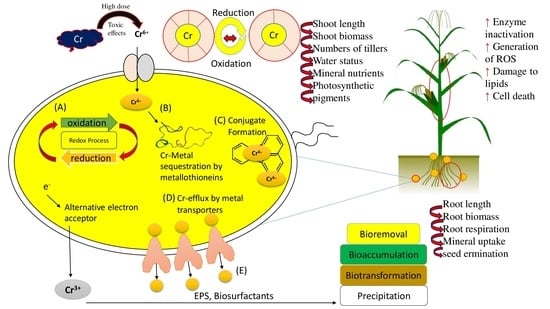
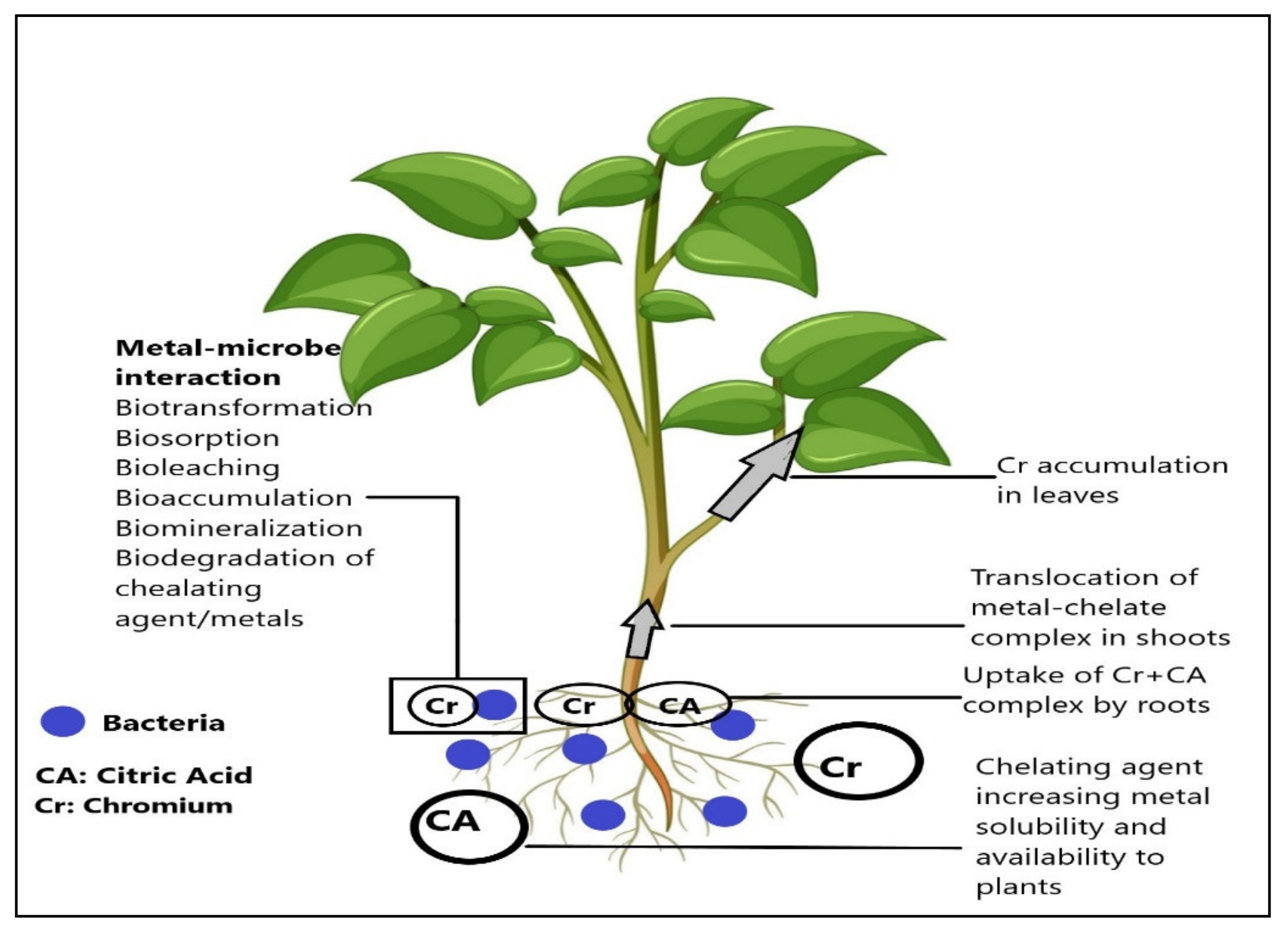
Combined Application of Citric Acid and Cr Resistant Microbes Improved Castor Bean Growth and Photosynthesis while It Alleviated Cr Toxicity by Reducing Cr+6 to Cr3+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Chromium (Cr) Resistant Bacteria
2.2. Bacterial Identification
2.3. Microbial Inoculum Preparation
2.4. Hydroponic Experiment
2.5. Plants Harvesting
2.6. Chlorophyll Contents and Gas Exchange Parameters
2.7. Estimation of MDA, EL, H2O2, and Antioxidants Enzymes
2.8. Estimation of Cr Contents
3. Results
3.1. Plants Growth and Biomass
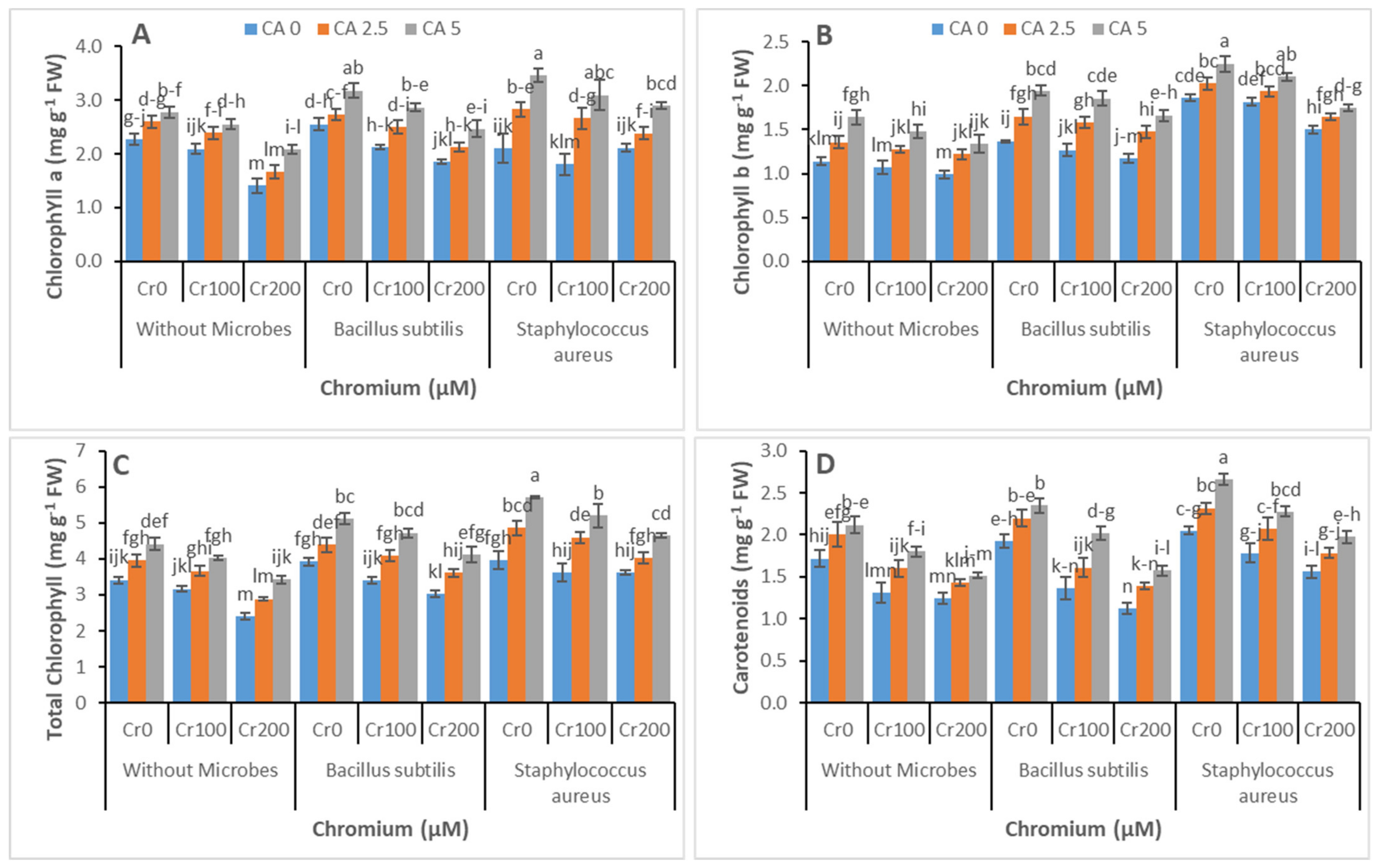
3.2. Chlorophyll Contents and Gas Exchange Parameters
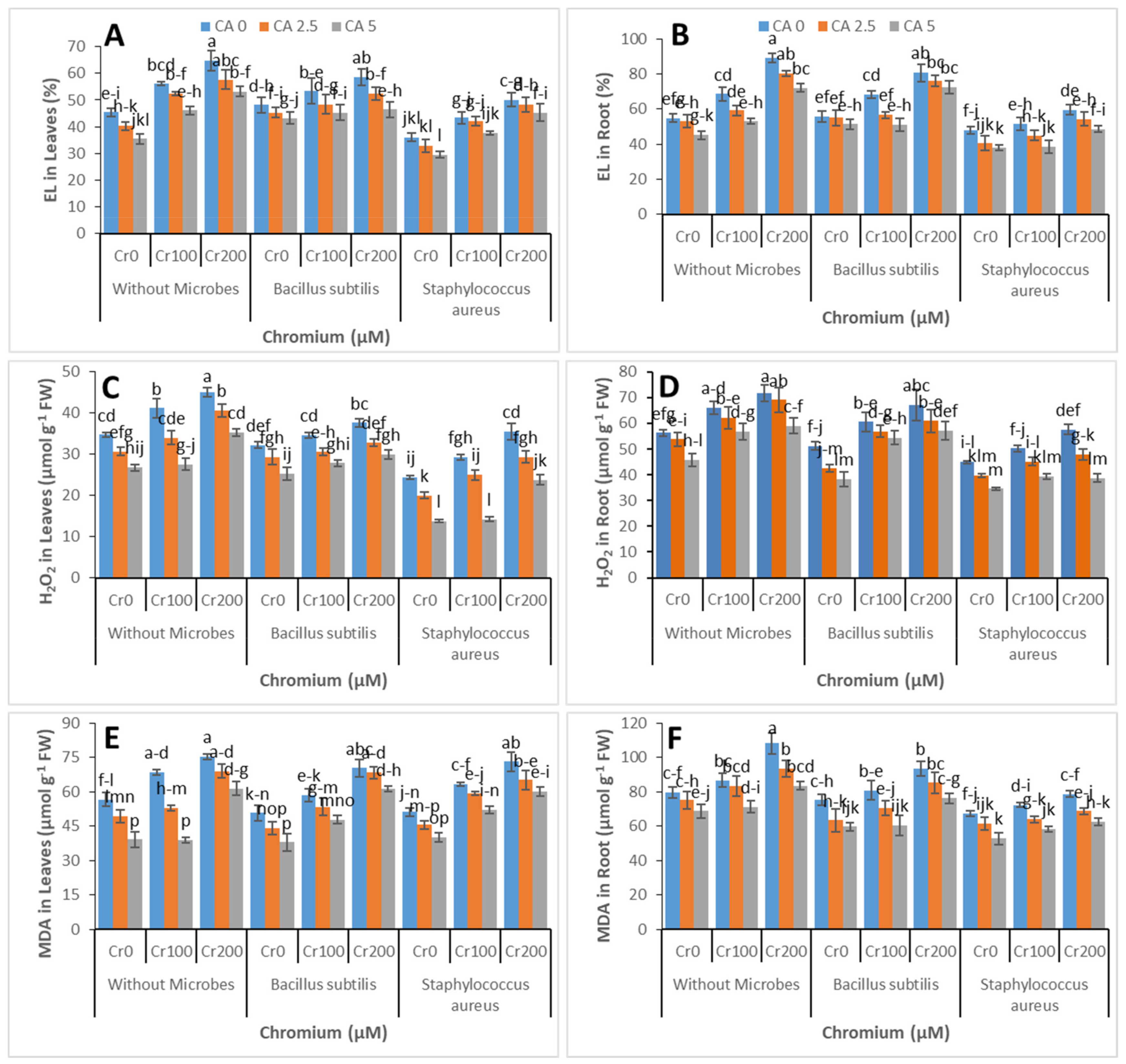
3.3. Electrolyte Leakage, MDA, and H2O2 Contents
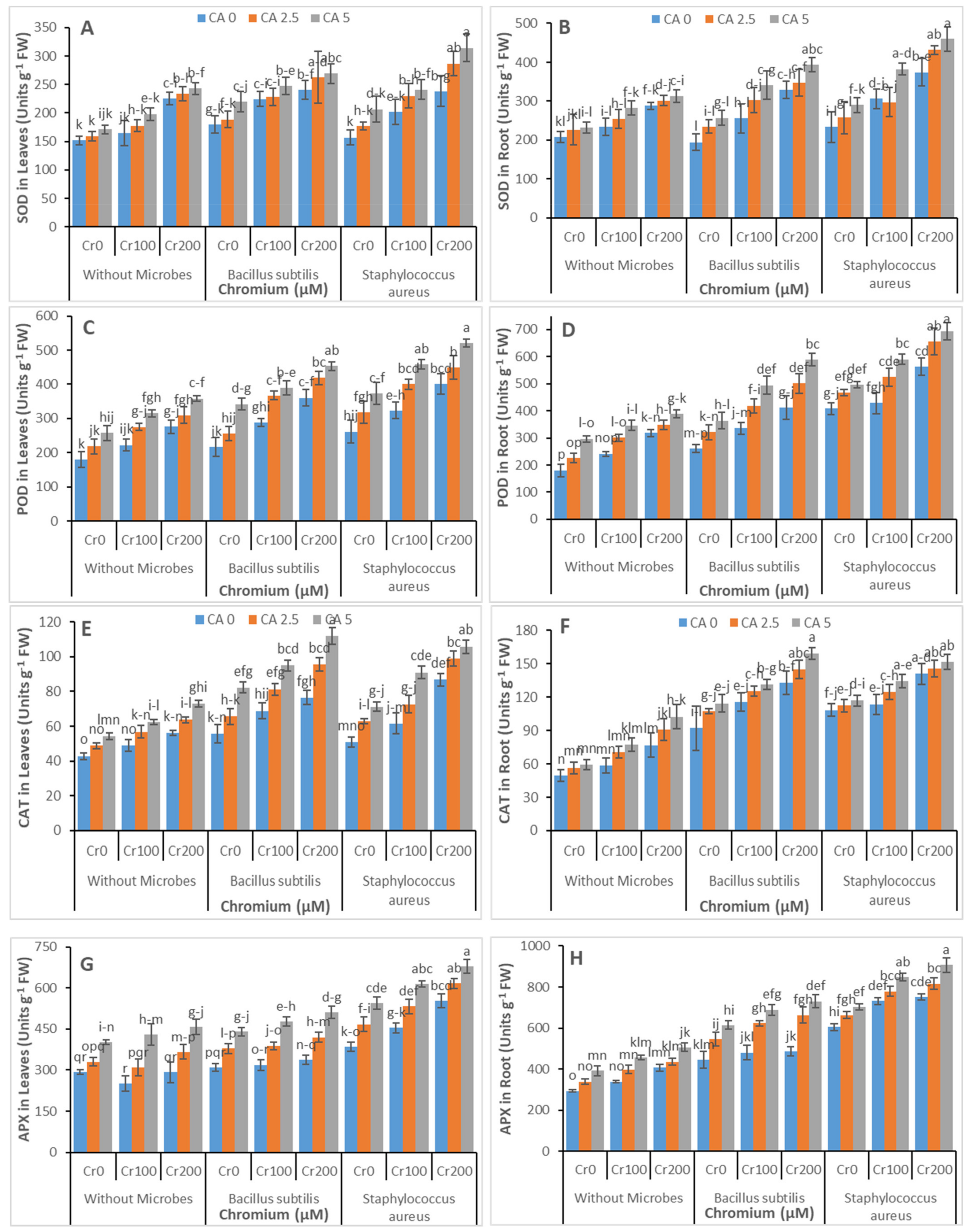
3.4. Antioxidants Enzymatic Activities
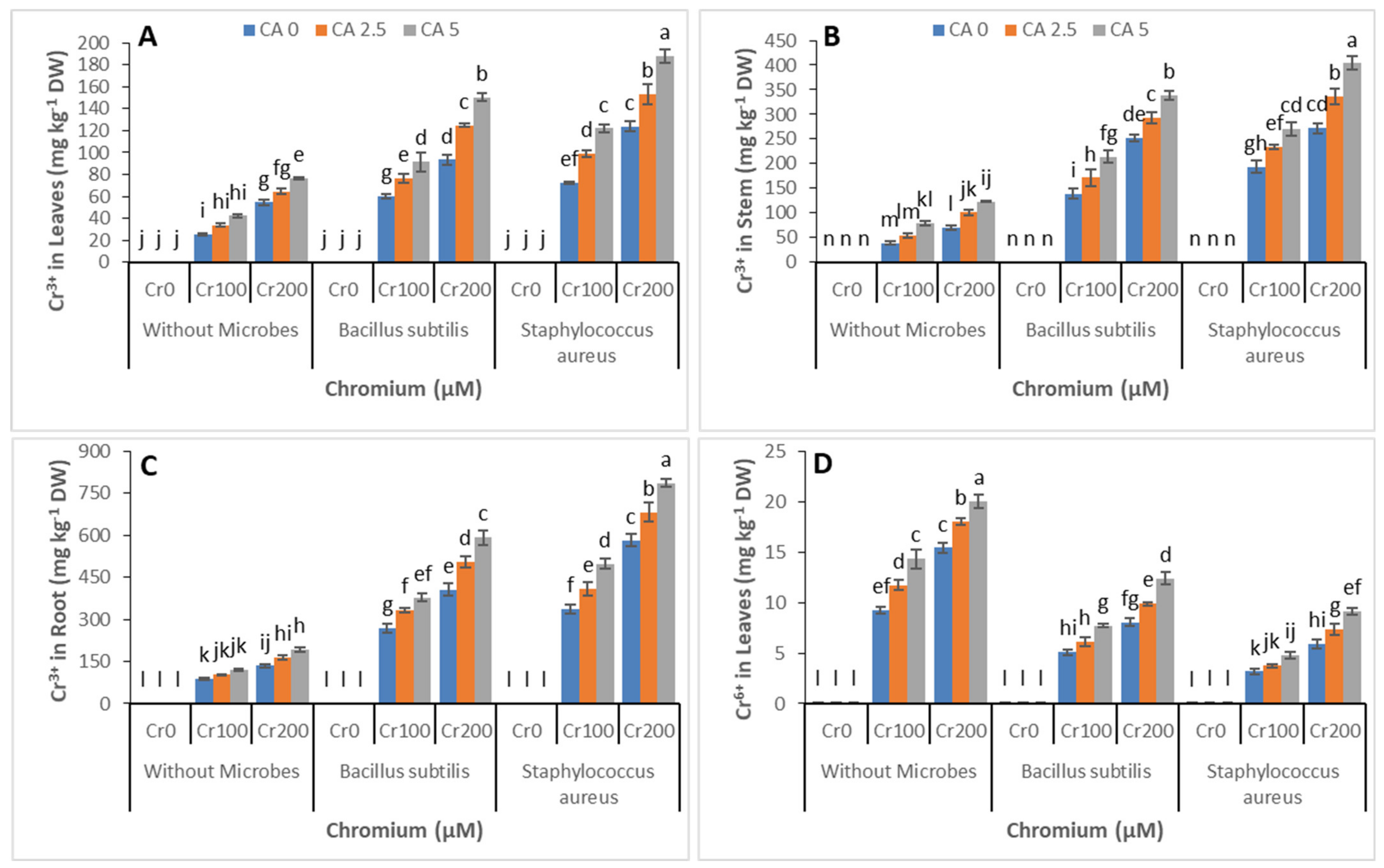
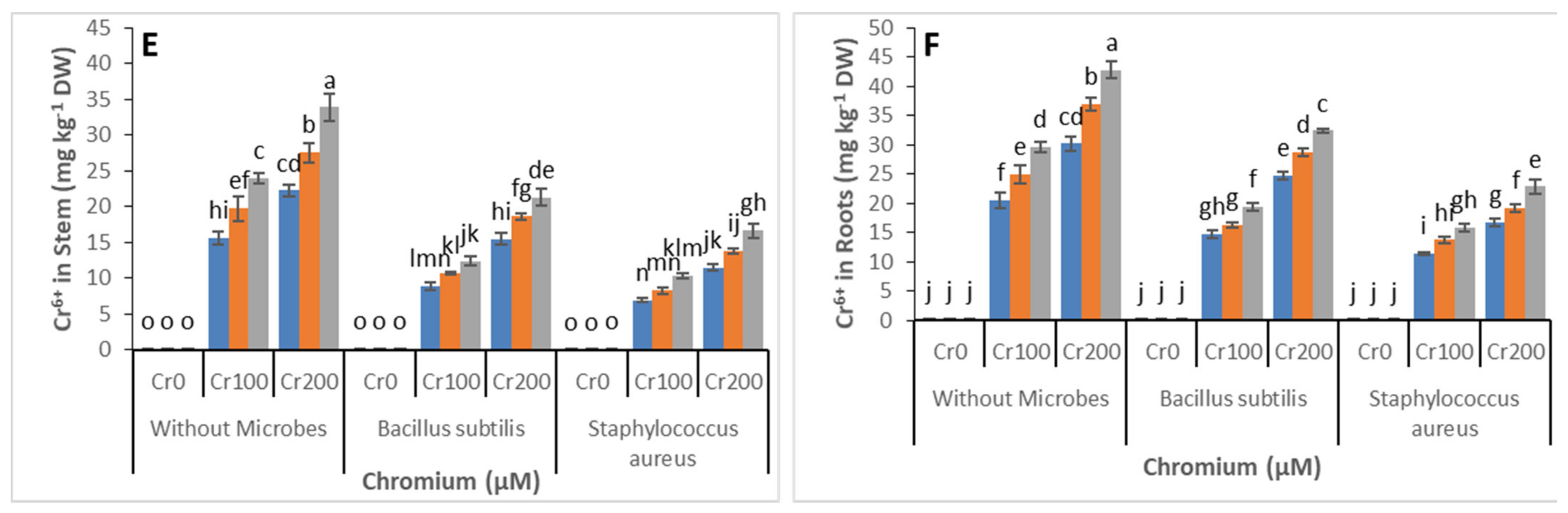
3.5. Chromium Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yoshinaga, M.; Ninomiya, H.; Al Hossain, M.M.A.; Sudo, M.; Akhand, A.A.; Ahsan, N.; Alim, M.A.; Khalequzzaman, M.; Iida, M.; Yajima, I. A comprehensive study including monitoring, assessment of health effects and development of a remediation method for chromium pollution. Chemosphere 2018, 201, 667–675. [Google Scholar] [CrossRef]
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef]
- Shekhawat, K.; Chatterjee, S.; Joshi, B. Chromium toxicity and its health hazards. Int. J. Adv. Res. 2015, 3, 167–172. [Google Scholar]
- Francisco, R.; Moreno, A.; Morais, P.V. Different physiological responses to chromate and dichromate in the chromium resistant and reducing strain Ochrobactrum tritici 5bvl1. Biometals 2010, 23, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.K.; Singh, N.R.; Rath, B.P.; Thatoi, H. Bacterial chromate reduction: A review of important genomic, proteomic, and bioinformatic analysis. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1659–1703. [Google Scholar] [CrossRef]
- Poljsak, B.; Pócsi, I.; Raspor, P.; Pesti, M. Interference of chromium with biological systems in yeasts and fungi: A review. J. Basic Microbiol. 2010, 50, 21–36. [Google Scholar] [CrossRef]
