History of COVID-19 Symptoms and Seroprevalence of SARS-CoV-2 Antibodies in HIV-Infected Patients in Northern France after the First Wave of the Pandemic
Abstract
:1. Introduction
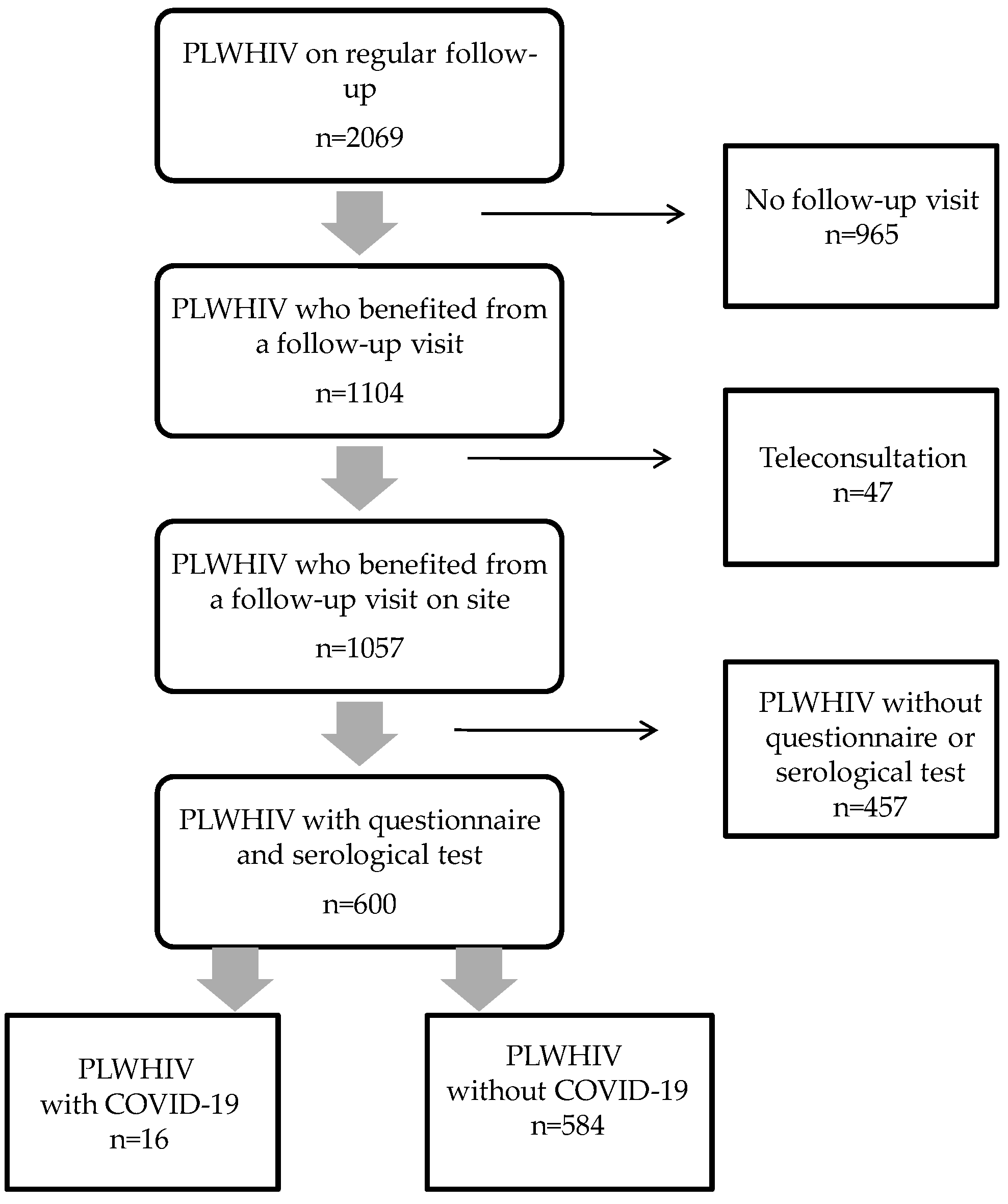
2. Materials and Methods
2.1. Patients and Hospital Setting
2.2. Data Collection
2.3. Biological Assays
2.4. Definitions and Endpoints
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. Exposure Factors, Symptoms, and Diagnostic Tests
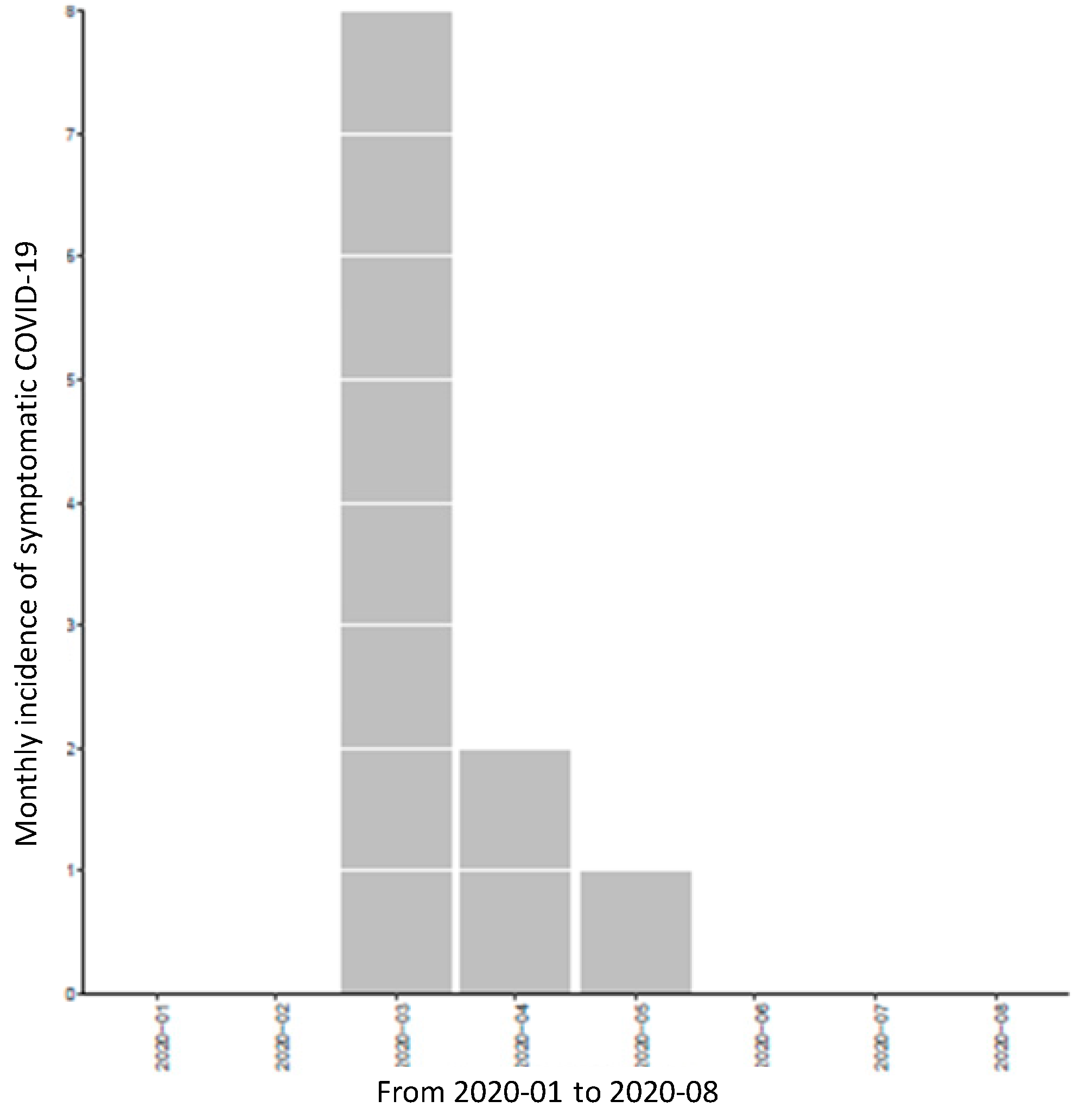
3.3. Prevalence and Incidence of COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhan, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T.; Li, P.; Zhou, Y.; Lin, Y.F.; Duan, Q.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 2020, 80, 656–665. [Google Scholar] [CrossRef]
- Calza, L.; Bon, I.; Tadolini, M.; Borderi, M.; Colangeli, V.; Badia, L.; Verruchi, G.; Rossini, G.; Vocale, C.; Gaibani, P.; et al. COVID-19 in patients with HIV-1 infection: A single-centre experience in northern Italy. Infection 2021, 49, 333–337. [Google Scholar] [CrossRef]
- Cooper, T.J.; Woodward, B.L.; Alom, S.; Harky, A. Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: A systematic review. HIV Med. 2020, 21, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Vizcarra, P.; Pérez-Elías, M.J.; Quereda, C.; Moreno, A.; Vivancos, M.J.; Dronda, F.; Casado, J.L. COVID-19 ID Team. Description of COVID-19 in HIV-infected individuals: A single-centre, prospective cohort. Lancet HIV 2020, 7, e554–e564. [Google Scholar] [CrossRef]
