, the adhesion and biofilm formation of S. enterica were significantly reduced in S. enterica ΔsdiA, as compared with WT and ΔssaV strains both in the presence and absence of AHL (p < 0.0001). The current results demonstrate that S. enterica adhesion to epithelial cells was not affected by mutation in ssaV. Furthermore, bacterial adhesion to abiotic surface and biofilm formation were not influenced by single mutation in ssaV.
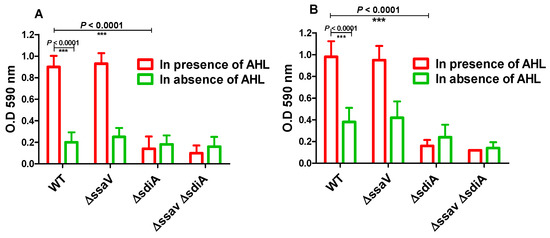
Figure 4.
Bacterial adhesion to abiotic surface and biofilm formation. S. enterica WT, ΔssaV, ΔsdiA and ΔssaVΔsdiA were cultured in presence or absence of AHL in polystyrene microtiter plate and incubated at 37 °C, either for 1 h (for evaluation of adhesion) or for 24 h (for evaluation of biofilm formation). (A) Adhering cells or (B) Biofilm forming cells were stained by crystal violet, ethanol was added and optical density was measured at 590 nm. Assays were performed in triplicate, and results were expressed as means ± standard deviations; p-value < 0.05 was considered statistically significant, using a two-tailed t-test (*** = p < 0.001).
3.3. Intercellular Survival of S. enterica ΔsdiA
S.enterica WT, ΔssaV, ΔsdiA and ΔssaVΔsdiA strains were cultured in SPI1-inducing conditions, and bacterial internalization within HeLa cells or macrophage was assessed by using the gentamicin protection assay. For invasion assays, Hela cells were washed and lysed after 1 h infection with 0.1% Triton X-100 (Figure 5A). The quorum sensing mediator AHL did not increase the invasiveness of Salmonella strains. Interference with sdiA did not affect bacterial invasiveness either in the absence or presence of AHL. However, AHL did not increase invasiveness of tested strains; the invasiveness of sdiA mutant was significantly reduced in comparison to the WT or ssaV mutant strain. On the other side, ssaV mutation did not influence bacterial invasiveness, as compared to S. enterica WT or sdiA mutant. For intracellular replication assays, bacteria-infected cells were washed and then lysed with 0.1% Triton-X-100 at 2 and 16 h post-infection (Figure 5B), and intracellular bacteria were counted. Interestingly, AHL did not enhance the invasion of Salmonella strains in HeLa cells or bacterial uptake by macrophage. Obviously, ssaV mutation significantly decreased the intercellular bacterial replication as compared to the WT and sdiA mutant (p = 0.0062 and 0.0094; respectively). Moreover, sdiA mutation did not increase Salmonella intracellular replication within raw macrophage, as compared to Salmonella WT (p = 0.44).
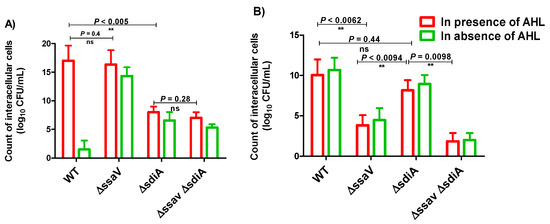
Figure 5.
Intercellular survival of S. enterica strains in HeLa cells and raw macrophages. S. enterica WT, ΔssaV, ΔsdiA and ΔssaVΔsdiA strains were cultured in suitable conditions to induce SPI1 genes. Bacterial strains were used to infect HeLa cells or raw macrophages in multiplicity of infection (MOI of 1). Non-internalized bacteria were removed by washing with PBS, and remaining extracellular bacteria were killed by using gentamicin (100 µg/mL). (A) Invasion assays; Hela cells were lysed with 0.1% Triton X-100 after 1 h infection, and intracellular bacteria were counted. The number of invading bacteria (1 h versus inoculum) was calculated. (B) Intracellular replication assays; infected cells were lysed with 0.1% Triton-X-100, and intracellular bacteria were counted at 2 and 16 h post-infection. Assays were performed in triplicate, and results were expressed as means ± standard deviations; p-value < 0.05 was considered statistically significant, using a two-tailed t-test (**= p < 0.01).
