Steroid Metabolism in Thermophilic Actinobacterium Saccharopolyspora hirsuta VKM Ac-666T
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism
2.3. Microorganism Cultivation and Cholesterol Conversion
2.4. Steroid Metabolite Isolation and Identification
2.5. Thin Layer Chromatography (TLC)
2.6. High-Performance Liquid Chromatography (HPLC)
2.7. Mass-Spectrometry (MS), 1H- and 13C-Nuclear Magnetic Resonance Spectroscopy (1H- and 13C-NMR Spectroscopy)
2.8. Genome Analysis
2.9. Phylogenetic Analysis
2.10. BLAST Search for Steroid Catabolism Genes
3. Results
3.1. Cholesterol and Lithocholic Acid Bioconversion
3.2. General Clustering of Steroid Catabolic Gene Homologs
3.3. BLAST Search for the Key Enzymes of Steroid Catabolism in 52 Thermophilic/Thermotolerant Strains
4. Discussion
4.1. Cholesterol Oxidases (ChOs)
4.2. Cyp 125
4.3. Side Chain Degradation
4.4. Steroid Nucleus Degradation
4.5. Steroid Core Degradation
4.6. Search for the Key Genes of Steroid Catabolism in the Genomes of Thermophilic/Thermotolerant Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bergstrand, L.H.; Cardenas, E.; Holert, J.; Van Hamme, J.D.; Mohn, W.W. Delineation of steroid-degrading microorganisms through comparative genomic analysis. mBio 2016, 7, e00166-16. [Google Scholar] [CrossRef] [Green Version]
- Holert, J.; Cardenas, E.; Bergstrand, L.H.; Zaikova, E.; Hahn, A.S.; Hallam, S.J.; Mohn, W.W. Metagenomes reveal global distribution of bacterial steroid catabolism in natural, engineered, and host environments. mBio 2018, 9, e02345-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philipp, B. Bacterial degradation of bile salts. Appl. Microbiol. Biotechnol. 2011, 89, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Donova, M.V.; Egorova, O.V. Microbial steroid transformations: Current state and prospects. Appl. Microbiol. Biotechnol. 2012, 94, 1423–1447. [Google Scholar] [CrossRef]
- Olivera, E.R.; Luengo, J.M. Steroids as environmental compounds recalcitrant to degradation: Genetic mechanisms of bacterial biodegradation pathways. Genes 2019, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, V.; Menéndez, P.; García-Carnelli, C. Microbial transformation of cholesterol: Reactions and practical aspects—An update. World J. Microbiol. Biotechnol. 2019, 35, 131. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.E.; Gawronski, J.D.; Dejesus, M.A.; Ioerger, T.R.; Akerley, B.J.; Sassetti, C.M. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011, 7, e1002251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Geize, R.; Yam, K.; Heuser, T.; Wilbrink, M.H.; Hara, H.; Anderton, M.C.; Sim, E.; Dijkhuizen, L.; Davies, J.E.; Mohn, W.W.; et al. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 1947–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, M.P.; Warren, R.L.; Hsiao, W.W.L.; Araki, N.; Myhre, M.; Fernandes, C.; Miyazawa, D.; Wong, W.; Lillquist, A.L.; Wang, D.; et al. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc. Natl. Acad. Sci. USA 2006, 103, 15582–15587. [Google Scholar] [CrossRef] [Green Version]
