Toxoplasmosis: Current and Emerging Parasite Druggable Targets
Abstract
1. Introduction
2. Current Treatment Modalities of Toxoplasmosis
3. Drug Resistance in Toxoplasma gondii Infections
4. Emerging Therapeutic Targets in Toxoplasma gondii Infections
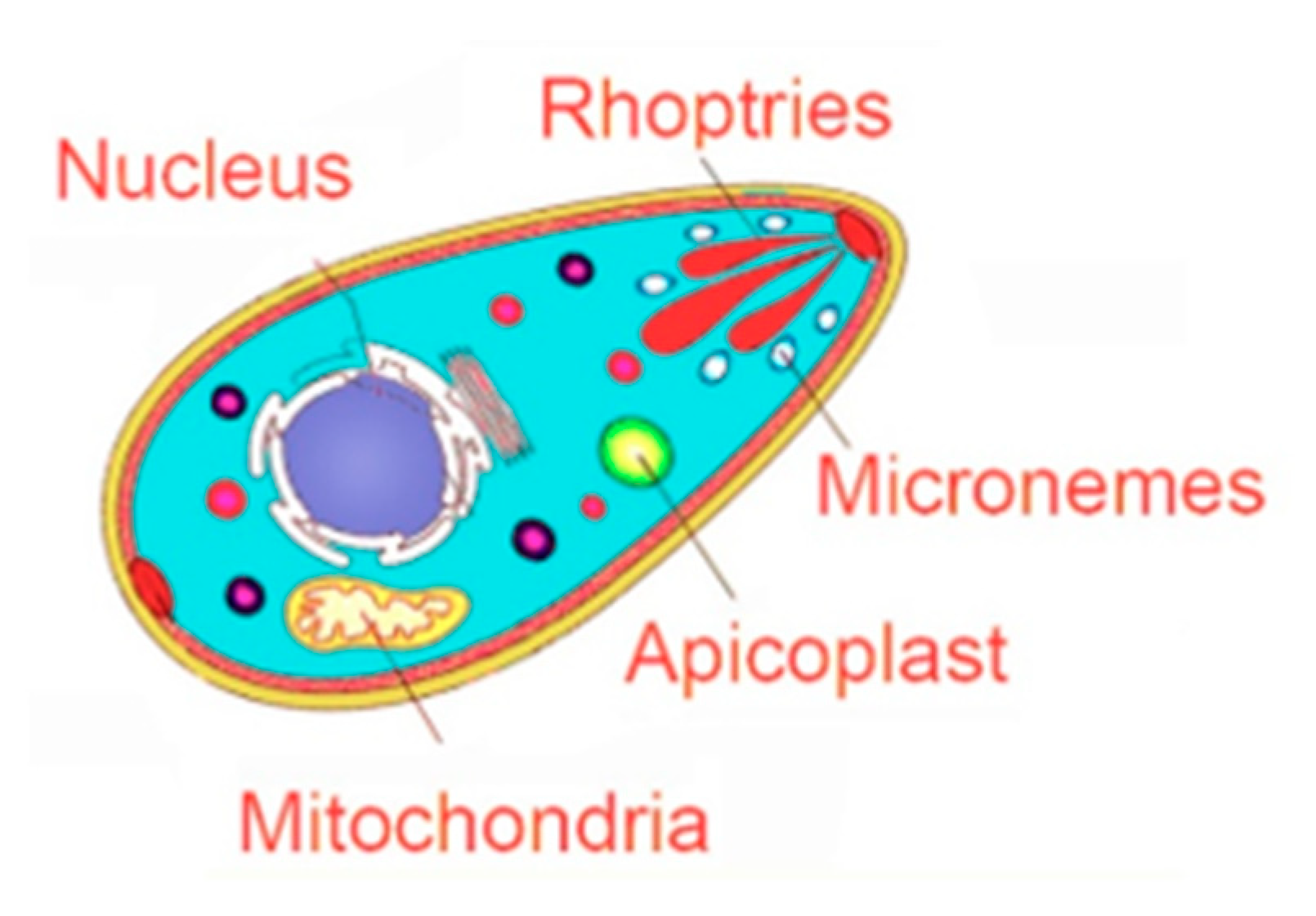
4.1. Targeting the Apicoplast
4.2. Targeting the Invasion Complex
4.2.1. Microneme Organelles
4.2.2. Rhoptry Organelles
4.3. Targeting the Parasite Mitochondrial Electron Transport Pathway
4.4. Targeting the Interconversion between Tachyzoites and Bradyzoites
5. Drug Repositioning: A Promising Approach against T. Gondii
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Ben-Harari, R.R.; Connolly, M.P. High burden and low awareness of toxoplasmosis in the United States. Postgrad. Med. 2019, 131, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Reza Yazdani, M.; Mehrabi, Z.; Ataei, B.; Baradaran Ghahfarokhi, A.; Moslemi, R.; Pourahmad, M. Frequency of sero-positivity in household members of the patients with positive toxoplasma serology. Rev. Esp. Quimioter. Publ. Of. Soc. Esp. Quimioter. 2018, 31, 506–510. [Google Scholar]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Toxoplasma gondii: The changing paradigm of congenital toxoplasmosis. Parasitology 2011, 138, 1829–1831. [Google Scholar] [CrossRef]
- Yamamoto, L.; Targa, L.S.; Sumita, L.M.; Shimokawa, P.T.; Rodrigues, J.C.; Kanunfre, K.A.; Okay, T.S. Association of Parasite Load Levels in Amniotic Fluid With Clinical Outcome in Congenital Toxoplasmosis. Obstet. Gynecol. 2017, 130, 335–345. [Google Scholar] [CrossRef]
- Robbins, J.R.; Zeldovich, V.B.; Poukchanski, A.; Boothroyd, J.C.; Bakardjiev, A.I. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect. Immun. 2012, 80, 418–428. [Google Scholar] [CrossRef]
- McAuley, J.B. Congenital Toxoplasmosis. J. Pediatr. Infect. Dis. Soc. 2014, 3 (Suppl. 1), S30–S35. [Google Scholar] [CrossRef]
- Singh, S. Congenital toxoplasmosis: Clinical features, outcomes, treatment, and prevention. Trop. Parasitol. 2016, 6, 113–122. [Google Scholar] [CrossRef]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef]
- Vasconcelos-Santos, D.V.; Dodds, E.M.; Orefice, F. Review for disease of the year: Differential diagnosis of ocular toxoplasmosis. Ocul. Immunol. Inflamm. 2011, 19, 171–179. [Google Scholar] [CrossRef]
- Nahouli, H.; El Arnaout, N.; Chalhoub, E.; Anastadiadis, E.; El Hajj, H. Seroprevalence of Anti-Toxoplasma gondii Antibodies Among Lebanese Pregnant Women. Vector Borne Zoonotic Dis. 2017, 17, 785–790. [Google Scholar] [CrossRef]
- Nowakowska, D.; Colón, I.; Remington, J.S.; Grigg, M.; Golab, E.; Wilczynski, J.; Sibley, L.D. Genotyping of Toxoplasma gondii by Multiplex PCR and Peptide-Based Serological Testing of Samples from Infants in Poland Diagnosed with Congenital Toxoplasmosis. J. Clin. Microbiol. 2006, 44, 1382. [Google Scholar] [CrossRef]
- Galal, L.; Sarr, A.; Cuny, T.; Brouat, C.; Coulibaly, F.; Sembène, M.; Diagne, M.; Diallo, M.; Sow, A.; Hamidović, A.; et al. The introduction of new hosts with human trade shapes the extant distribution of Toxoplasma gondii lineages. PLoS Negl. Trop. Dis. 2019, 13, e0007435. [Google Scholar] [CrossRef]
- Delhaes, L.; Ajzenberg, D.; Sicot, B.; Bourgeot, P.; Darde, M.L.; Dei-Cas, E.; Houfflin-Debarge, V. Severe congenital toxoplasmosis due to a Toxoplasma gondii strain with an atypical genotype: Case report and review. Prenat. Diagn. 2010, 30, 902–905. [Google Scholar] [CrossRef]
- Schlüter, D.; Barragan, A. Advances and Challenges in Understanding Cerebral Toxoplasmosis. Front. Immunol. 2019, 10, 242. [Google Scholar] [CrossRef]
- Blanchard, N.; Dunay, I.R.; Schlüter, D. Persistence of Toxoplasma gondii in the central nervous system: A fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol. 2015, 37, 150–158. [Google Scholar] [CrossRef]
- Matta, S.K.; Rinkenberger, N.; Dunay, I.R.; Sibley, L.D. Toxoplasma gondii infection and its implications within the central nervous system. Nat. Rev. Microbiol. 2021, 19, 467–480. [Google Scholar] [CrossRef]
- Madireddy, S.; Rivas Chacon, E.D.; Mangat, R. Toxoplasmosis; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Evans, A.K.; Strassmann, P.S.; Lee, I.P.; Sapolsky, R.M. Patterns of Toxoplasma gondii cyst distribution in the forebrain associate with individual variation in predator odor avoidance and anxiety-related behavior in male Long–Evans rats. Brain Behav. Immun. 2014, 37, 122–133. [Google Scholar] [CrossRef]
- Hermes, G.; Ajioka, J.W.; Kelly, K.A.; Mui, E.; Roberts, F.; Kasza, K.; Mayr, T.; Kirisits, M.J.; Wollmann, R.; Ferguson, D.J.; et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J. Neuroinflamm. 2008, 5, 48. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Gressitt, K.L.; He, H.; Kannan, G.; Schultz, T.L.; Svezhova, N.; Carruthers, V.B.; Pletnikov, M.V.; Yolken, R.H.; et al. Severance Cerebral complement C1q activation in chronic Toxoplasma infection. Brain Behav. Immun. 2016, 58, 52–56. [Google Scholar] [CrossRef]
- Ngô, H.M.; Zhou, Y.; Lorenzi, H.; Wang, K.; Kim, T.K.; Zhou, Y.; El Bissati, K.; Mui, E.; Fraczek, L.; Rajagopala, S.V.; et al. Toxoplasma Modulates Signature Pathways of Human Epilepsy, Neurodegeneration & Cancer. Sci. Rep. 2017, 7, 11496. [Google Scholar]
- Johnson, H.J.; Koshy, A.A. Latent Toxoplasmosis Effects on Rodents and Humans: How Much is Real and How Much is Media Hype? mBio 2020, 11, e02164-19. [Google Scholar] [CrossRef]
- Johnson, S.K.; Johnson, P.T.J. Toxoplasmosis: Recent Advances in Understanding the Link Between Infection and Host Behavior. Annu. Rev. Anim. Biosci. 2021, 9, 249–264. [Google Scholar] [CrossRef]
- Bannoura, S.; El Hajj, R.; Khalifeh, I.; El Hajj, H. Acute disseminated encephalomyelitis and reactivation of cerebral toxoplasmosis in a child: Case report. IDCases 2018, 13, e00434. [Google Scholar] [CrossRef]
- Basavaraju, A. Toxoplasmosis in HIV infection: An overview. Trop. Parasitol. 2016, 6, 129–135. [Google Scholar] [CrossRef]
- Kodym, P.; MalÝ, M.; Beran, O.; Jilich, D.; Rozsypal, H.; Machala, L.; Holub, M. Incidence, immunological and clinical characteristics of reactivation of latent Toxoplasma gondii infection in HIV-infected patients. Epidemiol. Infect. 2015, 143, 600–607. [Google Scholar] [CrossRef]