3.4. Assessment the Expression of SPI2 Effectors S. enterica Strains
To evaluate the capability of tested strains to cope the drastic conditions inside the Salmonella containing vacuole (SCV) and survive in order to induce efficient immunologic response, the delivery of SPI2-effector proteins from SCV to outside by live attenuated Salmonella mutants (ΔssaV and/or ΔsdiA) was used as indicator. Expression cassettes that contain sseJ promoter were constructed with genes encoding SPI2 effector. They were used to express SPI2-T3SS translocated effector proteins SseJ tagged with HA (Figure 6A). In vitro culture conditions were used to induce both the expression of SsrAB regulon and synthesis of SPI2 effector proteins. The synthesis of SPI2-effector fusion protein tagged with HA was quantified (Figure 6B). Western blotting was employed to quantify the amounts of recombinant protein, using the Odyssey detection system and DnaK as control protein. Importantly, the expression level of recombinant protein was significantly reduced in S. enterica ΔssaV and ΔsdiA mutants relative to WT.
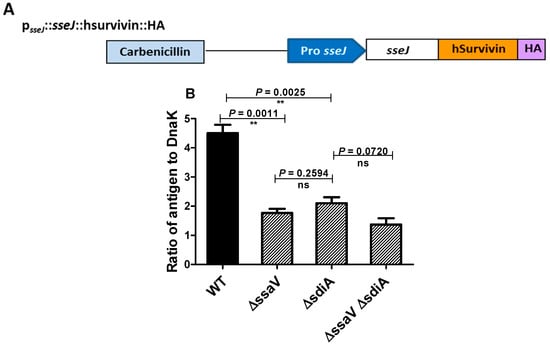
Figure 6.
Expression of SPI2 effectors. (A) Plasmid-encoding SPI2 effector tagged with HA was constructed in order to test the efficacy of SPI2-TTSS-dependent translocation. (B) Expression of translocated proteins was evaluated as ratios of the HA to DnaK signals (**= p < 0.01).
The efficiency of the SPI2-T3SS-dependent translocation in ΔsdiA strain was investigated herein. Salmonella tested strains harboring the constructed plasmid were used to infect HeLa cells or macrophages (in presence of AHL) and then were processed for immunostaining, and the fluorescence intensities of tagged protein were measured (Figure 7A,B). The translocated proteins were significantly reduced in S. enterica ∆ssaV and/or sdiA mutants, as compared to WT. Furthermore, the SPI2 effector translocation was significantly reduced in ΔssaV or ΔssaVΔsdiA strains when compared to ΔsdiA strains. There was no difference in the SPI2-effector translocation efficacy between S. enterica ΔssaV and ΔssaVΔsdiA (Figure 7C,D).
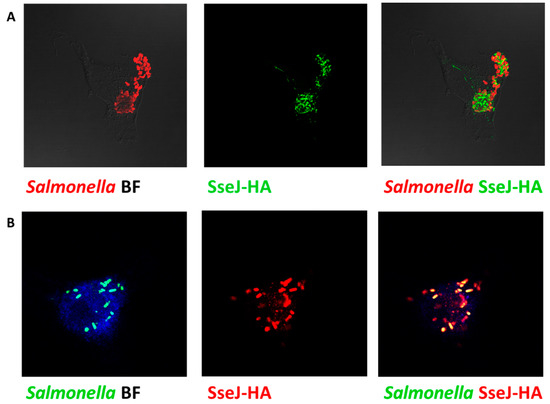
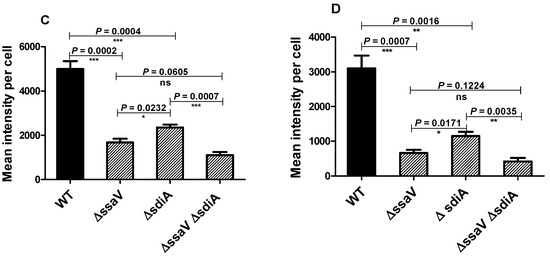
Figure 7.
Assessment of SPI2-TTSS translocation of effector proteins. Translocation of SPI2 effectors into the cytoplasm of infected HeLa cells or raw macrophage cells (in presence of AHL) with equal number of S. enterica WT, ΔssaV, ΔsdiA or ΔssaVΔsdiA harboring constructed plasmid was evaluated. Salmonella Lipopolysaccharide (rabbit anti-Salmonella O antigen) and HA epitope tag were immuno-stained and analyzed by Leica laser-scanning confocal microscope both in (A) HeLa cells and(B) macrophages. Fluorescence intensities of tagged protein were measured by using J-image program both in (C) HeLa cells and (D) macrophages. (*** = p < 0.001; **= p < 0.01; * p < 0.05).