- Uhía, I.; Galán, B.; Kendall, S.L.; Stoker, N.G.; García, J.L. Cholesterol metabolism in Mycobacterium smegmatis: Cholesterol pathway. Environ. Microbiol. Rep. 2012, 4, 168–182. [Google Scholar] [CrossRef]
- Drzyzga, O.; de las Heras, L.F.; Morales, V.; Navarro Llorens, J.M.; Perera, J. Cholesterol degradation by Gordonia cholesterolivorans. Appl. Environ. Microbiol. 2011, 77, 4802–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtratnikova, V.Y.; Schelkunov, M.I.; Fokina, V.V.; Bragin, E.Y.; Lobastova, T.G.; Shutov, A.A.; Kazantsev, A.V.; Donova, M.V. Genome-wide transcriptome profiling provides insight on cholesterol and lithocholate degradation mechanisms in Nocardioides simplex VKM Ac-2033D. Genes 2020, 11, 1229. [Google Scholar] [CrossRef]
- Mohn, W.W.; Wilbrink, M.H.; Casabon, I.; Stewart, G.R.; Liu, J.; van der Geize, R.; Eltis, L.D. Gene cluster encoding cholate catabolism in Rhodococcus spp. J. Bacteriol. 2012, 194, 6712–6719. [Google Scholar] [CrossRef] [Green Version]
- Feller, F.M.; Holert, J.; Yücel, O.; Philipp, B. Degradation of bile acids by soil and water bacteria. Microorganisms 2021, 9, 1759. [Google Scholar] [CrossRef]
- Barrientos, Á.; Merino, E.; Casabon, I.; Rodríguez, J.; Crowe, A.M.; Holert, J.; Philipp, B.; Eltis, L.D.; Olivera, E.R.; Luengo, J.M. Functional analyses of three acyl-CoA synthetases involved in bile acid degradation in Pseudomonas putida DOC21. Environ. Microbiol. 2015, 17, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, M.; Koshino, H.; Malon, M.; Hirota, H.; Hayashi, T. Steroid degradation in Comamonas testosteroni TA441: Identification of the entire β-oxidation cycle of the cleaved B ring. Appl. Environ. Microbiol. 2019, 85, e01204-19. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Puopolo, R.; Carbonaro, M.; Maresca, E.; Fiorentino, G. Extremophiles, a nifty tool to face environmental pollution: From exploitation of metabolism to genome engineering. Int. J. Environ. Res. Public Health 2021, 18, 5228. [Google Scholar] [CrossRef]
- Ishino, S.; Ishino, Y. DNA polymerases as useful reagents for biotechnology–the history of developmental research in the field. Front. Microbiol. 2014, 5, 465. [Google Scholar] [CrossRef] [Green Version]
- Sideso, O.; Williams, R.A.D.; Welch, S.G.; Smith, K.E. Progesterone 6-hydroxylation is catalysed by cytochrome P-450 in the moderate thermophile Bacillus thermoglucosidasius strain 12060. J. Steroid Biochem. Mol. Biol. 1998, 67, 163–169. [Google Scholar] [CrossRef]
- Al-Tamimi, S.; Al-Awadi, S.; Oommen, S.; Afzal, M. Modification of progesterone and testosterone by a food-borne thermophile Geobacillus kaustophilus. Int. J. Food Sci. Nutr. 2010, 61, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Sodano, G.; Trabucco, A.; De Rosa, M.; Gambacorta, A. Microbiological reduction of steroidal ketones using the thermophilic bacterium Caldariella acidophila. Experientia 1982, 38, 1311–1312. [Google Scholar] [CrossRef]