- Achmad, R.T.; Auerkari, E.I. Effects of chromium on human body. Annu. Res. Rev. Biol. 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ranjit, P.; Jhansi, V.; Reddy, K.V. Conventional Wastewater Treatment Processes. In Advances in the Domain of Environmental Biotechnology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 455–479. [Google Scholar]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; Tarpani, R.R.Z. Life cycle assessment of wastewater treatment in developing countries: A review. Water Res. 2019, 153, 63–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [Green Version]
- Salgueiro, J.L.; Perez, L.; Maceiras, R.; Sanchez, A.; Cancela, A. Bioremediation of wastewater using Chlorella vulgaris microalgae: Phosphorus and organic matter. Int. J. Environ. Res. 2016, 10, 465–470. [Google Scholar]
- Allan, G.; Williams, A.; Rabinowicz, P.D.; Chan, A.P.; Ravel, J.; Keim, P. Worldwide genotyping of castor bean germplasm (Ricinus communis L.) using AFLPs and SSRs. Genet. Resour. Crop. Evol. 2008, 55, 365–378. [Google Scholar] [CrossRef]
- Kiran, B.R.; Prasad, M.N.V. Ricinus communis L. (Castor bean), a potential multi-purpose environmental crop for improved and integrated phytoremediation. EuroBiotech J. 2017, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bursztyn Fuentes, A.L.; José, C.; de Los Ríos, A.; do Carmo, L.I.; de Iorio, A.F.; Rendina, A.E. Phytoextraction of heavy metals from a multiply contaminated dredged sediment by chicory (Cichorium intybus L.) and castor bean (Ricinus communis L.) enhanced with EDTA, NTA, and citric acid application. Int. J. Phytoremediat. 2018, 20, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Dipu, S.; Kumar, A.A.; Thanga, S.G. Effect of chelating agents in phytoremediation of heavy metals. Remediat. J. 2012, 22, 133–146. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Sinegani, A.A.S. Improving the efficiency of phytoremediation using electrically charged plant and chelating agents. Environ. Sci. Pollut. Res. 2016, 23, 2479–2486. [Google Scholar] [CrossRef]