- Gay, J.; Gendron, N.; Verney, C.; Joste, V.; Dardé, M.L.; Loheac, C.; Vrtovsnik, F.; Argy, N.; Houze, S. Disseminated toxoplasmosis associated with hemophagocytic syndrome after kidney transplantation: A case report and review. Transpl. Infect. Dis. 2019, 21, e13154. [Google Scholar] [CrossRef]
- Kollu, V.; Magalhaes-Silverman, M.; Tricot, G.; Ince, D. Toxoplasma Encephalitis following Tandem Autologous Hematopoietic Stem Cell Transplantation: A Case Report and Review of the Literature. Case Rep. Infect. Dis. 2018, 2018, 9409121. [Google Scholar] [CrossRef]
- Paccoud, O.; Guitard, J.; Labopin, M.; Surgers, L.; Malard, F.; Battipaglia, G.; Duléry, R.; Hennequin, C.; Mohty, M.; Brissot, E. Features of Toxoplasma gondii reactivation after allogeneic hematopoietic stem-cell transplantation in a high seroprevalence setting. Bone Marrow Transplant. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Ramanan, P.; Scherger, S.; Benamu, E.; Bajrovic, V.; Jackson, W.; Hage, C.A.; Hakki, M.; Baddley, J.W.; Abidi, M.Z. Toxoplasmosis in non-cardiac solid organ transplant recipients: A case series and review of literature. Transpl. Infect. Dis. 2020, 22, e13218. [Google Scholar] [CrossRef] [PubMed]
- Ramchandar, N.; Pong, A.; Anderson, E. Identification of disseminated toxoplasmosis by plasma next-generation sequencing in a teenager with rapidly progressive multiorgan failure following haploidentical stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28205. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Meroni, V.; Dupont, D.; Botterel, F.; Garcia, J.M.A.; Brenier-Pinchart, M.-P.; Accoceberry, I.; Akan, H.; Abbate, I.; Boggian, K.; et al. Toxoplasmosis in Transplant Recipients, Europe, 2010–2014. Emerg. Infect. Dis. 2018, 24, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, R.O.; Sherman, A.; Spicer, J.O.; Messina, J.A.; Steinbrink, J.M.; Sexton, M.E.; Lyon, G.M.; Mehta, A.K.; Phadke, V.K.; Woodworth, M.H. Clinical characteristics and outcomes of toxoplasmosis among transplant recipients at two US academic medical centers. Transpl. Infect. Dis. 2021, 23, e13636. [Google Scholar] [CrossRef] [PubMed]
- La Hoz, R.M.; Morris, M.I.; Infectious Diseases Community of Practice of the American Society of Transplantation. Infectious Diseases Community of Practice of the American Society of Tissue and blood protozoa including toxoplasmosis, Chagas disease, leishmaniasis, Babesia, Acanthamoeba, Balamuthia, and Naegleria in solid organ transplant recipients- Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13546. [Google Scholar] [PubMed]
- Holland, M.S.; Sharma, K.; Lee, B.C. Cerebral toxoplasmosis after rituximab therapy for splenic marginal zone lymphoma: A case report and review of the literature. JMM Case Rep. 2015, 2, e005010. [Google Scholar] [CrossRef]
- Lee, E.B.; Ayoubi, N.; Albayram, M.; Kariyawasam, V.; Motaparthi, K. Cerebral toxoplasmosis after rituximab for pemphigus vulgaris. JAAD Case Rep. 2019, 6, 37–41. [Google Scholar] [CrossRef][Green Version]
- Morjaria, S.; Epstein, D.J.; Romero, F.A.; Taur, Y.; Seo, S.K.; Papanicolaou, G.A.; Hatzoglou, V.; Rosenblum, M.; Perales, M.-A.; Scordo, M.; et al. Toxoplasma Encephalitis in Atypical Hosts at an Academic Cancer Center. Open Forum Infect. Dis. 2016, 3, ofw070. [Google Scholar] [CrossRef]
- Safa, G.; Darrieux, L. Cerebral Toxoplasmosis After Rituximab Therapy. JAMA Intern. Med. 2013, 173, 924–926. [Google Scholar] [CrossRef][Green Version]
- Rajapakse, S.; Weeratunga, P.; Rodrigo, C.; de Silva, N.L.; Fernando, S.D. Prophylaxis of human toxoplasmosis: A systematic review. Pathog. Glob. Health 2017, 111, 333–342. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stajner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef]
- Blume, M.; Seeber, F. Metabolic interactions between Toxoplasma gondii and its host. F1000Research 2018, 7, 1719. [Google Scholar] [CrossRef]
- Lapinskas, P.J.; Ben-Harari, R.R. Perspective on current and emerging drugs in the treatment of acute and chronic toxoplasmosis. Postgrad. Med. 2019, 131, 589–596. [Google Scholar] [CrossRef]
- Remington, J.S.; Thulliez, P.; Montoya, J.G. Recent Developments for Diagnosis of Toxoplasmosis. J. Clin. Microbiol. 2004, 42, 941. [Google Scholar] [CrossRef]
- Silva, D.R.; Sardi, J.D.C.O.; Freires, I.A.; Silva, A.C.B.; Rosalen, P.L. In silico approaches for screening molecular targets in Candida albicans: A proteomic insight into drug discovery and development. Eur. J. Pharmacol. 2019, 842, 64–69. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef]
- Katlama, C.; Mouthon, B.; Gourdon, D.; Lapierre, D.; Rousseau, F. Atovaquone as long-term suppressive therapy for toxoplasmic encephalitis in patients with AIDS and multiple drug intolerance. Atovaquone Expanded Access Group. Aids 1996, 10, 1107–1112. [Google Scholar]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R D 2017, 17, 523–544. [Google Scholar] [CrossRef]
- Ardabili, S.; Kohl, J.; Gul, G.; Hodel, M. What obstetricians should be aware of: Serious side effects of antibiotic toxoplasmosis treatment in pregnancy. BMJ Case Rep. 2021, 14, e240809. [Google Scholar] [CrossRef]
- Shammaa, A.M.; Powell, T.G.; Benmerzouga, I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Paquet, C.; Yudin, M.H. No. 285-Toxoplasmosis in Pregnancy: Prevention, Screening, and Treatment. J. Obstet. Gynaecol. Can. 2018, 40, e687–e693. [Google Scholar] [CrossRef] [PubMed]
- Demar, M.P.; Ajzenberg, D.; Maubon, D.; Djossou, F.; Panchoe, D.; Punwasi, W.; Valery, N.; Peneau, C.; Daigre, J.; Aznar, C.; et al. Fatal outbreak of human toxoplasmosis along the Maroni River: Epidemiological, clinical, and parasitological aspects. Clin. Infect. Dis. 2007, 45, e88–e95. [Google Scholar] [CrossRef] [PubMed]
- Demar, M.; Hommel, D.; Djossou, F.; Peneau, C.; Boukhari, R.; Louvel, D.; Bourbigot, A.M.; Nasser, V.; Ajzenberg, D.; Darde, M.L.; et al. Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin. Microbiol. Infect. 2012, 18, E221–E331. [Google Scholar] [CrossRef]
- Rajapakse, S.; Chrishan Shivanthan, M.; Samaranayake, N.; Rodrigo, C.; Deepika Fernando, S. Antibiotics for human toxoplasmosis: A systematic review of randomized trials. Pathog. Glob. Health 2013, 107, 162–169. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Hogh, B.; Andersen, O.; Hansen, S.H.; Dalhoff, K.; Petersen, E. Treatment of infants with congenital toxoplasmosis: Tolerability and plasma concentrations of Sulfadiazine and Pyrimethamine. Eur. J. Pediatr. 2006, 165, 19–25. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef]