- Lacey, J.; Goodfellow, M.J. A novel actinomycete from sugar-cane bagasse: Saccharopolyspora hirsuta gen. et. sp. nov. J. Gen. Microbiol. 1975, 88, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollerov, V.V.; Monti, D.; Deshcherevskaya, N.O.; Lobastova, T.G.; Ferrandi, E.E.; Larovere, A.; Gulevskaya, S.A.; Riva, S.; Donova, M.V. Hydroxylation of lithocholic acid by selected actinobacteria and filamentous fungi. Steroids 2013, 78, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Lobastova, T.G.; Khomutov, S.M.; Shutov, A.A.; Donova, M.V. Microbiological synthesis of stereoisomeric 7(α/β)-hydroxytestololactones and 7(α/β)-hydroxytestolactones. Appl. Microbiol. Biotechnol. 2019, 103, 4967–4976. [Google Scholar] [CrossRef] [PubMed]
- Lobastova, T.G.; Fokina, V.V.; Bragin, E.Y.; Shtratnikova, V.Y.; Starodumova, I.P.; Tarlachkov, S.V.; Donova, M.V. Draft genome sequence of the moderately thermophilic actinobacterial steroid-transforming Saccharopolyspora hirsuta subsp. hirsuta strain VKM Ac-666T. Microbiol. Resour. Announc. 2020, 9, e01327-19. [Google Scholar] [CrossRef] [Green Version]
- Park, N.S.; Myeong, J.S.; Park, H.J.; Han, K.B.; Kim, S.N.; Kim, E.S. Characterization and culture optimization of regiospecific cyclosporin hydroxylation in rare actinomycetes species. J. Microbiol. Biotechnol. 2005, 15, 188–191. [Google Scholar]
- Jork, H.; Funk, W.; Fischer, W.; Wimmer, H. Thin-Layer Chromatography. Reagents and Detection Methods. Physical and Chemical Detection Methods: Fundamentals, Reagents I; VCH: Weinheim, Germany, 1990; Volume 1a, pp. 333–336. [Google Scholar]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; Dejongh, M.; Disz, T. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [Green Version]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Shivlata, L.; Satyanarayana, T. Thermophilic and alkaliphilic Actinobacteria: Biology and potential applications. Front. Microbiol. 2015, 6, 1014. [Google Scholar] [CrossRef]
- Nesbitt, N.M.; Yang, X.; Fontán, P.; Kolesnikova, I.; Smith, I.; Sampson, N.S.; Dubnau, E. A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Infect. Immun. 2010, 78, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.T.; VanderVen, B.C.; Sherman, D.R.; Russell, D.G.; Sampson, N.S. Pathway profiling in Mycobacterium tuberculosis: Elucidation of cholesterol-derived catabolite and enzymes that catalyze its metabolism. J. Biol. Chem. 2011, 286, 43668–43678. [Google Scholar] [CrossRef] [Green Version]