- Charre, C.; Icard, V.; Pradat, P.; Brochier, C.; Lina, B.; Chidiac, C.; Cotte, L. COVID-19 attack rate in HIV-infected patients and in PrEP users. AIDS 2020, 34, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Nichol, K.L. Excess mortality due to pneumonia or influenza durin influenza seasons among persons with acquired immunodeficiency syndrome. Arch. Intern. Med. 2001, 161, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. Trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Ribavirin, remdesivir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (PdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef] [PubMed]
- DICOM_Jocelyne, M. Dans les Etablissements de Santé: Recommandations COVID-19 et Prise en Charge [Internet] Ministère des Solidarités et de la Santé. 2020. Available online: https://solidarite-sante.gouv.fr/soins-et-maladies/maladies-infectieuses/coronavirus/professionnels-de-sante/article/dans-les-etablissements-de-sante-recommandations-covid-19-et-de-prise-en-charge (accessed on 10 May 2020).
- Pugliese, P.; Cuzin, L.; Enel, P.; Agher, R.; Alfandari, S.; Billaud, E.; Druard, P.; Duvivier, C.; Perez, M.; Salmi, D.; et al. NADIS 2000, development of an electronic medical record for patients infected by HIV, HBV and HCV. Presse Med. 2003, 32, 299–303. [Google Scholar] [PubMed]
- Grzelak, L.; Temmam, S.; Planchais, C.; Demeret, C.; Tondeur, L.; Huon, C.; Guivel-Benhassine, F.; Staropoli, I.; Chazal, M.; Dufloo, J.; et al. SARS-CoV-2 serological analysis of COVID-19 hospitalized patients, pauci-symptomatic individuals and blood donors. MedRxiv 2020, preprint. [Google Scholar] [CrossRef] [Green Version]
- Grzelak, L.; Temmam, S.; Planchais, C.; Demeret, C.; Tondeur, L.; Huon, C.; Guivel-Benhassine, F.; Staropoli, I.; Chazal, M.; Dufloo, J.; et al. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci. Transl. Med. 2020, 12, eabc3103. [Google Scholar] [CrossRef] [PubMed]