3.5. MICs and MBICs of S. enterica Mutant Strains
The influence of the mutations on S. enterica resistance to antibiotics was investigated herein. The MICs and MBICs of tested antibiotics were determined by the broth microdilution method, and the results are represented in Table 2. It is shown that the MICs and MBIC were markedly decreased in S. enterica ΔssaV, ΔsdiA and ΔssaVΔsdiA mutants in comparison to WT. This indicates that the mutation in ssaV and/or sdiA genes may increase the susceptibility and decrease the resistance to tested antibiotics.

Table 2.
MICs and MBICs (µg/mL) of tested antibiotics against S. Typhimurium strains *.
3.6. Mutation in sdiA and/or ssaV Genes Decreases S. enterica Virulence In Vivo
The impact of mutation on S. enterica virulence was evaluated by using mice infection models. All mice in the negative control groups survived. Similarly, all mice survived in the groups injected with S. enterica ΔssaV, ΔsdiA and ΔssaVΔsdiA. On the other side, only five mice out 10 survived in the mice group injected with S. enterica WT. The mice survival was observed over five days and plotted by the Kaplan–Meier method, where significance (p < 0.05) was assessed by using the Log-rank test (Figure 8). These findings obviously show that the sdiaA and/or ssaV mutations markedly decreased the capacity of S. enterica to kill mice (p = 0.0069).

Figure 8.
Mutation of S. enterica sdiA and/or ssaV genes significantly reduced bacterial virulence in mice. Mice (n = 10 mice/group) were injected with 100 μL of bacterial cells (2 × 106 CFU/mL) of S. Typhimurium WT, ΔssaV, ΔsdiA or ΔssaVΔsdiA strains. No death was reported for mice in negative controls, either uninfected or injected with PBS. Similarly, all mice survived in groups injected with S. enterica ΔssaV, ΔsdiA and ΔssaVΔsdiA. In contrast, mice injected with S. enterica WT showed a higher mortality rate; 5 mice killed out of 10 mice (*** = p < 0.001).
4. Discussion
S. enterica is an intracellular bacteria of special interest which could be engineered to deliver heterologous antigens that induce efficient cellular and humoral immune responses [18]. For this purpose, the development of specific mutations in S. enterica chromosome is a critical requirement [1]. In this context, this study aimed to evaluate the influence of sdiA mutation on the virulence of both S. enterica WT and ssaV mutant. The present findings would be valuable and extend our knowledge about employing S. enterica as a vector for delivering antigens and stimulating immune system.
The DNA sequences’ altering possibility within the cell in a controlled fashion greatly helps understand gene function. Importantly, the CRISPR prokaryotic immunity system has led to the identification of nucleases whose sequence specificity is programmed by small RNAs [25]. The type II CRISPR-Cas system is characterized by RNA-guided Cas9 endonuclease activity. It is the simplest of all Cas systems to be used to interfere or even edit both eukaryotic and prokaryotic genomes [31,40,41].
In the current work, a CRISPR-Cas9 system approach was used to target S. enterica sdiA, achieving efficient interference with targeted genes in two different sites. The mutation induction can be facilitated by a co-selection of transformable cells and use of dual-RNA:Cas9 cleavage to induce a small induction of recombination at the target locus [25,41]. We tried to edit a Salmonella chromosome to be used as a carrier for vaccination (unpublished data). Lambda red-mediated gene replacement was used to induce specific mutations; however, it was difficult to select proper tetracycline sensitive clones. In comparison, the CRISPR-Cas9 system has the advantage of being more efficient and easier as a bacterial chromosome targeting tool. These results are comparable with those reported in other studies [27,36,37,41,60].