- Sinegani, A.A.S.; Tahmasbian, I.; Sinegani, M.S. Chelating agents and heavy metal phytoextraction. In Heavy Metal Contamination of Soils; Springer: Berlin/Heidelberg, Germany, 2015; pp. 367–393. [Google Scholar]
- Mallhi, A.I.; Chatha, S.A.S.; Hussain, A.I.; Rizwan, M.; Bukhar, S.A.H.; Hussain, A.; Mallhi, Z.I.; Ali, S.; Hashem, A.; Abd_Allah, E.F. Citric acid assisted phytoremediation of chromium through sunflower plants irrigated with tannery wastewater. Plants 2020, 9, 380. [Google Scholar] [CrossRef] [Green Version]
- Ehsan, S.; Ali, S.; Noureen, S.; Mahmood, K.; Farid, M.; Ishaque, W.; Shakoor, M.B.; Rizwan, M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014, 106, 164–172. [Google Scholar] [CrossRef]
- Gonzalez, D.J.; Haste, N.M.; Hollands, A.; Fleming, T.C.; Hamby, M.; Pogliano, K.; Nizet, V.; Dorrestein, P.C. Microbial competition between Bacillus subtilis and Staphylococcus aureus monitored by imaging mass spectrometry. Microbiology 2011, 157, 2485. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Bano, A. Modulation of phytoremediation and plant growth by the treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int. J. Phytoremediat. 2016, 18, 1258–1269. [Google Scholar] [CrossRef]
- Belal, E.B.; Kamel, S.M.H.; Hassan, M.M. Production of antimicrobial metabolites by Bacillus subtilis and their applications. Biotechnology 2013, 12, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Hameed, A.; Ahmed, S. Physicochemical characterization and bioremediation perspective of textile effluent, dyes and metals by indigenous bacteria. J. Hazard. Mater. 2009, 164, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int. J. Phytorem. 2016, 18, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shah, M. Characterisation and bioremediation of wastewater: A review exploring bioremediation as a sustainable technique for pharmaceutical wastewater. Groundw. Sustain. Dev. 2020, 11, 100383. [Google Scholar] [CrossRef]
- Turki, Y.; Mehri, I.; Lajnef, R.; Rejab, A.B.; Khessairi, A.; Cherif, H.; Ouzari, H.; Hassen, A. Biofilms in bioremediation and wastewater treatment: Characterization of bacterial community structure and diversity during seasons in municipal wastewater treatment process. Environ. Sci. Pollut. Res. 2017, 24, 3519–3530. [Google Scholar] [CrossRef]