- Montazeri, M.; Sharif, M.; Sarvi, S.; Mehrzadi, S.; Ahmadpour, E.; Daryani, A. A Systematic Review of In vitro and In vivo Activities of Anti-Toxoplasma Drugs and Compounds (2006–2016). Front. Microbiol. 2017, 8, 25. [Google Scholar] [CrossRef]
- Luft, B.J.; Remington, J.S. Toxoplasmic Encephalitis in AIDS. Clin. Infect. Dis. 1992, 15, 211–222. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Y.-M.; Liu, M.; Lu, Y.-Q.; Liu, X.-Y.; Zhang, Y.-L.; Jiang, Z.-S.; Yang, T.-T.; Sun, Y.; Lan, K.; et al. Development of a risk scoring system for prognostication in HIV-related toxoplasma encephalitis. BMC Infect. Dis. 2020, 20, 923. [Google Scholar] [CrossRef]
- Porter, S.B.; Sande, M.A. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N. Engl. J. Med. 1992, 327, 1643–1648. [Google Scholar] [CrossRef]
- Wei, H.X.; Wei, S.S.; Lindsay, D.S.; Peng, H.J. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma gondii Medicines in Humans. PLoS ONE 2015, 10, e0138204. [Google Scholar]
- Podzamczer, D.; Miró, J.M.; Ferrer, E.; Gatell, J.M.; Ramón, J.M.; Ribera, E.; Sirera, G.; Cruceta, A.; Knobel, H.; Domingo, P.; et al. Thrice-weekly Sulfadiazine-Pyrimethamine for maintenance therapy of toxoplasmic encephalitis in HIV-infected patients. Spanish Toxoplasmosis Study Group. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 89–95. [Google Scholar] [CrossRef]
- Vidal, J.E.; Hernandez, A.V.; de Oliveira, A.C.; Dauar, R.F.; Barbosa, S.P., Jr.; Focaccia, R. Cerebral toxoplasmosis in HIV-positive patients in Brazil: Clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS 2005, 19, 626–634. [Google Scholar] [CrossRef]
- Connolly, M.P.; Goodwin, E.; Schey, C.; Zummo, J. Toxoplasmic encephalitis relapse rates with Pyrimethamine-based therapy: Systematic review and meta-analysis. Pathog. Glob. Health 2017, 111, 31–44. [Google Scholar] [CrossRef][Green Version]
- Connolly, M.P.; Haitsma, G.; Hernández, A.V.; Vidal, J.E. Systematic review and meta-analysis of secondary prophylaxis for prevention of HIV-related toxoplasmic encephalitis relapse using trimethoprim-sulfamethoxazole. Pathog. Glob. Health 2017, 111, 327–331. [Google Scholar] [CrossRef]
- Mayor, A.M.; Santos, D.M.F.; Dworkin, M.S.; Ríos-Olivares, E.; Hunter-Mellado, R.F. Toxoplasmic encephalitis in an AIDS cohort at Puerto Rico before and after highly active antiretroviral therapy (HAART). Am. J. Trop. Med. Hyg. 2011, 84, 838–841. [Google Scholar] [CrossRef][Green Version]
- Martin-Iguacel, R.; Ahlström, M.G.; Touma, M.; Engsig, F.N.; Stærke, N.B.; Stærkind, M.; Obel, N.; Rasmussen, L.D. Incidence, presentation and outcome of toxoplasmosis in HIV infected in the combination antiretroviral therapy era. J. Infect. 2017, 75, 263–273. [Google Scholar] [CrossRef]
- Mele, A.; Paterson, P.J.; Prentice, H.G.; Leoni, P.; Kibbler, C.C. Toxoplasmosis in bone marrow transplantation: A report of two cases and systematic review of the literature. Bone Marrow Transplant. 2002, 29, 691–698. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Binisti, P.; Antonetti, D.; Brezin, A.; Yera, H.; Dupouy-Camet, J. Usefulness of immunoblotting and Goldmann-Witmer coefficient for biological diagnosis of toxoplasmic retinochoroiditis. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 34–38. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.C.; Martínez-Grueiro, M.M.; Martínez-Fernández, A.R. Comparative activity of several antibiotics against Toxoplasma gondii in a mouse model. Enferm. Infect. Microbiol. Clin. 1993, 11, 543–546. [Google Scholar]
- Doliwa, C.; Escotte-Binet, S.; Aubert, D.; Sauvage, V.; Velard, F.; Schmid, A.; Villena, I. Sulfadiazine resistance in Toxoplasma gondii: No involvement of overexpression or polymorphisms in genes of therapeutic targets and ABC transporters. Parasite 2013, 20, 19. [Google Scholar] [CrossRef]
- Doliwa, C.; Escotte-Binet, S.; Aubert, D.; Velard, F.; Schmid, A.; Geers, R.; Villena, I. Induction of Sulfadiazine resistance in vitro in Toxoplasma gondii. Exp. Parasitol. 2013, 133, 131–136. [Google Scholar] [CrossRef]
- Oliveira, C.; Meurer, Y.S.; Andrade, J.; Costa, M.E.; Andrade, M.; Silva, L.A.; Lanza, D.C.; Vítor, R.W.; Andrade-Neto, V.F. Pathogenicity and phenotypic Sulfadiazine resistance of Toxoplasma gondii isolates obtained from livestock in northeastern Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 391–398. [Google Scholar] [CrossRef]
- Aspinall, T.V.; Joynson, D.H.; Guy, E.; Hyde, J.E.; Sims, P.F. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J. Infect. Dis. 2002, 185, 1637–1643. [Google Scholar] [CrossRef]
- Silva, L.A.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Vitor, R.W. Genetic Polymorphisms and Phenotypic Profiles of Sulfadiazine-Resistant and Sensitive Toxoplasma gondii Isolates Obtained from Newborns with Congenital Toxoplasmosis in Minas Gerais, Brazil. PLoS ONE 2017, 12, e0170689. [Google Scholar] [CrossRef]
- Meneceur, P.; Bouldouyre, M.A.; Aubert, D.; Villena, I.; Menotti, J.; Sauvage, V.J.; Garin, F.; Derouin, F. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to Pyrimethamine, Sulfadiazine, and Atovaquone. Antimicrob. Agents Chemother. 2008, 52, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Doliwa, C.; Xia, D.; Escotte-Binet, S.; Newsham, E.L.; Aubert, D.; Randle, N.; Wastling, J.M.; Villena, I. Identification of differentially expressed proteins in Sulfadiazine resistant and sensitive strains of Toxoplasma gondii using difference-gel electrophoresis (DIGE). Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Escotte-Binet, S.; Huguenin, A.; Aubert, D.; Martin, A.P.; Kaltenbach, M.; Florent, I.; Villena, I. Metallopeptidases of Toxoplasma gondii: In silico identification and gene expression. Parasite 2018, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Benmerzouga, I.; Checkley, L.A.; Ferdig, M.T.; Arrizabalaga, G.; Wek, R.C.; Sullivan, W.J., Jr. Guanabenz repurposed as an antiparasitic with activity against acute and latent toxoplasmosis. Antimicrob. Agents Chemother. 2015, 59, 6939–6945. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.M.; Zach, S.J.; Wang, X.; Potluri, L.-P.; Neville, A.; Vennerstrom, J.L.; Davis, P.H. Review of Experimental Compounds Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2016, 60, 7017–7034. [Google Scholar] [CrossRef]