In order to evaluate the role of SdiA in S. enterica pathogenesis at different stages of infection, the sdiA gene was targeted as described in Materials and Methods. S. enterica adhesion to epithelial cells and abiotic surfaces was greatly enhanced in the presence of AHL. Bacterial adhesion is the first step in biofilm formation; as AHL increases bacterial adhesion, the bacterial biofilm formation increases significantly. In order to assess the influence of AHL/SdiA on early stages of S. enterica invasion, the experimental conditions were adjusted to induce SPI1 effectors. As previously mentioned, SdiA is a sensor to AHL; therefore, any mutation or interference within sdiA would impact bacterial QS. S. enterica ΔsdiA lacked the capability to adhere to epithelial cells or abiotic surfaces, and its biofilm formation diminished significantly. The decreased S. enterica biofilm formation upon sdiA mutation relative to WT could account for the lowered MICs and MBICs of tested antibiotics. These findings are in great compliance with several studies that investigated the significant role of SdiA in Salmonella adhesion [15,16,17,58].
Moreover, S. enterica ΔsdiA exhibited a significant decreased invasion capacity within HeLa cells, regardless the presence or absence of AHL. The present results meet those of an independent work in which the increased bacterial invasion was found to be sdiA-dependent [61]. In addition, SdiA is known to regulate seven genes in S. enterica upon the activation of the SdiA transcription factor by AHL. These genes are located in two different loci: the rck locus and the srgE locus [17]. The rck operon includes srgA, which encodes a disulfide bond oxidoreductase, while SrgA plays a role in folding of fimbrial subunit (PefA) that could affect adhesion [62]. The present findings clearly indicate a role of AHL-SdiA (inducer–receptor) not only in S. enterica adhesion but also in biofilm formation. It is worth mentioning that AHL presence did not enhance S. enterica WT pathogenesis; both bacterial invasion within HeLa cells and intracellular replication in raw macrophage did not increase. However, S. enterica invasion was shown to be influenced by sdiA mutation, which may lead us to ask if SdiA can be involved directly or indirectly in the SPI1-TTSS functions. Interestingly, current data show that mutation in sdiA did not affect S. enterica intracellular replication.
Furthermore, the effect of sdiA mutation on the functionality of SPI2-TTSS translocation system was explored. The translocation of HA-tagged SPI2-fusion protein in both S. enterica WT and ΔsdiA mutant was investigated herein. Cells were infected with an equal number of bacteria, and translocated proteins were quantified. Surprisingly, mutation in sdiA significantly influenced SPI2 effector translocation. That is in compliance with the fact that srgA in rck operon, which is regulated by SdiA, plays a role in folding of outer membrane component of SPI2-TTSS [63]. Moreover, the deficient adhesion and invasion may diminish the internalized bacterial cells, and, as a consequence, the expression and translocation may be reduced. SsaV is a vital component for SPI2-TT3SS machinery and essential for secretion of a lot of TTSS effectors [7]. As predicted for S. enterica ΔssaV, adherence and invasion within epithelial cells were not affected. In contrast, bacterial intracellular replication and translocation of SPI2 effectors were significantly reduced; these findings are in agreement with previous results [32,43]. For more convenience, the virulence characteristics of S. enterica ΔsdiAΔssaV were evaluated. The adhesion, invasion and intercellular replication of the double mutant ΔsdiAΔssaV were greatly diminished, regardless of the presence or absence of AHL. Importantly, double mutation in sdiA and ssaV genes confers a significate protection to the infected mice.
Attenuated S. enterica has been used as a carrier for heterologous antigens, activating both humoral and cellular responses. Previous studies showed that SPI2-T3SS-deficient S. enterica was weakened enough and could provide protection from further challenges with WT and induces the production of both secretory IgA and serum IgG against somatic O-antigen in C57BL/6mice [19]. Moreover, S. enterica mutants in ssaV or any of SPI1-TTSS genes has been efficiently used in preparation of vaccines against typhoid fever [64] or to induce chemokines [65]. On the other hand, S. enterica ΔssaV was found to be virulent in immunocompromised C57BL/6 mice [66]. In this study, we showed the ability of tested mutants, especially S. enterica ΔsdiAΔssaV, to confer a significant mitigation of S. enterica pathogenesis, in comparison with WT or ssaV mutant strains. Thus, we need further investigations to evaluate the possibility of using this mutant as a vaccine itself or as a suitable attenuated carrier for heterologous antigens. Targeting bacterial virulence may ease the eradication of virulent bacteria by the host’s immune system [5,67].