- Lucious, S.; Reddy, E.S.; Anuradha, V.; Vijaya, P.P.; Ali, M.S.; Yogananth, N.; Rajan, R.; Parveen, P.K. Heavy metal tolerance and antibiotic sensitivity of bacterial strains isolated from tannery effluent. Asian J. Exp. Bio. Sci. 2013, 4, 597–606. [Google Scholar]
- Zhang, X.; Krumholz, L.R.; Yu, Z.; Chen, Y.; Liu, P.; Li, X. A novel subspecies of Staphylococcus aureus from sediments of Lanzhou reach of the Yellow River aerobically reduces hexavalent chromium. J. Bioremediat. Biodegrad. 2013, 4. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef]
- Lõoke, M.; Kristjuhan, K.; Kristjuhan, A. Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques 2011, 50, 325–328. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.-Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. JoVE J. Vis. Exp. 2012, 62, e3923. [Google Scholar] [CrossRef]
- Clarridge, J.E., III. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.J.; Arslan, M.; Siddique, M.; Ali, S.; Tahseen, R.; Afzal, M. Potentialities of floating wetlands for the treatment of polluted water of river Ravi, Pakistan. Ecol. Eng. 2019, 133, 167–176. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytorem. 2018, 20, 405–414. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Lace, A.; Ryan, D.; Bowkett, M.; Cleary, J. Chromium monitoring in water by colorimetry using optimised 1, 5-diphenylcarbazide method. Int. J. Environ. Res. Public Health 2019, 16, 1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqbool, A.; Ali, S.; Rizwan, M.; Ishaque, W.; Rasool, N.; ur Rehman, M.Z.; Bashir, A.; Abid, M.; Wu, L. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. Res. 2018, 25, 10848–10856. [Google Scholar] [CrossRef]
- Mustapha, M.U.; Halimoon, N. Screening and isolation of heavy metal tolerant bacteria in industrial effluent. Procedia Environ. Sci. 2015, 30, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Abbas, S.; Ahmed, I.; Kudo, T.; Iqbal, M.; Lee, Y.-J.; Fujiwara, T.; Ohkuma, M. A heavy metal tolerant novel bacterium, Bacillus malikii sp. nov., isolated from tannery effluent wastewater. Antonie Van Leeuwenhoek 2015, 108, 1319–1330. [Google Scholar] [CrossRef]