- Carere, J.; McKenna, S.E.; Kimber, M.S.; Seah, S.Y.K. Characterization of an aldolase-dehydrogenase complex from the cholesterol degradation pathway of Mycobacterium tuberculosis. Biochemistry 2013, 52, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Casabon, I.; Crowe, A.M.; Liu, J.; Eltis, L.D. FadD3 is an acyl-CoA synthetase that initiates catabolism of cholesterol rings C and D in actinobacteria: Role of FadD3 in cholesterol catabolism. Mol. Microbiol. 2013, 87, 269–283. [Google Scholar] [CrossRef]
- Yang, M.; Lu, R.; Guja, K.E.; Wipperman, M.F.; St. Clair, J.R.; Bonds, A.C.; Garcia-Diaz, M.; Sampson, N.S. Unraveling cholesterol catabolism in Mycobacterium tuberculosis: ChsE4-ChsE5 α2β2 acyl-CoA dehydrogenase initiates β-oxidation of 3-oxo-cholest-4-en-26-oyl CoA. ACS Infect. Dis. 2015, 1, 110–125. [Google Scholar] [CrossRef] [Green Version]
- Crowe, A.M.; Casabon, I.; Brown, K.L.; Liu, J.; Lian, J.; Rogalski, J.C.; Hurst, T.E.; Snieckus, V.; Foster, L.J.; Eltis, L.D. Catabolism of the last two steroid rings in Mycobacterium tuberculosis and other bacteria. mBio 2017, 8, e00321-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadbery, J.E.; Round, J.W.; Yuan, T.; Wipperman, M.F.; Story, K.T.; Crowe, A.M.; Casabon, I.; Liu, J.; Yang, X.; Eltis, L.D.; et al. IpdE1-IpdE2 is a heterotetrameric acyl coenzyme A dehydrogenase that is widely distributed in steroid-degrading bacteria. Biochemistry 2020, 59, 1113–1123. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Schelkunov, M.I.; Fokina, V.V.; Bragin, E.Y.; Shutov, A.A.; Donova, M.V. Different genome-wide transcriptome responses of Nocardioides simplex VKM Ac-2033D to phytosterol and cortisone 21-acetate. BMC Biotechnol. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Werman, J.M.; Yin, X.; Yang, M.; Garcia-Diaz, M.; Sampson, N.S. Enzymatic β-oxidation of the cholesterol side chain in Mycobacterium tuberculosis bifurcates stereospecifically at hydration of 3-oxo-cholest-4,22-dien-24-oyl-CoA. ACS Infect. Dis. 2021, 7, 1739–1751. [Google Scholar] [CrossRef]
- Smith, K.E.; Williams, R.A.D.; Sideso, O. Transformation of progesterone by a thermophilic bacillus. FEMS Microbiol. Lett. 1992, 92, 29–34. [Google Scholar] [CrossRef]
- Donova, M.V. Transformation of steroids by actinobacteria: A review. Appl. Biochem. Microbiol. 2007, 43, 1–14. [Google Scholar] [CrossRef]
- Yam, K.C.; D’Angelo, I.; Kalscheuer, R.; Zhu, H.; Wang, J.-X.; Snieckus, V.; Ly, L.H.; Converse, P.J.; Jacobs, W.R., Jr.; Strynadka, N.; et al. Studies of a ring cleaving dioxygenase illuminate the role of cholesterol metabolism in the pathogenesis of Mycobacterium tuberculosis. PLoS Pathog. 2009, 5, e1000344. [Google Scholar] [CrossRef]
- Rosłoniec, K.Z.; Wilbrink, M.H.; Capyk, J.K.; Mohn, W.W.; Ostendorf, M.; van der Geize, R.; Dijkhuizen, L.; Eltis, L.D. Cytochrome P450 125 (CYP125) catalyses C26-hydroxylation to initiate sterol side-chain degradation in Rhodococcus jostii RHA1. Mol. Microbiol. 2009, 74, 1031–1043. [Google Scholar] [CrossRef] [Green Version]
- Kreit, J. Microbial catabolism of sterols: Focus on the enzymes that transform the sterol 3-hydroxy-5-en into 3-keto-4-en. FEMS Microbiol. Lett. 2017, 364, fnx007. [Google Scholar] [CrossRef]