5. Conclusions
In the current study, a CRISPR-Cas9 system was employed to target bacterial chromosome efficiently. Investigating the virulence characteristics of S. enterica ΔsdiAΔssaV demonstrates that ssaV mutation did not influence either adherence or invasion of the S. enterica ΔsdiA strain. Similarly, sdiA mutation did not affect the intracellular behavior of ΔssaV strain. These findings could suggest that these two virulence machineries work apart from each other, indicating that S. enterica ΔsdiAΔssaV requires more in vivo examination to evaluate its capability to be used as vaccine or carrier for vaccination. SdiA plays a key role in QS as a sensor to signaling AHL. Mutation of sdiA significantly affects bacterial adhesion and invasion, as well as biofilm formation. Current data clearly suggest that any agent that reduces SdiA transcription could be used as an antibiofilm agent that could help us control S. enterica infection.
Author Contributions
Conceptualization, M.A. and W.A.H.H.; methodology, M.A. and W.A.H.H.; software, A.S.A.L. and E.-S.K.; validation, K.A. and F.A.; formal analysis, A.S.A.L. and E.-S.K.; investigation, T.S.I. and A.J.A. resources, A.S.A.L. and E.-S.K.; data curation, M.A.; T.S.I. and A.J.A. writing—original draft preparation, M.A. and W.A.H.H.; writing—review and editing, M.A. and W.A.H.H.; visualization, M.A. and W.A.H.H.; supervision, W.A.H.H.; project administration, W.A.H.H.; funding acquisition, K.A. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia under grant no. RG-7-166-42.
Institutional Review Board Statement
This study did not comprise any studies with human participants. The institutional Review Board (ethical committee) at the Faculty of Pharmacy, Zagazig University approved animal experiments in this study. The procedures were performed in compliance with the ARRIVE guidelines, in compliance with the UK Animals (Scientific Procedures) Act, 1986, and related guidelines (ECAHZU, 23 August 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia funded this project under grant no. RG-7-166-42. The authors, therefore, gratefully acknowledge DSR technical and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hegazy, W.A.H.; Hensel, M. Salmonella enterica as a vaccine carrier. Futur. Microbiol. 2012, 7, 111–127. [Google Scholar] [CrossRef]
- Haraga, A.; Ohlson, M.B.; Miller, S.I. Salmonellae interplay with host cells. Nat. Rev. Genet. 2008, 6, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P. Process of protein transport by the Type III secretion system. Microbiol. Mol. Biol. Rev. 2004, 68, 771–795. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, R.G.; Hensel, M. Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics re-sistance of Salmonella enterica. Berl. Munch. Tierarztl. Wochenschr. 2007, 120, 317–327. [Google Scholar]
- Askoura, M.; Hegazy, W.A.H. Ciprofloxacin interferes with Salmonella Typhimurium intracellular survival and host virulence through repression of Salmonella pathogenicity island-2 (SPI-2) genes expression. Pathog. Dis. 2020, 78, 78. [Google Scholar] [CrossRef] [PubMed]
- Deiwick, J.; Salcedo, S.; Boucrot, E.; Gilliland, S.M.; Henry, T.; Petermann, N.; Waterman, S.R.; Gorvel, J.-P.; Holden, D.W.; Méresse, S. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect. Immun. 2006, 74, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.H.; Hasegawa, P.; Okamoto, S.; Fierer, J.; Guiney, D.G. Identification of Salmonella SPI-2 secretion system compo-nents required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol. Med. Microbiol. 2008, 52, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Wael, A.H.H.; Hisham, A.A.; Hegazy, W.A.H.; Abbas, H.A. Evaluation of the role of SsaV Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr. J. Biotechnol. 2017, 16, 718–726. [Google Scholar] [CrossRef]