- Sørensen, S.J.; Bailey, M.; Hansen, L.H.; Kroer, N.; Wuertz, S. Studying plasmid horizontal transfer in situ: A critical review. Nat. Rev. Microbiol. 2005, 3, 700–710. [Google Scholar] [CrossRef]
- Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Abd_Allah, E.F.; et al. Bacterial augmented floating treatmentwetlands for efficient treatment of synthetic textile dye wastewater. Sustainability 2020, 12, 3731. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YavaŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.F.; Ashraf, M.A.; Rasheed, R.; Ali, S.; Hussain, I.; Ahmed, A.; Iqbal, M. Organic chelates decrease phytotoxic effects and enhance chromium uptake by regulating chromium-speciation in castor bean (Ricinus communis L.). Sci. Total Environ. 2020, 716, 137061. [Google Scholar] [CrossRef]
- Mahmood Aulakh, A.; Qadir, G.; Hassan, F.U.; Hayat, R.; Sultan, T.; Billah, M.; Hussain, M.; Khan, N. Desert Soil Microbes as a Mineral Nutrient Acquisition Tool for Chickpea (Cicer arietinum L.) Productivity at Different Moisture Regimes. Plants 2020, 9, 1629. [Google Scholar]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Noor, I.; El-Esawi, M.A.; Hayat, K.; Rizwan, M.; Abbas, Z.; El-Sheikh, M.A.; Alyemeni, M.N. Iron–Lysine mediated alleviation of chromium toxicity in spinach (Spinacia oleracea L.) plants in relation to morpho-physiological traits and iron uptake when irrigated with tannery wastewater. Sustainability 2020, 12, 6690. [Google Scholar]
- ALKahtani, M.D.F.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.-D.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.-D.; Khan, N.; et al. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hurd, C.L. Seaweed nutrient physiology: Application of concepts to aquaculture and bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Merkl, N.; Schultze-Kraft, R.; Arias, M. Influence of fertilizer levels on phytoremediation of crude oil-contaminated soils with the tropical pasture grass Brachiaria brizantha (Hochst. ex a. rich.) stapf. Int. J. Phytoremediat. 2005, 7, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kram, K.E.; Finkel, S.E. Rich medium composition affects Escherichia coli survival, glycation, and mutation frequency during long-term batch culture. Appl. Environ. Microbiol. 2015, 81, 4442–4450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, X.; Tan, H.; Zhao, Y.; Hu, Y. Detoxification of chromium slag by chromate resistant bacteria. J. Hazard. Mater. 2006, 137, 836–841. [Google Scholar] [CrossRef]