- Saremy, S.; Boroujeni, M.E.; Bhattacharjee, B.; Mittal, V.; Chatterjee, J. Identification of potential apicoplast associated therapeutic targets in human and animal pathogen Toxoplasma gondii ME49. Bioinformation 2011, 7, 379–383. [Google Scholar] [CrossRef][Green Version]
- Sonda, S.; Hehl, A.B. Lipid biology of Apicomplexa: Perspectives for new drug targets, particularly for Toxoplasma gondii. Trends Parasitol. 2006, 22, 41–47. [Google Scholar] [CrossRef]
- Waller, R.; Keeling, P.; Donald, R.G.K.; Striepen, B.; Handman, E.; Lang-Unnasch, N.; Cowman, A.F.; Besra, G.; Roos, D.; McFadden, G.I. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1998, 95, 12352–12357. [Google Scholar] [CrossRef]
- Zuther, E.; Johnson, J.J.; Haselkorn, R.; McLeod, R.; Gornicki, P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. USA 1999, 96, 13387–13392. [Google Scholar] [CrossRef]
- Seeber, F.; Soldati-Favre, D. Metabolic pathways in the apicoplast of apicomplexa. Int. Rev. Cell Mol. Biol. 2010, 281, 161–228. [Google Scholar]
- Jomaa, H.; Wiesner, J.; Sanderbrand, S.; Altincicek, B.; Weidemeyer, C.; Hintz, M.; Turbachova, I.; Eberl, M.; Zeidler, J.; Lichtenthaler, H.K.; et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 1999, 285, 1573–1576. [Google Scholar] [CrossRef]
- Ling, Y.; Sahota, G.; Odeh, S.; Chan, J.M.; Araujo, F.G.; Moreno, S.N.; Oldfield, E. Bisphosphonate inhibitors of Toxoplasma gondi growth: In vitro, QSAR, and in vivo investigations. J. Med. Chem. 2005, 48, 3130–3140. [Google Scholar] [CrossRef]
- Clastre, M.; Goubard, A.; Prel, A.; Mincheva, Z.; Viaud-Massuart, M.-C.; Bout, D.; Rideau, M.; Velge-Roussel, F.; Laurent, F. The methylerythritol phosphate pathway for isoprenoid biosynthesis in coccidia: Presence and sensitivity to fosmidomycin. Exp. Parasitol. 2007, 116, 375–384. [Google Scholar] [CrossRef]
- Garcia-Estrada, C.; Prada, C.F.; Fernandez-Rubio, C.; Rojo-Vazquez, F.; Balana-Fouce, R. DNA topoisomerases in apicomplexan parasites: Promising targets for drug discovery. Proc. Biol. Sci. 2010, 277, 1777–1787. [Google Scholar] [CrossRef]
- Maxwell, A. DNA gyrase as a drug target. Biochem. Soc. Trans. 1999, 27, 48–53. [Google Scholar] [CrossRef]
- McFadden, G.I.; Roos, D.S. Apicomplexan plastids as drug targets. Trends Microbiol. 1999, 7, 328–333. [Google Scholar] [CrossRef]
- Gozalbes, R.; Brun-Pascaud, M.; Garcia-Domenech, R.; Galvez, J.; Girard, P.M.; Doucet, J.P.; Derouin, F. Anti-toxoplasma activities of 24 quinolones and fluoroquinolones in vitro: Prediction of activity by molecular topology and virtual computational techniques. Antimicrob. Agents Chemother. 2000, 44, 2771–2776. [Google Scholar] [CrossRef]
- Khan, A.A.; Slifer, T.; Araujo, F.G.; Remington, J.S. Trovafloxacin is active against Toxoplasma gondii. Antimicrob. Agents Chemother. 1996, 40, 1855–1859. [Google Scholar] [CrossRef]
- Reiff, S.B.; Vaishnava, S.; Striepen, B. The HU protein is important for apicoplast genome maintenance and inheritance in Toxoplasma gondii. Eukaryot. Cell 2012, 11, 905–915. [Google Scholar] [CrossRef]
- Pfefferkorn, E.R.; Nothnagel, R.F.; Borotz, S.E. Parasiticidal effect of Clindamycin on Toxoplasma gondii grown in cultured cells and selection of a drug-resistant mutant. Antimicrob. Agents Chemother. 1992, 36, 1091–1096. [Google Scholar] [CrossRef]
- Dubremetz, J.; Garcia-Réguet, N.; Conseil, V.; Fourmaux, M.N. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 1998, 28, 1007–1013. [Google Scholar] [CrossRef]
- Langsley, G.; Heussler, V.; Chaussepied, M.; Stanway, R.R.; Lüder, C.G.K. Intracellular survival of apicomplexan parasites and host cell modification. Int. J. Parasitol. 2009, 39, 163–173. [Google Scholar]
- Portes, J.; Barrias, E.; Travassos, R.; Attias, M.; De Souza, W. Toxoplasma gondii Mechanisms of Entry Into Host Cells. Front. Cell. Infect. Microbiol. 2020, 10, 294. [Google Scholar] [CrossRef]
- Cardew, E.M.; Verlinde, C.L.M.J.; Pohl, E. The calcium-dependent protein kinase 1 from Toxoplasma gondii as target for structure-based drug design. Parasitology 2018, 145, 210–218. [Google Scholar] [CrossRef]
- Murphy, R.C.; Ojo, K.K.; Larson, E.T.; Castellanos-Gonzalez, A.; Perera, B.G.; Keyloun, K.R.; Kim, J.E.; Bhandari, J.G.; Muller, N.R.; Verlinde, C.L.; et al. Discovery of Potent and Selective Inhibitors of Calcium-Dependent Protein Kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med. Chem. Lett. 2010, 1, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ojo, K.K.; Larson, E.T.; Keyloun, K.R.; Castaneda, L.J.; DeRocher, A.E.; Inampudi, K.K.; E Kim, J.; Arakaki, T.L.; Murphy, R.C.; Zhang, L.; et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010, 17, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Müller, J.; Aguado-Martínez, A.; Rahman, M.; Balmer, V.; Manser, V.; Ortega-Mora, L.M.; Ojo, K.K.; Fan, E.; Maly, D.J.; et al. In Vitro and In Vivo Effects of the Bumped Kinase Inhibitor 1294 in the Related Cyst-Forming Apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob. Agents Chemother. 2015, 59, 6361–6374. [Google Scholar] [CrossRef] [PubMed]
- Doggett, J.S.; Ojo, K.K.; Fan, E.; Maly, D.; Van Voorhis, W.C. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob. Agents Chemother. 2014, 58, 3547–3549. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martínez, A.; Ortega-Mora, L.M.; Moreno-Gonzalo, J.; Ferre, I.; Hulverson, M.A.; Choi, R.; McCloskey, M.C.; Barrett, L.K.; Maly, D.J.; et al. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J. Antimicrob. Chemother. 2017, 72, 2334–2341. [Google Scholar] [CrossRef]
- Schaefer, D.A.; Betzer, D.P.; Smith, K.D.; Millman, Z.G.; Michalski, H.C.; Menchaca, S.E.; Zambriski, J.A.; Ojo, K.K.; Hulverson, M.A.; Arnold, S.L.M.; et al. Novel Bumped Kinase Inhibitors Are Safe. and Effective Therapeutics in the Calf Clinical Model. for Cryptosporidiosis. J. Infect. Dis. 2016, 214, 1856–1864. [Google Scholar] [CrossRef]
- Vidadala, R.S.R.; Rivas, K.L.; Ojo, K.K.; Hulverson, M.A.; Zambriski, J.A.; Bruzual, I.; Schultz, T.L.; Huang, W.; Zhang, Z.; Scheele, S.; et al. Development of an Orally Available and Central Nervous System (CNS) Penetrant Toxoplasma gondii Calcium-Dependent Protein Kinase 1 (TgCDPK1) Inhibitor with Minimal Human Ether-a-go-go-Related Gene (hERG) Activity for the Treatment of Toxoplasmosis. J. Med. Chem. 2016, 59, 6531–6546. [Google Scholar] [CrossRef]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. hERG K(+) channels: Structure, function, and clinical significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef]