- Capyk, J.K.; Kalscheuer, R.; Stewart, G.R.; Liu, J.; Kwon, H.; Zhao, R.; Okamoto, S.; Jacobs, R.W., Jr.; Eltis, L.D.; Mohn, W.W. Mycobacterial cytochrome P450 125 (Cyp125) catalyzes the terminal hydroxylation of C27 steroids. J. Biol. Chem. 2009, 284, 35534–35542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouellet, H.; Guan, S.; Johnston, J.B.; Chow, E.D.; Kells, P.M.; Burlingame, A.L.; Cox, J.S.; Podust, L.M.; Ortiz de Montellano, P.R. Mycobacterium tuberculosis CYP125A1, a steroid C27 monooxygenase that detoxifies intracellularly generated cholest-4-en-3-one: CYP125A1 in cholesterol metabolism. Mol. Microbiol. 2010, 77, 730–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casabon, I.; Swain, K.; Crowe, A.M.; Eltis, L.D.; Mohn, W.W. Actinobacterial acyl coenzyme A synthetases involved in steroid side-chain catabolism. J. Bacteriol. 2014, 196, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Wilbrink, M.H.; van der Geize, R.; Dijkhuizen, L. Molecular characterization of ltp3 and ltp4, essential for C24-branched chain sterol-side-chain degradation in Rhodococcus rhodochrous DSM 43269. Microbiology 2012, 158, 3054–3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonds, A.C.; Yuan, T.; Werman, J.M.; Jang, J.; Lu, R.; Nesbitt, N.M.; Garcia-Diaz, M.; Sampson, N.S. Post-translational succinylation of Mycobacterium tuberculosis enoyl-CoA hydratase EchA19 slows catalytic hydration of cholesterol catabolite 3-oxo-chol-4,22-diene-24-oyl-CoA. ACS Infect. Dis. 2020, 6, 2214–2224. [Google Scholar] [CrossRef]
- Xu, L.-Q.; Liu, Y.-J.; Yao, K.; Liu, H.-H.; Tao, X.-Y.; Wang, F.-Q.; Wei, D.-Z. Unraveling and engineering the production of 23,24-bisnorcholenic steroids in sterol metabolism. Sci. Rep. 2016, 6, 21928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, C.M.; Lu, R.; Nesbitt, N.M.; Schiebel, J.; Sampson, N.S.; Kisker, C. FadA5 a thiolase from Mycobacterium tuberculosis: A steroid-binding pocket reveals the potential for drug development against tuberculosis. Structure 2015, 23, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Aggett, R.; Mallette, E.; Gilbert, S.E.; Vachon, M.A.; Schroeter, K.L.; Kimber, M.S.; Seah, S.Y.K. The steroid side-chain–cleaving aldolase Ltp2–ChsH2DUF35 is a thiolase superfamily member with a radically repurposed active site. J. Biol. Chem. 2019, 294, 11934–11943. [Google Scholar] [CrossRef]
- Itagaki, E.; Matushita, H.; Hatta, T. Steroid transhydrogenase activity of 3-ketosteroid-Δ1-dehydrogenase from Nocardia corallina. J. Biochem. 