- Mohamed, M.S.M.; El-Arabi, N.I.; El-Hussein, A.; El-Maaty, S.A.; Abdelhadi, A.A. Reduction of chromium-VI by chromium-resistant Escherichia coli FACU: A prospective bacterium for bioremediation. Folia Microbiol. 2020, 65, 687–696. [Google Scholar] [CrossRef]
- Nawaz, M.; Ishaq, S.; Ishaq, H.; Khan, N.; Iqbal, N.; Ali, S.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Salicylic Acid Improves Boron Toxicity Tolerance by Modulating the Physio-Biochemical Characteristics of Maize (Zea mays L.) at an Early Growth Stage. Agronomy 2020, 10, 2013. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, S.; Rizwan, M.; Hussain, M.B.; Hussain, A.; Alam, P.; Alqarawi, A.A.; Hashem, A.; AbdAllah, E.F. The ameliorative role of 5-aminolevulinic acid (ALA) under Cr stress in two maize cultivars showing differential sensitivity to Cr stress tolerance. J. Plant Growth Regul. 2019, 38, 788–798. [Google Scholar] [CrossRef]
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S.; Noor, Y.; Javed, M.; Athar, H.R.; Zafar, Z.U. Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 2020, 6, e04364. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Long, M.; Islam, F.; Farooq, M.A.; Wang, J.; Mwamba, T.M.; Shou, J.; Zhou, W. Synergistic effects of chromium and copper on photosynthetic inhibition, subcellular distribution, and related gene expression in Brassica napus cultivars. Environ. Sci. Pollut. Res. 2019, 26, 11827–11845. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Verma, S.; Pandith, S.A.; Masood, A.; Khan, N.A. Ethylene supplementation increases PSII efficiency and alleviates chromium-inhibited photosynthesis through increased nitrogen and sulfur assimilation in mustard. J. Plant Growth Regul. 2018, 37, 1300–1317. [Google Scholar] [CrossRef]