- Salje, H.; Tran Kiem, C.; Lefrancq, N.; Courtejoie, N.; Bosetti, P.; Paireau, J.; Andronico, A.; Hoze, N.; Richet, J.; Dubost, C.L.; et al. Estimating the burden of SARS-CoV-2 in France. Science 2020, 369, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Fafi-Kremer, S.; Bruel, T.; Madec, Y.; Grant, R.; Tondeur, L.; Grzelak, L.; Staropoli, I.; Anna, F.; Souque, P.; Fernandes-Pellerin, S.; et al. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. EbioMedicine 2020, 59, 102915. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [CrossRef]
- Huang, J.; Xie, N.; Hu, X.; Yan, H.; Ding, J.; Liu, P.; Ma, H.; Ruan, L.; Li, G.; He, N.; et al. Epidemiological, virological and serological features of COVID-19 cases in people living with HIV in Wuhan City: A population-based cohort study. Clin. Infect. Dis. 2020, ciaa1186. [Google Scholar] [CrossRef]
- Souty, C.; Amoros, P.; Falchi, A.; Capai, L.; Bonmarin, I.; van der Werf, S.; Masse, S.; Turbelin, C.; Rossignol, L.; Vilcu, A.M.; et al. Influenza epidemics observed in primary care from 1984 to 2017 in France: A decrease in epidemic size over time. Influenza Other Respir. Viruses 2019, 13, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelle, P.Y.; Souty, C.; Launay, T.; Guerrisi, C.; Turbelin, C.; Behillil, S.; Enouf, V.; Poletto, C.; Lina, B.; van der Werf, S.; et al. Excess cases of influenza-like illnesses synchronous with coronavirus disease (COVID-19) epidemic, France, March 2020. Eur. Surveill. 2020, 25, 2000326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; de Fanciscis, S.; Serra, R. Cardiovascular disease as a biomarker for an increased risk of COVID-19 infection and related poor prognosis. Biomark. Med. 2020, 14, 713–716. [Google Scholar]