- Rutaganira, F.U.; Barks, J.; Dhason, M.S.; Wang, Q.; Lopez, M.S.; Long, S.; Radke, J.B.; Jones, N.G.; Maddirala, A.R.; Janetka, J.W.; et al. Inhibition of Calcium Dependent Protein Kinase 1 (CDPK1) by Pyrazolopyrimidine Analogs Decreases Establishment and Reoccurrence of Central Nervous System Disease by Toxoplasma gondii. J. Med. Chem. 2017, 60, 9976–9989. [Google Scholar] [CrossRef]
- Imhof, D.; Anghel, N.; Winzer, P.; Balmer, V.; Ramseier, J.; Hänggeli, K.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Arnold, S.L.; et al. In vitro activity, safety and in vivo efficacy of the novel bumped kinase inhibitor BKI-1748 in non-pregnant and pregnant mice experimentally infected with Neospora caninum tachyzoites and Toxoplasma gondii oocysts. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 90–101. [Google Scholar] [CrossRef]
- Débare, H.; Moiré, N.; Baron, F.; Lantier, L.; Héraut, B.; Van Langendonck, N.; Denevault-Sabourin, C.; Dimier-Poisson, I.; Debierre-Grockiego, F. A Novel Calcium-Dependent Protein Kinase 1 Inhibitor Potently Prevents Toxoplasma gondii Transmission to Foetuses in Mouse. Molecules 2021, 26, 4203. [Google Scholar] [CrossRef]
- Hakimi, M.-A.; Olias, P.; Sibley, L.D. Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin. Microbiol. Rev. 2017, 30, 615–645. [Google Scholar] [CrossRef]
- Ihara, F.; Nishikawa, Y. Toxoplasma gondii manipulates host cell signaling pathways via its secreted effector molecules. Parasitol. Int. 2021, 83, 102368. [Google Scholar] [CrossRef]
- Niedelman, W.; Gold, D.A.; Rosowski, E.; Sprokholt, J.K.; Lim, D.; Arenas, A.; Melo, M.; Spooner, E.; Yaffe, M.B.; Saeij, J.P.J. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 2012, 8, e1002784. [Google Scholar] [CrossRef]
- El Hajj, H.; Demey, E.; Poncet, J.; Lebrun, M.; Wu, B.; Galéotti, N.; Fourmaux, M.N.; Mercereau-Puijalon, O.; Vial, H.; Labesse, G.; et al. The ROP2 family of Toxoplasma gondii rhoptry proteins: Proteomic and genomic characterization and molecular modeling. Proteomics 2006, 6, 5773–5784. [Google Scholar] [CrossRef]
- El Hajj, H.; Lebrun, M.; Arold, S.T.; Vial, H.; Labesse, G.; Dubremetz, J.F. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007, 3, e14. [Google Scholar] [CrossRef]
- Sinai, A.P.; Joiner, K.A. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J. Cell Biol. 2001, 154, 95–108. [Google Scholar] [CrossRef]
- Pernas, L.; Boothroyd, J.C. Association of host mitochondria with the parasitophorous vacuole during Toxoplasma infection is not dependent on rhoptry proteins ROP2/8. Int. J. Parasitol. 2010, 40, 1367–1371. [Google Scholar] [CrossRef]
- El Hajj, H.; Lebrun, M.; Fourmaux, M.N.; Vial, H.; Dubremetz, J.F. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cell. Microbiol. 2007, 9, 54–64. [Google Scholar] [CrossRef]
- Etheridge, R.D.; Alaganan, A.; Tang, K.; Lou, H.J.; Turk, B.E.; Sibley, L.D. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 2014, 15, 537–550. [Google Scholar] [CrossRef]
- Behnke, M.; Fentress, S.J.; Mashayekhi, M.; Li, L.X.; Taylor, G.A.; Sibley, L.D. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog. 2012, 8, e1002992. [Google Scholar] [CrossRef]
- Bernstein, M.; Pardini, L.; Bello Pede Castro, B.; Unzaga, J.M.; Venturini, M.C.; More, G. ROP18 and ROP5 alleles combinations are related with virulence of T. gondii isolates from Argentina. Parasitol. Int. 2021, 83, 102328. [Google Scholar] [CrossRef]
- Shwab, E.K.; Jiang, T.; Pena, H.F.; Gennari, S.M.; Dubey, J.P.; Su, C. The ROP18 and ROP5 gene allele types are highly predictive of virulence in mice across globally distributed strains of Toxoplasma gondii. Int. J. Parasitol. 2016, 46, 141–146. [Google Scholar] [CrossRef]
- Rêgo, W.; Costa, J.; Baraviera, R.; Pinto, L.; Bessa, G.; Lopes, R.; Vitor, R. Association of ROP18 and ROP5 was efficient as a marker of virulence in atypical isolates of Toxoplasma gondii obtained from pigs and goats in Piaui, Brazil. Vet. Parasitol. 2017, 247, 19–25. [Google Scholar] [CrossRef]
- Wei, F.; Wang, W.; Liu, Q. Protein kinases of Toxoplasma gondii: Functions and drug targets. Parasitol. Res. 2013, 112, 2121–2129. [Google Scholar] [CrossRef]
- Grzybowski, M.M.; Dziadek, B.; Gatkowska, J.M.; Dzitko, K.; Dlugonska, H. Towards vaccine against toxoplasmosis: Evaluation of the immunogenic and protective activity of recombinant ROP5 and ROP18 Toxoplasma gondii proteins. Parasitol. Res. 2015, 114, 4553–4563. [Google Scholar] [CrossRef]
- Grzybowski, M.M.; Gatkowska, J.M.; Dziadek, B.; Dzitko, K.; Długońska, H. Human toxoplasmosis: A comparative evaluation of the diagnostic potential of recombinant Toxoplasma gondii ROP5 and ROP18 antigens. J. Med. Microbiol. 2015, 64, 1201–1207. [Google Scholar] [CrossRef]
- Behnke, M.; Khan, A.; Wootton, J.C.; Dubey, J.P.; Tang, K.; Sibley, L.D. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc. Natl. Acad. Sci. USA 2011, 108, 9631–9636. [Google Scholar] [CrossRef]
- Blader, I.J.; Saeij, J.P. Communication between Toxoplasma gondii and its host: Impact on parasite growth, development, immune evasion, and virulence. APMIS 2009, 117, 458–476. [Google Scholar] [CrossRef]
- Taylor, S.; Barragan, A.; Su, C.; Fux, B.; Fentress, S.J.; Tang, K.; Beatty, W.L.; El Hajj, H.; Jerome, M.; Behnke, M.S.; et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 2006, 314, 1776–1780. [Google Scholar] [CrossRef]
- Fentress, S.J.; Behnke, M.S.; Dunay, I.R.; Mashayekhi, M.; Rommereim, L.M.; Fox, B.A.; Bzik, D.J.; Taylor, G.A.; Turk, B.E.; Lichti, C.F.; et al. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 2010, 8, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Steinfeldt, T.; Konen-Waisman, S.; Tong, L.; Pawlowski, N.; Lamkemeyer, T.; Sibley, L.D.; Hunn, J.P.; Howard, J.C. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010, 8, e1000576. [Google Scholar] [CrossRef] [PubMed]