- Ali, J.; Mahmood, T.; Hayat, K.; Afridi, M.S.; Ali, F.; Chaudhary, H.J. Phytoextraction of Cr by maize (Zea mays L.): The role of plant growth promoting endophyte and citric acid under polluted soil. Arch. Environ. Prot. 2018, 44. [Google Scholar]
- Zeng, F.; Zahoor, M.; Waseem, M.; Anayat, A.; Rizwan, M.; Ahmad, A.; Yasmeen, T.; Ali, S.; El-Sheikh, M.A.; Alyemeni, M.N. Influence of metal-resistant staphylococcus aureus strain K1 on the alleviation of chromium stress in wheat. Agronomy 2020, 10, 1354. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ma, L.Q.; Cao, X.; Rathinasabapathi, B. Effects of heavy metals on growth and arsenic accumulation in the arsenic hyperaccumulator Pteris vittata L. Environ. Pollut. 2004, 132, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Choudhury, S.; Patra, H.K. Heavy-metal-induced oxidative stress in plants: Physiological and molecular perspectives. Abiotic Stress Response Plants 2016, 1, 219–232. [Google Scholar]
- Islam, F.; Yasmeen, T.; Ali, Q.; Mubin, M.; Ali, S.; Arif, M.S.; Hussain, S.; Riaz, M.; Abbas, F. Copper-resistant bacteria reduces oxidative stress and uptake of copper in lentil plants: Potential for bacterial bioremediation. Environ. Sci. Pollut. Res. 2016, 23, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Hua, S.; Shamsi, I.H.; Jilani, G.; Li, Y.; Jiang, L. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul. 2009, 58, 47–59. [Google Scholar]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar]
- Qiu, B.; Zeng, F.; Cai, S.; Wu, X.; Haider, S.I.; Wu, F.; Zhang, G. Alleviation of chromium toxicity in rice seedlings by applying exogenous glutathione. J. Plant Physiol. 2013, 170, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.-B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031. [Google Scholar]
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Saqib, M.; Ayub, M.A. Combined effects of citric acid and 5-aminolevulinic acid in mitigating chromium toxicity in sunflower (Helianthus annuus L.) grown in Cr spiked soil. Pak. J. Agric. Sci. 2020, 57, 477–488. [Google Scholar]
- Sallah-Ud-Din, R.; Farid, M.; Saeed, R.; Ali, S.; Rizwan, M.; Tauqeer, H.M.; Bukhari, S.A.H. Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. Environ. Sci. Pollut. Res. 2017, 24, 17669–17678. [Google Scholar] [CrossRef] [PubMed]