1990, 108, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Bragin, E.Y.; Shtratnikova, V.Y.; Dovbnya, D.V.; Schelkunov, M.I.; Pekov, Y.A.; Malakho, S.G.; Egorova, O.V.; Ivashina, T.V.; Sokolov, S.L.; Ashapkin, V.V.; et al. Comparative analysis of genes encoding key steroid core oxidation enzymes in fast-growing Mycobacterium spp. strains. J. Steroid Biochem. Mol. Biol. 2013, 138, 41–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, Y.; He, J.; Cheng, S.; Yuan, J.; Ge, F.; Li, W.; Zhang, Y.; Xie, G. Multiplicity of 3-ketosteroid Δ1-dehydrogenase enzymes in Gordonia neofelifaecis NRRL B-59395 with preferences for different steroids. Ann. Microbiol. 2015, 65, 1961–1971. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Schelkunov, M.I.; Fokina, V.V.; Pekov, Y.A.; Ivashina, T.; Donova, M.V. Genome-wide bioinformatics analysis of steroid metabolism associated genes in Nocardioides simplex VKM Ac-2033D. Curr. Genet. 2016, 62, 643–656. [Google Scholar] [CrossRef]
- Guevara, G.; Fernández de las Heras, L.; Perera, J.; Navarro Llorens, J.M. Functional differentiation of 3-ketosteroid Δ1-dehydrogenase isozymes in Rhodococcus ruber strain Chol-4. Microb. Cell Factories 2017, 16, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Liu, X.; Wang, Y.; Han, Y.; Sun, J.; Shi, J.; Zhang, B. Identification, function, and application of 3-ketosteroid Δ1-dehydrogenase isozymes in Mycobacterium neoaurum DSM 1381 for the production of steroidic synthons. Microb. Cell Factories 2018, 17, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capyk, J.K.; D’Angelo, I.; Strynadka, N.C.; Eltis, L.D. Characterization of 3-ketosteroid 9α-hydroxylase, a Rieske oxygenase in the cholesterol degradation pathway of Mycobacterium tuberculosis. J. Biol. Chem. 2009, 284, 9937–9946. [Google Scholar] [CrossRef] [Green Version]
- Bragin, E.Y.; Shtratnikova, V.Y.; Schelkunov, M.I.; Dovbnya, D.V.; Donova, M.V. Genome-wide response on phytosterol in 9-hydroxyandrostenedione-producing strain of Mycobacterium sp. VKM Ac-1817D. BMC Biotechnol. 2019, 19, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrusma, M.; Hessels, G.; Dijkhuizen, L.; van der Geize, R. Multiplicity of 3-ketosteroid-9α-hydroxylase enzymes in Rhodococcus rhodochrous DSM43269 for specific degradation of different classes of steroids. J. Bacteriol. 2011, 193, 3931–3940. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.; Giovannini, P.P.; Fantin, G.; Medici, A.; Pedrini, P. New 9,10-secosteroids from biotransformations of hyodeoxycholic acid with Rhodococcus spp. Helv. Chim. Acta 2013, 96, 1062. [Google Scholar] [CrossRef]
- Costa, S.; Giovannini, P.P.; Fantin, G.; Medici, A.; Pedrini, P. New 9,10-Secosteroids from biotransformations of bile acids with Rhodococcus ruber. Helv. Chim. Acta 2013, 96, 2124. [Google Scholar] [CrossRef]
- García, J.L.; Uhía, I.; Galάn, B. Catabolism and biotechnological applications of cholesterol degrading bacteria: Cholesterol degradation. Microb. Biotechnol. 2012, 5, 679–699. [Google Scholar] [CrossRef]
- Dresen, C.; Lin, L.Y.-C.; D’Angelo, I.; Tocheva, E.I.; Strynadka, N.; Eltis, L.D. A Flavindependent monooxygenase from Mycobacterium tuberculosis involved in cholesterol catabolism. J. Biol. Chem. 2010, 285, 22264–22275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