- Butcher, B.A.; Fox, B.A.; Rommereim, L.M.; Kim, S.G.; Maurer, K.J.; Yarovinsky, F.; Herbert, D.B.R.; Bzik, D.J.; Denkers, E.Y. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011, 7, e1002236. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Christian, D.A.; Kochanowsky, J.A.; Phan, A.T.; Clark, J.T.; Wang, S.; Berry, C.; Oh, J.; Chen, X.; Roos, D.S.; et al. The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J. Exp. Med. 2020, 217, e20181757. [Google Scholar] [CrossRef]
- Kochanowsky, J.A.; Thomas, K.K.; Koshy, A.A. ROP16-Mediated Activation of STAT6 Suppresses Host Cell Reactive Oxygen Species Production, Facilitating Type III Toxoplasma gondii Growth and Survival. mBio 2021, 12, e03305-20. [Google Scholar] [CrossRef]
- Sabou, M.; Doderer-Lang, C.; Leyer, C.; Konjic, A.; Kubina, S.; Lennon, S.; Rohr, O.; Viville, S.; Cianférani, S.; Candolfi, E.; et al. Toxoplasma gondii ROP16 kinase silences the cyclin B1 gene promoter by hijacking host cell UHRF1-dependent epigenetic pathways. Cell. Mol. Life Sci. 2020, 77, 2141–2156. [Google Scholar] [CrossRef]
- Simpson, C.; Jones, N.G.; Hull-Ryde, E.A.; Kireev, D.; Stashko, M.; Tang, K.; Janetka, J.W.; Wildman, S.A.; Zuercher, W.J.; Schapira, M.; et al. Identification of small molecule inhibitors that block the Toxoplasma gondii rhoptry kinase ROP18. ACS Infect. Dis. 2016, 2, 194–206. [Google Scholar] [CrossRef]
- Molina, D.; Cossio-Pérez, R.; Rocha-Roa, C.; Pedraza, L.; Cortes, E.; Hernández, A.; Gómez-Marín, J.E. Protein targets of thiazolidinone derivatives in Toxoplasma gondii and insights into their binding to ROP18. BMC Genom. 2018, 19, 856. [Google Scholar] [CrossRef]
- Maclean, A.E.; Bridges, H.R.; Silva, M.F.; Ding, S.; Ovciarikova, J.; Hirst, J.; Sheiner, L. Complexome profile of Toxoplasma gondii mitochondria identifies divergent subunits of respiratory chain complexes including new subunits of cytochrome bc1 complex. PLoS Pathog. 2021, 17, e1009301. [Google Scholar] [CrossRef]
- Al-Anouti, F.; Tomavo, S.; Parmley, S.; Ananvoranich, S. The expression of lactate dehydrogenase is important for the cell cycle of Toxoplasma gondii. J. Biol. Chem. 2004, 279, 52300–52311. [Google Scholar] [CrossRef]
- Alday, P.H.; Bruzual, I.; Nilsen, A.; Pou, S.; Winter, R.; Ben Mamoun, C.; Riscoe, M.K.; Doggett, J.S. Genetic Evidence for Cytochrome b Qi Site Inhibition by 4(1H)-Quinolone-3-Diarylethers and Antimycin in Toxoplasma gondii. Antimicrob. Agents Chemother. 2017, 61, e01866-16. [Google Scholar] [CrossRef]
- McConnell, E.V.; Bruzual, I.; Pou, S.; Winter, R.; Dodean, R.A.; Smilkstein, M.J.; Krollenbrock, A.; Nilsen, A.; Zakharov, L.N.; Riscoe, M.K.; et al. Targeted Structure-Activity Analysis of Endochin-like Quinolones Reveals Potent Qi and Qo Site Inhibitors of Toxoplasma gondii and Plasmodium falciparum Cytochrome bc1 and Identifies ELQ-400 as a Remarkably Effective Compound against Acute Experimental Toxoplasmosis. ACS Infect. Dis. 2018, 4, 1574–1584. [Google Scholar]
- Doggett, J.S.; Nilsen, A.; Forquer, I.; Wegmann, K.W.; Jones-Brando, L.; Yolken, R.H.; Bordón, C.; Charman, S.A.; Katneni, K.; Schultz, T.; et al. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15936–15941. [Google Scholar] [CrossRef]
- Secrieru, A.; Costa, I.C.C.; O’Neill, P.M.; Cristiano, M.L.S. Antimalarial Agents as Therapeutic Tools against Toxoplasmosis—A Short Bridge. between Two Distant Illnesses. Molecules 2020, 25, 1574. [Google Scholar] [CrossRef]
- Bougdour, A.; Maubon, D.; Baldacci, P.; Ortet, P.; Bastien, O.; Bouillon, A.; Barale, J.-C.; Pelloux, H.; Ménard, R.; Hakimi, M.-A. Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites. J. Exp. Med. 2009, 206, 953–966. [Google Scholar] [CrossRef]
- Maubon, D.; Bougdour, A.; Wong, Y.-S.; Brenier-Pinchart, M.-P.; Curt, A.; Hakimi, M.-A.; Pelloux, H. Activity of the histone deacetylase inhibitor FR235222 on Toxoplasma gondii: Inhibition of stage conversion of the parasite cyst form and study of new derivative compounds. Antimicrob. Agents Chemother. 2010, 54, 4843–4850. [Google Scholar] [CrossRef]
- Afifi, M.A.; Al-Rabia, M.W. The immunomodulatory effects of rolipram abolish drug-resistant latent phase of Toxoplasma gondii infection in a murine model. J. Microsc. Ultrastruct. 2015, 3, 86–91. [Google Scholar] [CrossRef]
- Wei, S.; Marches, F.; Daniel, B.; Sonda, S.; Heidenreich, K.; Curiel, T. Pyridinylimidazole p38 mitogen-activated protein kinase inhibitors block intracellular Toxoplasma gondii replication. Int. J. Parasitol. 2002, 32, 969–977. [Google Scholar] [CrossRef]
- Brumlik, M.J.; Pandeswara, S.; Ludwig, S.M.; Jeansonne, D.P.; Lacey, M.R.; Murthy, K.; Daniel, B.J.; Wang, R.F.; Thibodeaux, S.R.; Church, K.M.; et al. TgMAPK1 is a Toxoplasma gondii MAP kinase that hijacks host MKK3 signals to regulate virulence and interferon-gamma-mediated nitric oxide production. Exp. Parasitol. 2013, 134, 389–399. [Google Scholar] [CrossRef]
- Brumlik, M.J.; Wei, S.; Finstad, K.; Nesbit, J.; Hyman, L.E.; Lacey, M.; Burow, M.E.; Curiel, T.J. Identification of a novel mitogen-activated protein kinase in Toxoplasma gondii. Int. J. Parasitol. 2004, 34, 1245–1254. [Google Scholar] [CrossRef]
- Sun, H.; Zhuo, X.; Zhao, X.; Yang, Y.; Chen, X.; Yao, C.; Du, A. The heat shock protein 90 of Toxoplasma gondii is essential for invasion of host cells and tachyzoite growth. Parasite 2017, 24, 22. [Google Scholar] [CrossRef]
- Lyons, R.E.; Johnson, A.M. Heat shock proteins of Toxoplasma gondii. Parasite Immunol. 1995, 17, 353–359. [Google Scholar] [CrossRef]
- Toursel, C.; Dzierszinski, F.; Bernigaud, A.; Mortuaire, M.; Tomavo, S. Molecular cloning, organellar targeting and developmental expression of mitochondrial chaperone HSP60 in Toxoplasma gondii. Mol. Biochem. Parasitol. 2000, 111, 319–332. [Google Scholar] [CrossRef]
- Dobbin, C.A.; Smith, N.C.; Johnson, A.M. Heat shock protein 70 is a potential virulence factor in murine toxoplasma infection via immunomodulation of host NF-kappa B and nitric oxide. J. Immunol. 2002, 169, 958–965. [Google Scholar] [CrossRef]
- Ashwinder, K.; Kho, M.T.; Chee, P.M.; Lim, W.Z.; Yap, I.K.S.; Choi, S.B.; Yam, W.K. Targeting Heat Shock Proteins 60 and 70 of Toxoplasma gondii as a Potential Drug Target.: In Silico Approach. Interdiscip. Sci. 2016, 8, 374–387. [Google Scholar] [CrossRef]
- Boyom, F.F.; Fokou, P.V.T.; Tchokouaha, L.R.Y.; Spangenberg, T.; Mfopa, A.N.; Kouipou, R.M.T.; Mbouna, C.J.; Donfack, V.F.D.; Zollo, P.H.A. Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob. Agents Chemother. 2014, 58, 5848–5854. [Google Scholar] [CrossRef]