- Miłobędzka, A.; Witeska, A.; Muszyński, A. Factors affecting population of filamentous bacteria in wastewater treatment plants with nutrients removal. Water Sci. Technol. 2016, 73, 790–797. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Danish, M.; Singh, R.S.; Rafatullah, M.; HPS, A.K. Exploiting microbial biomass in treating azo dyes contaminated wastewater: Mechanism of degradation and factors affecting microbial efficiency. J. Water Process Eng. 2021, 43, 102255. [Google Scholar] [CrossRef]
- Kalantari, N. Evaluation of toxicity of iron, chromium and cadmium on Bacillus cereus growth. Iran. J. Basic Med. Sci. 2008, 222–228. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ali, S.; Refay, Y.; Rizwan, M.; Alhammad, B.A.; El-Hendawy, S.E. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 2020, 250, 126239. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Waseem, M.; Hussain, A.; Rizwan, M.; Ahmad, A.; Khan, N. Combined Application of Citric Acid and Cr Resistant Microbes Improved Castor Bean Growth and Photosynthesis while It Alleviated Cr Toxicity by Reducing Cr+6 to Cr3+. Microorganisms 2021, 9, 2499. https://doi.org/10.3390/microorganisms9122499
Ali S, Waseem M, Hussain A, Rizwan M, Ahmad A, Khan N. Combined Application of Citric Acid and Cr Resistant Microbes Improved Castor Bean Growth and Photosynthesis while It Alleviated Cr Toxicity by Reducing Cr+6 to Cr3+. Microorganisms. 2021; 9(12):2499. https://doi.org/10.3390/microorganisms9122499
Chicago/Turabian StyleAli, Shafaqat, Muhammad Waseem, Afzal Hussain, Muhammad Rizwan, Awais Ahmad, and Naeem Khan. 2021. "Combined Application of Citric Acid and Cr Resistant Microbes Improved Castor Bean Growth and Photosynthesis while It Alleviated Cr Toxicity by Reducing Cr+6 to Cr3+" Microorganisms 9, no. 12: 2499. https://doi.org/10.3390/microorganisms9122499
APA StyleAli, S., Waseem, M., Hussain, A., Rizwan, M., Ahmad, A., & Khan, N. (2021). Combined Application of Citric Acid and Cr Resistant Microbes Improved Castor Bean Growth and Photosynthesis while It Alleviated Cr Toxicity by Reducing Cr+6 to Cr3+. Microorganisms, 9(12), 2499. https://doi.org/10.3390/microorganisms9122499