- Abbas, H.A.; Hegazy, W.A.H. Repurposing anti-diabetic drug “Sitagliptin” as a novel virulence attenuating agent in Serratia marcescens. PLoS ONE 2020, 15, e0231625. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Khayat, M.T.; Ibrahim, T.S.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Repurposing anti-diabetic drugs to cripple Quorum sensing in Pseudomonas aeruginosa. Microorganisms 2020, 8, 1285. [Google Scholar] [CrossRef] [PubMed]
- Askoura, M.; Youns, M.; Hegazy, W.A.H. Investigating the influence of iron on Campylobacter jejuni transcriptome in re-sponse to acid stress. Microb. Pathog. 2020, 138, 103777. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef]
- Jiang, T.; Li, M. Quorum sensing inhibitors: A patent review. Expert Opin. Ther. Patents 2013, 23, 867–894. [Google Scholar] [CrossRef]
- Khayyat, A.; Hegazy, W.; Shaldam, M.; Mosbah, R.; Almalki, A.; Ibrahim, T.; Khayat, M.; Khafagy, E.-S.; Soliman, W.; Abbas, H. Xylitol inhibits growth and blocks virulence in Serratia marcescens. Microorganism 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J.C.A.; Metzger, K.; Daniels, R.; Ptacek, D.; Verhoeven, T.; Habel, L.W.; Vanderleyden, J.; De Vos, D.E.; De Keersmaecker, S.C.J. Synthesis of N-Acyl Homoserine Lactone analogues reveals strong activators of SdiA, the Salmonella en-terica Serovar Typhimurium LuxR Homologue. Appl. Environ. Microbiol. 2007, 73, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Smith, J.N.; Swift, S.; Heffron, F.; Ahmer, B.M.M. SdiA of Salmonella enterica is a LuxR Homolog that detects mixed microbial communities. J. Bacteriol. 2001, 183, 5733–5742. [Google Scholar] [CrossRef]
- Smith, J.N.; Ahmer, B.M.M. Detection of other microbial species by Salmonella: Expression of the SdiA regulon. J. Bacteriol. 2003, 185, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Xu, X.; Metelitsa, L.; Hensel, M. Evaluation of Salmonella enterica Type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect. Immun. 2012, 80, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hegazy, W.; Guo, L.; Gao, X.; Courtney, A.N.; Kurbanov, S.; Liu, D.; Tian, G.; Manuel, E.; Diamond, D.; et al. Effective cancer vaccine platform based on attenuated Salmonella and a Type III secretion system. Cancer Res. 2014, 74, 6260–6270. [Google Scholar] [CrossRef]
- Theys, J.; Barbe, S.; Landuyt, W.; Nuyts, S.; Mellaert, L.; Wouters, B.; Anné, J.; Lambin, P. Tumor-specific gene delivery using genetically engineered bacteria. Curr. Gene Ther. 2003, 3, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Wiedenheft, B.; Sternberg, S.H.; Doudna, J.A. RNA-guided genetic silencing systems in bacteria and archaea. Nat. Cell Biol. 2012, 482, 331–338. [Google Scholar] [CrossRef]
- Charpentier, E.; Doudna, J.A. Rewriting a genome. Nat. Cell Biol. 2013, 495, 50–51. [Google Scholar] [CrossRef]
- Deveau, H.; Garneau, J.E.; Moineau, S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu. Rev. Microbiol. 2010, 64, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Barrangou, R. CRISPR/Cas, the immune system of bacteria and Archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, A.L. RNA-guided editing of bacterial genomes using CRISPR-Cas sys-tems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Van Pijkeren, J.-P. CRISPR–Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res. 2014, 42, e131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Seo, S.-O.; Choi, K.; Lu, T.; Jin, Y.-S.; Blaschek, H.P. Markerless chromosomal gene deletion in Clostrid-ium beijerinckii using CRISPR/Cas9 system. J. Biotechnol. 2015, 200, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chung, J.-H.; Kim, H.M.; Kim, D.-W.; Kim, H.H. Designed nucleases for targeted genome editing. Plant. Biotechnol. J. 2015, 14, 448–462. [Google Scholar] [CrossRef]
- Xie, K.; Yang, Y. RNA-guided genome editing in plants using a CRISPR–Cas system. Mol. Plant. 2013, 6, 1975–1983. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Braukmann, M.; Methner, U.; Berndt, A. Immune reaction and survivability of Salmonella Typhimurium and Salmonella Infantis after infection of primary avian macrophages. PLoS ONE 2015, 10, e0122540. [Google Scholar] [CrossRef]