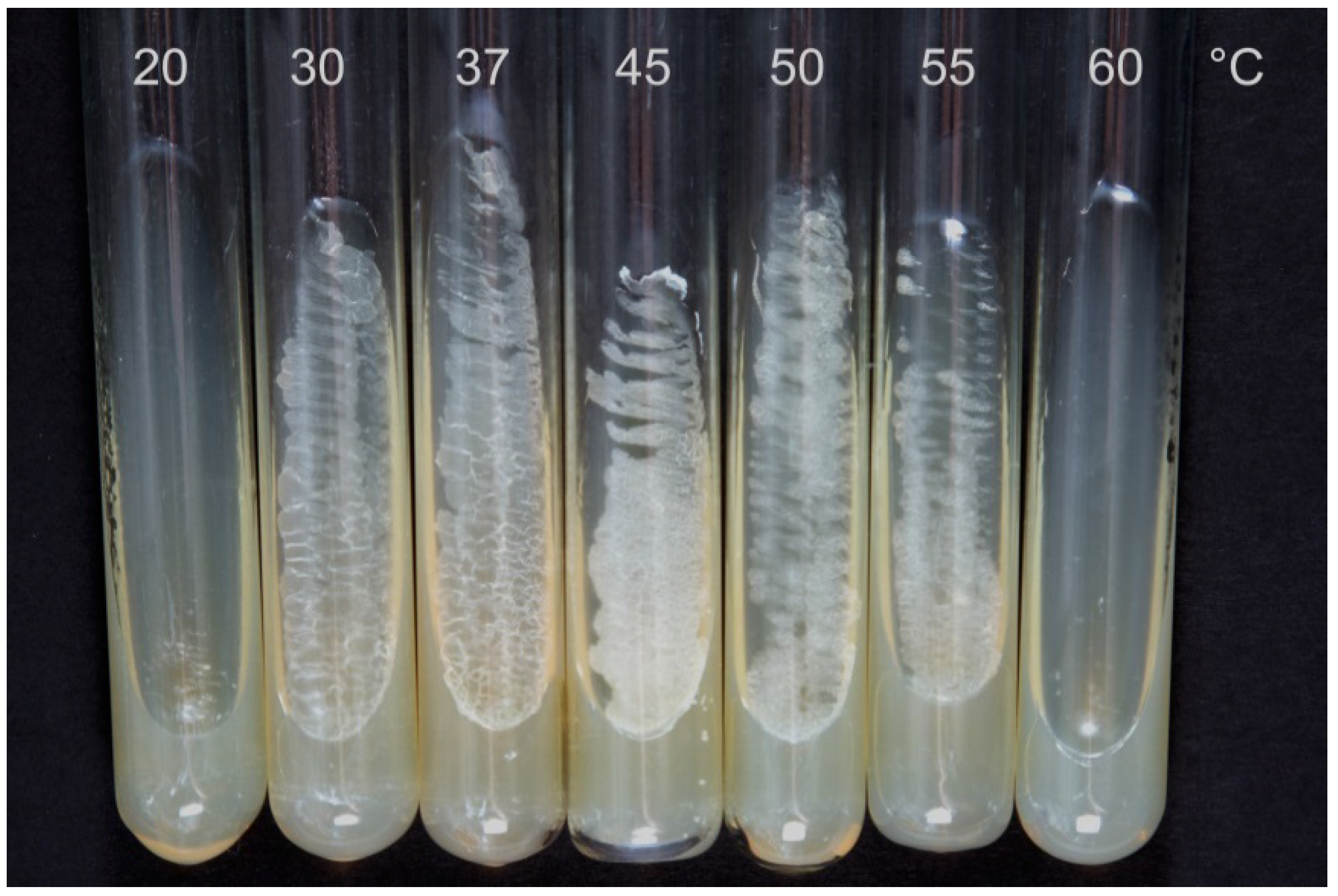
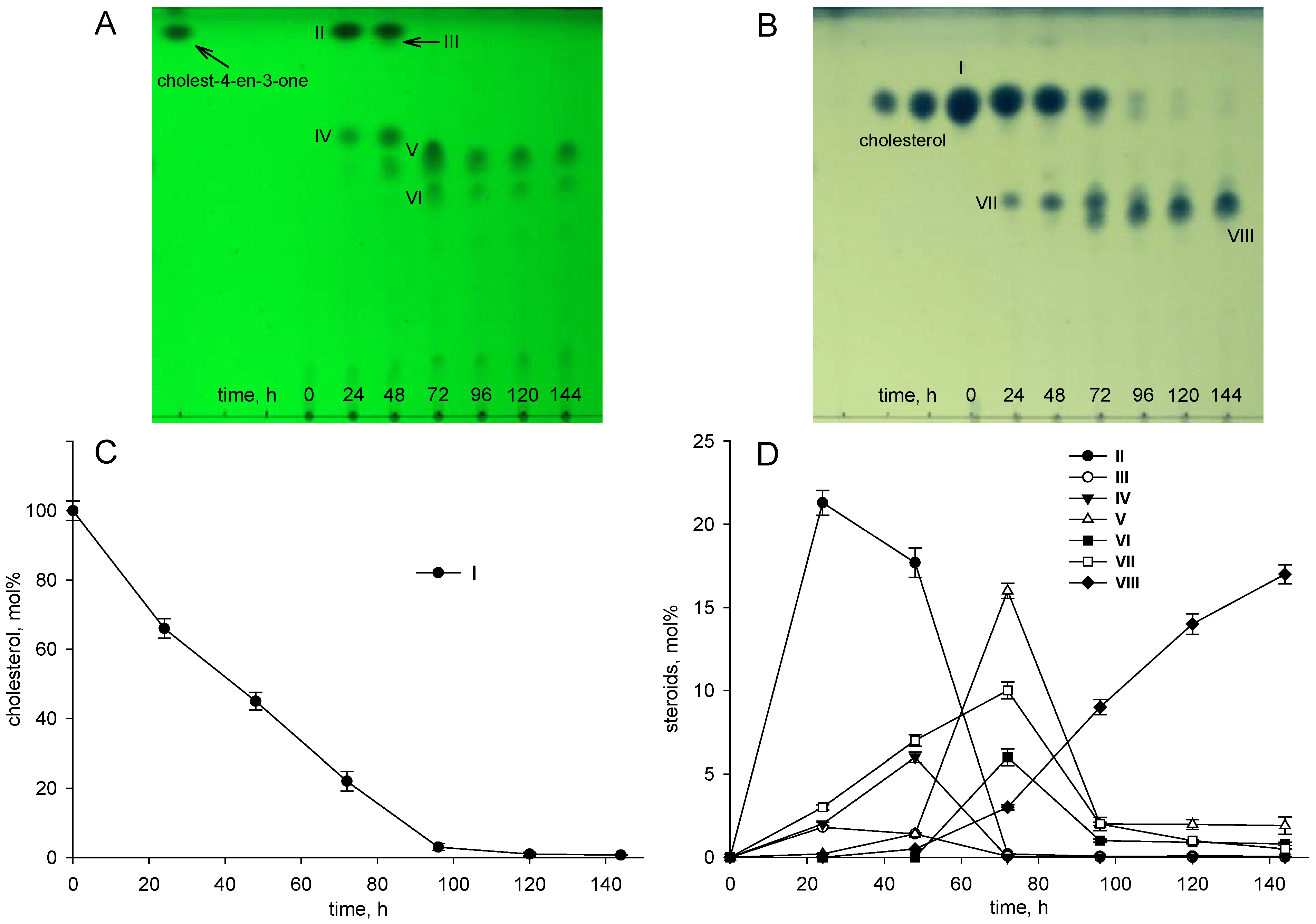


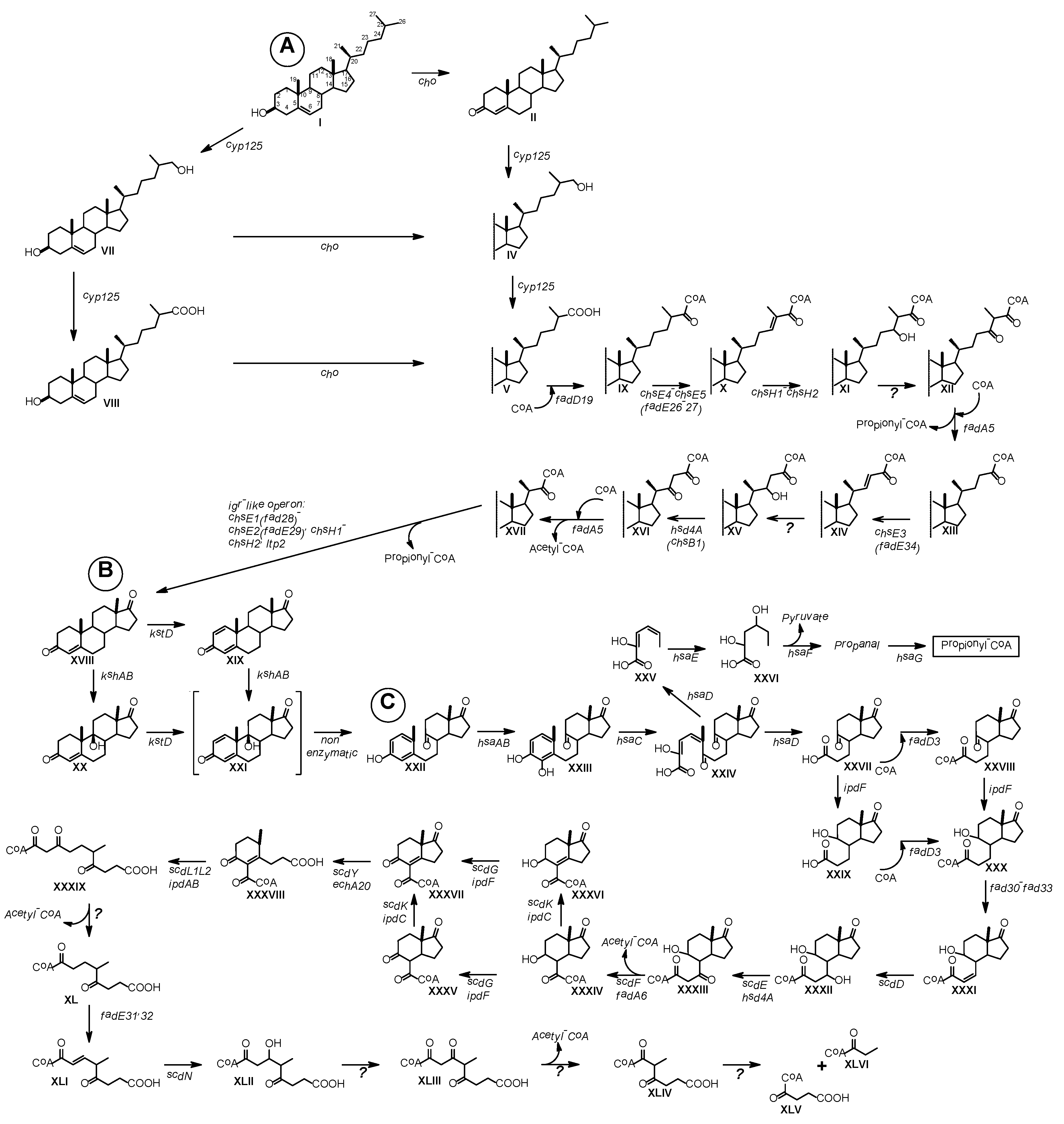
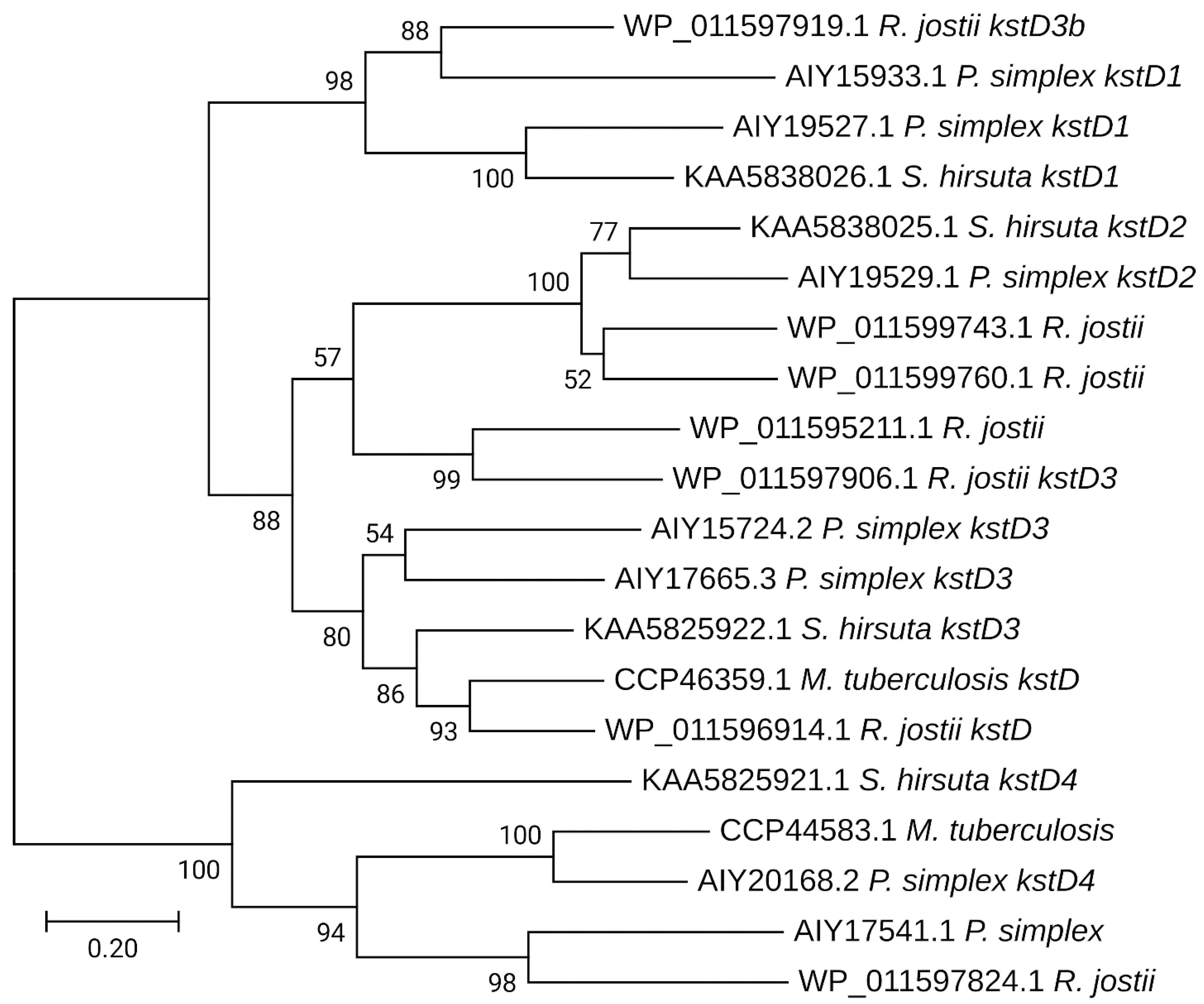
| Cultivation Temperature, °C | |||||||
|---|---|---|---|---|---|---|---|
| 20 | 30 | 37 | 45 | 50 | 55 | 60 | |
| Dried biomass *, g/L | 0.12 ± 0.01 | 0.36 ± 0.07 | 1.21 ± 0.12 | 1.86 ± 0.21 | 1.73 ± 0.19 | 0.28 ± 0.08 | 0 |
| Number | Name and Chemical Structure (mol wt) | High-Performance Liquid Chromatography (HPLC), Mass-Spectrometry (MS), 1H- and 13C- Nuclear Magnetic Resonance Spectroscopy (1H- and 13C-NMR Spectroscopy) Data |
|---|---|---|
| II | Cholest-4-en-3-one (384)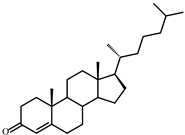 | Rt (mobile phase acetonitrile:2-propanol:water 50:45:5 vol/vol/vol, λ 240 nm) 8.9 min; MS (intensity,%) [M + H]+: 385(100), 279(5), 226(9), 149(3), 109(4) |
| III | Cholesta-1,4-dien-3-one (382)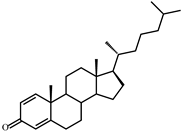 | Rt (mobile phase acetonitrile:2-propanol:water 50:45:5 vol/vol/vol, λ 240 nm) 7.2 min; MS (intensity,%) [M + H]+ (collision energy 33 eV): 383(90), 279(5), 365(55), 325(133), 271(15), 247(100), 175(40), 163(48), 135(11), 121(8) |
| IV | 26-Hydroxycholest-4-en-3-one (400)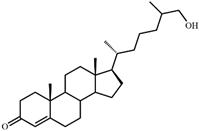 | Rt (mobile phase acetonitrile:2-propanol:water 50:45:5 vol/vol/vol, λ 240 nm) 3.9 min; Rt (mobile phase acetonitrile:water:acetic acid (60:40:0.01 vol/vol/vol, λ 240 nm) 81.8 min; MS (intensity,%) [M + H]+ (collision energy 33 eV): 401(100), 369(33). 1H-NMR (CDCl3) δ: 5.73 (s, 1H, H-4), 1.18 (s, 3H, 19-CH3), 0.92 (d, J = 6.7 Hz, 3H, 21-CH3), 0.87 (d, J = 6.6 Hz, 3H, 26(27)-CH3), 0.86 (d, J = 6.6 Hz, 3H, 26(27)-CH3), 0.71 (s, 3H, 18-CH3) |
| V | 3-Oxo-cholest-4-en-26-oic acid (414) | Rt (mobile phase acetonitrile:2-propanol:water 50:45:5 vol/vol/vol, λ 240 nm) 3.7 min; Rt (mobile phase acetonitrile:water:acetic acid (60:40:0.01 vol/vol/vol, λ 240 nm) 50.9 min; HRMS-ESI (m/z): [M-H]+ calcd for C27H41O3 413,3056; found 413,3059. 1H-NMR (CDCl3) δ: 5.73 (br. s., 1H, 4-H), 1.18 (s, 3H, 19-CH3), 1.17 (d, J = 7.0 Hz, 3H, 27-CH3), 0.91 (d, J = 6.5 Hz, 3H, 21-CH3), 0.70 (s, 3H, 18-CH3). 13C-NMR (CDCl3) δ: 199.9 (C-3), 182.3 (C-26), 171.9 (C-5), 123.7 (C-4), 56.0, 55.8, 53.8, 42.4, 39.6, 39.2, 38.6, 35.63, 35.56, 33.92, 33.86, 32.9, 32.0, 28.1, 24.1, 23.6, 21.0, 18.5, 17.3, 16.7, 11.9 |
| VI | 3-Oxo-cholesta-1,4-dien-26-oic (412)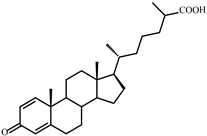 | Rt (mobile phase acetonitrile:2-propanol:water 50:45:5 vol/vol/vol, λ 240 nm) 3.3 min; Rt (mobile phase acetonitrile:water:acetic acid (60:40:0.01 vol/vol/vol, λ 240 nm) 32.2 min; HRMS-ESI (m/z): [M-H]+ calcd for C27H39O3 411,2899; found 411, 2903. 1H-NMR (CDCl3) δ: 7.06 (d, J = 10.1 Hz, 1H, 1-H), 6.24 (dd, J = 1.9, 10.1 Hz, 1H, 2-H), 6.08 (br. s., 1H. 4-H), 1.23 (s, 3H, 19-CH3), 1,17 (d, J = 7.0 Hz, 3H, 27-CH3), 0.91 (d, J = 6.5 Hz, 3H, 21-CH3), 0.73 (s, 3H, 18-CH3). 13C-NMR (CDCl3) δ: 186.6 (C-3), 182.3 (C-26), 169.8 (C-5), 156.3 (C-1), 127.4 (C-2), 123.7 (C-4), 56.0, 55.4, 52.3, 43.7, 42.6, 39.4, 39.2, 35.6, 35.5, 35.4, 33.9, 33.7, 32.9, 28.1, 24.4, 23.6, 22.8, 18.6, 18.5, 16.7, 12.0 |