- Spalenka, J.; Escotte-Binet, S.; Bakiri, A.; Hubert, J.; Renault, J.-H.; Velard, F.; Duchateau, S.; Aubert, D.; Huguenin, A.; Villena, I. Discovery of New Inhibitors of Toxoplasma gondii via the Pathogen Box. Antimicrob. Agents Chemother. 2018, 62, e01640-17. [Google Scholar] [CrossRef]
- Murata, Y.; Sugi, T.; Weiss, L.M.; Kato, K. Identification of compounds that suppress Toxoplasma gondii tachyzoites and bradyzoites. PLoS ONE 2017, 12, e0178203. [Google Scholar] [CrossRef]
- Opsenica, I.; Verbić, T.; Tot, M.; Sciotti, R.; Pybus, B.S.; Djurković-Djaković, O.; Slavic, K.; Šolaja, B.A. Investigation into novel thiophene- and furan-based 4-amino-7-chloroquinolines afforded antimalarials that cure mice. Bioorg. Med. Chem. 2015, 23, 2176–2186. [Google Scholar] [CrossRef]
- Eissa, M.M.; Barakat, A.M.; Amer, E.I.; Younis, L.K. Could miltefosine be used as a therapy for toxoplasmosis? Exp. Parasitol. 2015, 157, 12–22. [Google Scholar] [CrossRef]
- Liu, S.; Wu, M.; Hua, Q.; Lu, D.; Tian, Y.; Yu, H.; Cheng, L.; Chen, Y.; Cao, J.; Hu, X.; et al. Two old drugs, NVP-AEW541 and GSK-J4, repurposed against the Toxoplasma gondii RH strain. Parasites Vectors 2020, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, A.J.; Drozda, A.A.; Blader, I.J. Drug Repurposing Screening Identifies Novel Compounds That Effectively Inhibit Toxoplasma gondii Growth. mSphere 2016, 1, e00042-15. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.O.; Vanagas, L.; Ruiz, D.M.; Cristaldi, C.; Cartagena, A.M.S.; Sullivan, W.J.J. Emerging Therapeutic Targets Against Toxoplasma gondii: Update on DNA Repair Response Inhibitors and Genotoxic Drugs. Front. Cell. Infect. Microbiol. 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.; Atolani, O.; Awakan, O.J.; Olaolu, T.D.; Nwonuma, C.O.; Alejolowo, O.; Otohinoyi, D.A.; Rotimi, D.; Owolabi, A.; Batiha, G. In Vitro Screening to Identify Anti-Toxoplasma Compounds and In Silico Modeling for Bioactivities and Toxicity. Yale J. Biol. Med. 2019, 92, 369–383. [Google Scholar]
- Barbosa, B.F.; Gomes, A.O.; Ferro, E.; Napolitano, D.R.; Mineo, J.R.; Silva, N.M. Enrofloxacin is able to control Toxoplasma gondii infection in both in vitro and in vivo experimental models. Vet. Parasitol. 2012, 187, 44–52. [Google Scholar] [CrossRef]
- Khan, A.A.; Slifer, T.R.; Araujo, F.G.; Remington, J.S. Activity of gatifloxacin alone or in combination with Pyrimethamine or gamma interferon against Toxoplasma gondii. Antimicrob. Agents Chemother. 2001, 45, 48–51. [Google Scholar] [CrossRef]
- Jones-Brando, L.; D’Angelo, J.; Posner, G.H.; Yolken, R. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob. Agents Chemother. 2006, 50, 4206–4208. [Google Scholar] [CrossRef]
- Dunay, I.R.; Chan, W.C.; Haynes, R.; Sibley, L.D. Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrob. Agents Chemother. 2009, 53, 4450–4456. [Google Scholar] [CrossRef]
- D’Angelo, J.G.; Bordón, C.; Posner, G.H.; Yolken, R.; Jones-Brando, L. Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. J. Antimicrob. Chemother. 2009, 63, 146–150. [Google Scholar] [CrossRef]
- Schultz, T.L.; Hencken, C.P.; Woodard, L.E.; Posner, G.H.; Yolken, R.H.; Jones-Brando, L.; Carruthers, V.B. A thiazole derivative of artemisinin moderately reduces Toxoplasma gondii cyst burden in infected mice. J. Parasitol. 2014, 100, 516–521. [Google Scholar] [CrossRef]
- Alomar, M.L.; Rasse-Suriani, F.A.; Ganuza, A.; Coceres, V.M.; Cabrerizo, F.M.; Angel, S.O. In vitro evaluation of beta-carboline alkaloids as potential anti-Toxoplasma agents. BMC Res. Notes 2013, 6, 193. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Strobl, J.S.; Lindsay, D.S. Evaluation of five antischizophrenic agents against Toxoplasma gondii in human cell cultures. J. Parasitol. 2011, 97, 148–151. [Google Scholar] [CrossRef]
- Messina, M.; Niesman, I.; Mercier, C.; Sibley, L. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene 1995, 165, 213–217. [Google Scholar] [CrossRef]
- Tenorio, R.P.; Carvalho, C.S.; Pessanha, C.S.; de Lima, J.G.; de Faria, A.R.; Alves, A.J.; de Melo, E.J.; Goes, A.J. Synthesis of thiosemicarbazone and 4-thiazolidinone derivatives and their in vitro anti-Toxoplasma gondii activity. Bioorg. Med. Chem. Lett. 2005, 15, 2575–2578. [Google Scholar] [CrossRef]
- Jones-Brando, L.; Torrey, E.F.; Yolken, R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr. Res. 2003, 62, 237–244. [Google Scholar] [CrossRef]
- Miller, R.L.; Gerster, J.F.; Owens, M.L.; Slade, H.B.; Tomai, M.A. Imiquimod applied topically: A novel immune response modifier and new class of drug. Int. J. Immunopharmacol. 1999, 21, 1–14. [Google Scholar] [CrossRef]
- Miranda-Verastegui, C.; Tulliano, G.; Gyorkos, T.W.; Calderon, W.; Rahme, E.; Ward, B.; Cruz, M.; Llanos-Cuentas, A.; Matlashewski, G. First-line therapy for human cutaneous leishmaniasis in Peru using the TLR7 agonist imiquimod in combination with pentavalent antimony. PLoS Negl. Trop. Dis. 2009, 3, e491. [Google Scholar] [CrossRef]
- Raman, V.S.; Duthie, M.S.; Fox, C.B.; Matlashewski, G.; Reed, S.G. Adjuvants for Leishmania vaccines: From models to clinical application. Front. Immunol. 2012, 3, 144. [Google Scholar] [CrossRef]
- El Hajj, R.; Youness, H.B.; Lachaud, L.; Bastien, P.; Masquefa, C.; Bonnet, P.-A.; El Hajj, H.; Khalifeh, I. EAPB0503: An Imiquimod analog with potent in vitro activity against cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica. PLoS Negl. Trop. Dis. 2018, 12, e0006854. [Google Scholar] [CrossRef]
- Miranda-Verastegui, C.; Llanos-Cuentas, A.; Arevalo, I.; Ward, B.J.; Matlashewski, G. Randomized, double-blind clinical trial of topical imiquimod 5% with parenteral meglumine antimoniate in the treatment of cutaneous leishmaniasis in Peru. Clin. Infect. Dis. 2005, 40, 1395–1403. [Google Scholar] [CrossRef]
- Hamie, M.; Najm, R.; Deleuze-Masquefa, C.; Bonnet, P.A.; Dubremetz, J.F.; El Sabban, M.; El Hajj, H. Imiquimod Targets Toxoplasmosis Through Modulating Host Toll-Like Receptor-MyD88 Signaling. Front. Immunol. 2021, 12, 629917. [Google Scholar] [CrossRef]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef]
- Choi, W.H.; Lee, I.A. The Mechanism of Action of Ursolic Acid as a Potential Anti-Toxoplasmosis Agent, and Its Immunomodulatory Effects. Pathogens 2019, 8, 61. [Google Scholar] [CrossRef]