- Hegazy, W.; Youns, M. TALENs construction: Slowly but surely. Asian Pac. J. Cancer Prev. 2016, 17, 3329–3334. [Google Scholar]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S.D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151, 2551–2561. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Pourcel, C.; Salvignol, G.; Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bac-teriophage DNA, and provide additional tools for evolutionary studies. Microbiology 2005, 151, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR interference limits horizontal gene transfer in Staphylococci by targeting DNA. Sci-ence 2008, 322, 1843–1845. [Google Scholar] [CrossRef]
- Heler, R.; Marraffini, L.A.; Bikard, D. Adapting to new threats: The generation of memory by CRISPR-Cas immune systems. Mol. Microbiol. 2014, 93, 1–9. [Google Scholar] [CrossRef]
- Makarova, K.S.; Haft, D.H.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Horvath, P.; Moineau, S.; Mojica, F.J.; Wolf, Y.; Ya-kunin, A.; et al. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Genet. 2011, 9, 467–477. [Google Scholar] [CrossRef]
- Gratz, S.J.; Wildonger, J.; Harrison, M.M.; O’Connor-Giles, K.M. CRISPR/Cas9-mediated genome engineering and the promise of designer flies on demand. Fly 2013, 7, 249–255. [Google Scholar] [CrossRef]
- Jiang, W.; Marraffini, L.A. CRISPR-Cas: New tools for genetic manipulations from bacterial immunity systems. Annu. Rev. Microbiol. 2015, 69, 209–228. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, G.; Jiang, W.; Hu, H.; Lu, Y. One-step high-efficiency CRISPR/Cas9-mediated genome editing in Strepto-myces. Acta Biochim. Biophys. Sin. 2015, 47, 231–243. [Google Scholar] [CrossRef]
- Fierer, J.; Okamoto, S.; Banerjee, A.; Guiney, D.G. Diarrhea and Colitis in mice require the Salmonella Pathogenicity Island 2-Encoded secretion function but Not SifA or Spv effectors. Infect. Immun. 2012, 80, 3360–3370. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Pace, L.; Lillehoj, H.; Zhang, S. Functions exerted by the virulence-associated type-three secretion systems during Salmonella enterica serovar Enteritidis invasion into and survival within chicken oviduct epithelial cells and macro-phages. Avian Pathol. 2009, 38, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Vesterlund, S.; Paltta, J.; Karp, M.; Ouwehand, A. Measurement of bacterial adhesion—In vitro evaluation of different meth-ods. J. Microbiol. Methods 2005, 60, 225–233. [Google Scholar] [CrossRef]
- Schmidt, M.; Olejnik-Schmidt, A.K.; Myszka, K.; Borkowska, M.; Grajek, W. Evaluation of quantitative PCR Measurement of bacterial colonization of Epithelial cells. Pol. J. Microbiol. 2010, 59, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, A.; Abbas, H.; Mohamed, M.; Asfour, H.; Khayat, M.; Ibrahim, T.; Youns, M.; Khafagy, E.-S.; Abu Lila, A.; Safo, M.; et al. Not only antimicrobial: Metronidazole Mitigates the virulence of Proteus mirabilis isolated from macerated diabetic foot ulcer. Appl. Sci. 2021, 11, 6847. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Al Saqr, A.; Aldawsari, M.F.; Khafagy, E.-S.; Shaldam, M.A.; Hegazy, W.A.H.; Abbas, H.A. A novel use of Allopurinol as a quorum-sensing inhibitor in Pseudomonas aeruginosa. Antibiotics 2021, 10, 1385. [Google Scholar] [CrossRef]
- Hölzer, S.U.; Hensel, M. Divergent roles of Salmonella Pathogenicity Island 2 and metabolic traits during interaction of S. en-terica Serovar Typhimurium with host cells. PLoS ONE 2012, 7, e33220. [Google Scholar] [CrossRef]