| VII | 26-Hydroxycholesterol (cholest-5-ene-3β,26-diol) (402) 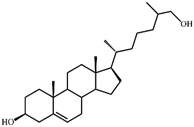 | Rt (mobile phase acetonitrile:2-propanol:water 50:45:5 vol/vol/vol, λ 200 nm) 3.9 min; Rt (mobile phase acetonitrile:water:acetic acid (60:40:0.01 vol/vol/vol, λ 200 nm) 78.9 min; HRMS-ESI (m/z): [M-H]+ calcd for C27H45O2 401,3420; found 401,3415. 1H-NMR (CDCl3) δ: 5.36 (br. s., 1H, 6-H), 3.53 (m, 1H, 3α-H), 3.51 (dd, J = 6.0, 10.6 Hz, 1H, CH2OH), 3.43 (dd, J = 6.4, 10.6 Hz, 1H, CH2OH), 1.01 (s, 3H, 19-CH3), 0.92 (d, J = 6.5 Hz, 3H, 21-CH3), 0.91 (d, J = 6.7 Hz, 3H, 27-CH3), 0.68 (s, 3H, 18-CH3). 13C-NMR (CDCl3) δ: 140.8 (C-5), 121.7 (C-6), 71.8 (C-3), 68.5 (C-26), 56.8, 56.1, 50.1, 42.32, 42.28, 39.8, 37.2, 36.5, 36.1, 35.8, 35.7, 33.5, 31.9, 31.7, 28.2, 24.3, 23.4, 21.1, 19.4, 18.7, 16.5, 11.9 |
| VIII | 3β-Hydroxy-cholest-5-en-26-oic acid (416) | Rt (mobile phase acetonitrile:2-propanol:water 50:45:5 vol/vol/vol, λ 200 nm) 3.6 min; Rt (mobile phase acetonitrile:water:acetic acid (60:40:0.01 vol/vol/vol, λ 200 nm) 45.9 min; HRMS-ESI (m/z): [M-H]+ calcd for C27H43O3 415,3212; found 415,3217. 1H-NMR (CD3OD) δ: 5.33 (d, J = 5.2 Hz, 1H, 6-H), 3.39 (m, 1H, 3α-H), 1.12 (d, J = 7.0 Hz, 3H, 27-CH3), 1.01 (s, 3H, 19-CH3), 0.93 (d, J = 6.5 Hz, 3H, 21-CH3), 0.71 (s, 3H, 18-CH3). 13C-NMR (CD3OD) δ: 180.8 (C-26), 142.2 (C-5), 122.5 (C-6), 72.4 (C-3), 58.2, 57.5, 51.7, 43.5, 43.0, 41.2, 40.7, 38.6, 37.7, 37.0, 35.4, 33.3, 33.0, 32.3, 29.3, 25.3, 24.8, 22.2, 19.9, 19.2, 17.6, 12.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobastova, T.; Fokina, V.; Tarlachkov, S.; Shutov, A.; Bragin, E.; Kazantsev, A.; Donova, M. Steroid Metabolism in Thermophilic Actinobacterium Saccharopolyspora hirsuta VKM Ac-666T. Microorganisms 2021, 9, 2554. https://doi.org/10.3390/microorganisms9122554
Lobastova T, Fokina V, Tarlachkov S, Shutov A, Bragin E, Kazantsev A, Donova M. Steroid Metabolism in Thermophilic Actinobacterium Saccharopolyspora hirsuta VKM Ac-666T. Microorganisms. 2021; 9(12):2554. https://doi.org/10.3390/microorganisms9122554
Chicago/Turabian StyleLobastova, Tatyana, Victoria Fokina, Sergey Tarlachkov, Andrey Shutov, Eugeny Bragin, Alexey Kazantsev, and Marina Donova. 2021. "Steroid Metabolism in Thermophilic Actinobacterium Saccharopolyspora hirsuta VKM Ac-666T" Microorganisms 9, no. 12: 2554. https://doi.org/10.3390/microorganisms9122554
APA StyleLobastova, T., Fokina, V., Tarlachkov, S., Shutov, A., Bragin, E., Kazantsev, A., & Donova, M. (2021). Steroid Metabolism in Thermophilic Actinobacterium Saccharopolyspora hirsuta VKM Ac-666T. Microorganisms, 9(12), 2554. https://doi.org/10.3390/microorganisms9122554