| Toxoplasmosis | Currently Used Drugs | Mechanism(s) of Action |
|---|---|---|
| Congenital toxoplasmosis | ||
| Maternal congenital toxoplasmosis or confirmed infection of neonate or fetus following congenital toxoplasmosis | 1 Pyrimethamine (Inhibitor of dihydrofolate reductase (DHFR)) | Inhibition of the biosynthesis of parasitic folate, interrupting nucleic acid synthesis and parasite replication |
| + 1 Sulfadiazine (Inhibitor of dihydropteroate synthase) | ||
| + Folinic acid (leucovorin) | Reduction of the harmful side effects (i.e., bone marrow myelosuppression) | |
| Suspected congenital toxoplasmosis | Spiramycin | Inhibition of protein synthesis |
| Toxoplasmosis in immunocompetent patients | ||
| Acute toxoplasmosis | 2 Pyrimethamine (Inhibitor of dihydrofolate reductase (DHFR)) | Inhibition of the biosynthesis of parasitic folate, interrupting nucleic acid synthesis and parasite replication |
| + 2 Sulfadiazine (Inhibitor of dihydropteroate synthase) | ||
| + Folinic acid (leucovorin) | ||
| Reduction of the harmful side effects (i.e., bone marrow myelosuppression) | ||
| 2 Pyrimethamine | Inhibition of the biosynthesis of parasitic folate | |
| +3 Clindamycin | Inhibition of protein synthesis | |
| + Folinic acid | Reduction of the harmful side effects (i.e., bone marrow myelosuppression) | |
| 2 Pyrimethamine | Inhibition of the biosynthesis of parasitic folate | |
| + Folinic acid | Reduction of the harmful side effects (i.e., bone marrow myelosuppression) | |
| + Atovaquone | Targeting the mitochondrial electron transport and the mitochondrial cytochrome bc1 complex | |
| 2 Pyrimethamine | Inhibition of the biosynthesis of parasitic folate | |
| Reduction of the harmful side effects (i.e., bone marrow myelosuppression) | ||
| + Folinic acid | ||
| Inhibition of protein synthesis | ||
| + 4 Azithromycin | ||
| 2 Trimethoprim | Inhibition of the biosynthesis of parasitic folate | |
| + Sulfamethoxazole | Bacteriostatic sulfonamide interfering with folic acid synthesis | |
| Ocular toxoplasmosis | ||
| Pyrimethamine | Inhibition of the biosynthesis of parasitic folate, interrupting nucleic acid synthesis and parasite replication | |
| + Sulfadiazine | ||
| +/− 5 Steroids | ||
| Intravitreal Clindamycin | Inhibition of protein synthesis | |
| + 5 Steroids | ||
| Trimethoprim | Inhibition of the biosynthesis of parasitic folate | |
| + Sulfamethoxazole | Bacteriostatic sulfonamide interfering with folic acid synthesis | |
| + 2 Steroids | ||
| Atovaquone | Targeting the mitochondrial electron transport and the mitochondrial cytochrome bc1 complex | |
| 4 Azitromycin+/− Pyrimethamine | Inhibition of protein synthesis+/− biosynthesis of parasite folate | |
| Toxoplasmosis in immunocompromised patients | ||
| (cycles of different doses in induction and maintenance therapy) | ||
| 2 Pyrimethamine | Inhibition of the biosynthesis of parasitic folate, interrupting nucleic acid synthesis and parasite replication | |
| + 2 Sulfadiazine | Reduction of the harmful side effects | |
| + Folinic acid (leucovorin) | ||
| 2 Pyrimethamine | Inhibition of the biosynthesis of parasitic folate | |
| + 3 Clindamycin | Inhibition of protein synthesis | |
| + Folinic acid | Reduction of the harmful side effects | |
| 2 Trimethoprim | Inhibition of the biosynthesis of parasitic folate | |
| + Sulfamethoxazole | Bacteriostatic sulfonamide interfering with folic acid synthesis | |
| 2 Pyrimethamine | Inhibition of the biosynthesis of parasitic folate | |
| + Folinic acid | Reduction of the harmful side effects (i.e., bone marrow myelosuppression) | |
| Targeting the mitochondrial electron transport and the mitochondrial cytochrome bc1 complex | ||
| + Atovaquone | ||
| 2 Sulfadiazine | Inhibition of the biosynthesis of parasitic folate | |
| + Atovaquone | Targeting the mitochondrial electron transport and the mitochondrial cytochrome bc1 complex | |
| 2 Pyrimethamine | Inhibition of the biosynthesis of parasitic folate | |
| + Folinic acid | Reduction of the harmful side effects (i.e., bone marrow myelosuppression) | |
| Inhibition of protein synthesis | ||
| + 4 Azithromycin | ||
| Parasite Targets | ||||
|---|---|---|---|---|
| Apicoplast | Micronemes | Rhoptries | Mitochondria | Nucleus |
| Inhibitors of Fatty Acid synthesis: -Clodinafop -Thiolactomycin -Triclosan | BKI targeting TgCDPK1: -BKI-1294 -BKI-1294 analogs: Compounds 24 and 32) -BKI-1748 | Oxindoles | Targeting HSP60 | Topo-isomerase 2 inhibitors: -Daunorubicin -Trovafloxacin -Enrofloxacin -Gatofloxacin |
| Inhibitors of 2-Isoprenoid synthesis: -Fosmidomycin | SP230 | 6-azaquinazolines | Atovaquone | Topo-isomerase 1 inhibitors: -Artemisinin -Artemisone -Artemiside -Artemether -Harmane -Harmine -Non-harmane |
| Inhibitors of DNA gyrase: -Quinolones -Fuoroquinolones -Ciprofloxacin -Trovafloxacin -Ofloxacin -Temafloxacin | Pyrazolopyridines | ELQ-271 | DNA-intercalating agents: -Fluphenasine -Thioridazine -Trifluoperazine -Hycanton -Phleomycin -Mitomycin C | |
| Inhibitors of Protein synthesis: -Clindamycin -Spiramycin -Azithromycin | Chemical scaffolds | ELQ-316 | Ribonucleotide reductase inhibitors: -Thiosemicarbazones -Hydroxyurea | |
| Thiazolidinone derivatives | ELQ-400 | Oxidative DNA damage/DNA binding: -Resveratrol -Valproic acid | ||
| Naphtoquinones | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajj, R.E.; Tawk, L.; Itani, S.; Hamie, M.; Ezzeddine, J.; El Sabban, M.; El Hajj, H. Toxoplasmosis: Current and Emerging Parasite Druggable Targets. Microorganisms 2021, 9, 2531. https://doi.org/10.3390/microorganisms9122531
Hajj RE, Tawk L, Itani S, Hamie M, Ezzeddine J, El Sabban M, El Hajj H. Toxoplasmosis: Current and Emerging Parasite Druggable Targets. Microorganisms. 2021; 9(12):2531. https://doi.org/10.3390/microorganisms9122531
Chicago/Turabian StyleHajj, Rana El, Lina Tawk, Shaymaa Itani, Maguy Hamie, Jana Ezzeddine, Marwan El Sabban, and Hiba El Hajj. 2021. "Toxoplasmosis: Current and Emerging Parasite Druggable Targets" Microorganisms 9, no. 12: 2531. https://doi.org/10.3390/microorganisms9122531
APA StyleHajj, R. E., Tawk, L., Itani, S., Hamie, M., Ezzeddine, J., El Sabban, M., & El Hajj, H. (2021). Toxoplasmosis: Current and Emerging Parasite Druggable Targets. Microorganisms, 9(12), 2531. https://doi.org/10.3390/microorganisms9122531