- Aldawsari, M.; Khafagy, E.-S.; Saqr, A.; Alalaiwe, A.; Abbas, H.; Shaldam, M.; Hegazy, W.; Goda, R. Tackling virulence of Pseudomonas aeruginosa by the Natural Furanone Sotolon. Antibiotics 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Khafagy, E.-S.; Alalaiwe, A.; Aldawsari, M.; Alshahrani, S.; Anwer, K.; Khan, S.; Lila, A.; Arab, H.; Hegazy, W. Synthesis of gold nanoparticles by using green machinery: Characterization and in vitro toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, A.N.; Abbas, H.A.; Khayat, M.T.; Shaldam, M.A.; Askoura, M.; Asfour, H.Z.; Khafagy, E.-S.; Abu Lila, A.S.; Allam, A.N.; Hegazy, W.A.H. Secnidazole is a promising imidazole mitigator of Serratia marcescens virulence. Microorganism 2021, 9, 2333. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.; Khayat, M.T.; Ibrahim, T.S.; Youns, M.; Mosbah, R.; Soliman, W.E. Repurposing of antidiabetics as Serratia marcescens virulence inhibitors. Braz. J. Microbiol. 2021, 52, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.H.; Awad, N.F.S.; Alshareef, W.A.; Al Omar, S.Y.; Zaitone, S.A.; El-Hamid, M.I.A. Thymol Nanoemulsion: A new therapeutic option for extensively drug resistant foodborne pathogens. Antibiotics 2020, 10, 25. [Google Scholar] [CrossRef]
- Rychlik, I.; Barrow, P.A. Salmonella stress management and its relevance to behaviour during intestinal colonisation and infec-tion. FEMS Microbiol. Rev. 2005, 29, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.J.; Ahmer, B.M. Detection of acyl-homoserine lactones by Escherichia and Salmonella. Curr. Opin. Microbiol. 2011, 14, 188–193. [Google Scholar] [CrossRef]
- Smith, J.N.; Dyszel, J.L.; Soares, J.A.; Ellermeier, C.D.; Altier, C.; Lawhon, S.D.; Adams, L.G.; Konjufca, V.; Curtiss, R.; Slauch, J.M.; et al. SdiA, an N-Acylhomoserine lactone receptor, becomes active during the transit of Salmonella enterica through the gastrointestinal tract of turtles. PLoS ONE 2008, 3, e2826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Que, F.; Liao, L.; Zhou, M.; You, L.; Zhao, Q.; Li, Y.; Niu, H.; Wu, S.; Huang, R. Study on the promotion of bacterial biofilm formation by a Salmonella Conjugative Plasmid and the Underlying mechanism. PLoS ONE 2014, 9, e109808. [Google Scholar] [CrossRef]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat. Rev. Genet. 2010, 11, 181–190. [Google Scholar] [CrossRef]
- Nesse, L.L.; Berg, K.; Vestby, L.K.; Olsaker, I.; Djønne, B. Salmonella Typhimurium invasion of HEp-2 epithelial cells in vitro is increased by N-acylhomoserine lactone quorum sensing signals. Acta Vet. Scand. 2011, 53, 44. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, C.W.; Kohli, M.; Killoran, A.; Touchie, G.A.; Kadner, R.J.; Martin, N.L. Characterization of SrgA, a Salmonella en-terica Serovar Typhimurium virulence plasmid-encoded paralogue of the Disulfide Oxidoreductase DsbA, essential for bio-genesis of plasmid-encoded fimbriae. J. Bacteriol. 2003, 185, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Okada, N.; Danbara, H. Two periplasmic disulfide oxidoreductases, DsbA and SrgA, target outer membrane protein SpiA, a component of the Salmonella Pathogenicity Island 2 Type III secretion system. J. Biol. Chem. 2004, 279, 34631–34642. [Google Scholar] [CrossRef] [PubMed]
- Pati, N.B.; Vishwakarma, V.; Selvaraj, S.K.; Dash, S.; Saha, B.; Singh, N.; Suar, M. Salmonella Typhimurium TTSS-2 deficient mig-14 mutant shows attenuation in immunocompromised mice and offers protection against wild-type Salmonella Typhi-murium infection. BMC Microbiol. 2013, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, M.Z.; Yan, L.; Lillehoj, H.; Pace, L.W.; Zhang, S. Induction of CXC Chemokine messenger-RNA expression in chicken oviduct epithelial cells by Salmonella enterica serovar Enteritidis via the type three secretion system–1. Avian Dis. 2009, 53, 396–404. [Google Scholar] [CrossRef]
- Periaswamy, B.; Maier, L.; Vishwakarma, V.; Slack, E.; Kremer, M.; Andrews-Polymenis, H.L.; McClelland, M.; Grant, A.J.; Suar, M.; Hardt, W.-D. Live attenuated S. Typhimurium vaccine with improved safety in immuno-compromised mice. PLoS ONE 2012, 7, e45433. [Google Scholar] [CrossRef]
- Aldawsari, M.; Alalaiwe, A.; Khafagy, E.-S.; Al Saqr, A.; Alshahrani, S.; Alsulays, B.; Alshehri, S.; Abu Lila, A.; Rizvi, S.D.; Hegazy, W. Efficacy of SPG-ODN 1826 Nanovehicles in inducing M1 phenotype through TLR-9 activation in murine alveolar J774A.1 cells: Plausible nano-immunotherapy for lung carcinoma. Int. J. Mol. Sci. 2021, 22, 6833. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).