- Maggiolo, F.; Zoboli, F.; Arosio, M.; Valenti, D.; Guarneri, D.; Sangriorgio, L.; Ripamonti, D.; Callegaro, A. SARS-CoV-2 infection in persons living with HIV: A single center prospective cohort. J. Med. Virol. 2020, 93, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.; Vitoria, M.; Rangaraj, A.; Norris, S.L.; Calmy, A.; Doherty, M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: Initial assessment. J. Int. AIDS Soc. 2020, 23, e25489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, J.L.; Ambrosioni, J.; Garcia, F.; Martínez, E.; Soriano, A.; Mallolas, J.; Miro, J.M. COVID-19 in HIV Investigators. COVID-19 in patients with HIV: Clinical case series. Lancet HIV 2020, 7, e314–e316. [Google Scholar] [CrossRef]
- Härter, G.; Spinner, C.D.; Roider, J.; Bickel, M.; Krznaric, I.; Grunwald, S.; Schabaz, F.; Gillor, D.; Postel, N.; Mueller, M.C.; et al. COVID-19 in people living with human immunodeficiency virus: A case series of 33 patients. Infection 2020, 48, 681–686. [Google Scholar] [CrossRef]
- Guo, W.; Ming, F.; Dong, Y.; Zhang, Q.; Zhang, X.; Mo, P.; Feng, Y.; Liang, K. A Survey for COVID-19 among HIV/AIDS Patients in Two Districts of Wuhan, China. Lancet 2020, 7, e524–e526. [Google Scholar] [CrossRef]
- Dandachi, D.; Geiger, G.; Montgomery, M.W.; Karmen-Tuohy, S.; Golzy, M.; Antar, A.A.R.; Llibre, J.M.; Camazine, M.; Diaz-De Santiago, A.; Carlucci, M.P.; et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin. Infect. Dis. 2021, 73, e1964–e1972. [Google Scholar] [CrossRef]
- Karmen-Tuohy, S.; Carlucci, P.M.; Zervou, F.N.; Zacharioudakis, I.M.; Rebick, G.; Klein, E.; Reich, J.; Jones, S.; Rahimian, J. Outcomes among HIV-Positive patients hospitalized with COVID-19. J. Acquir. Immune Defic. Syndr. 2020, 85, 6–10. [Google Scholar] [CrossRef]
- Gervasoni, C.; Meraviglia, P.; Riva, A.; Giacomelli, A.; Oreni, L.; Minisci, D.; Atzori, C.; Ridolfo, A.; Cattaneo, D. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- BHIVA; DAIG; EACS; GESIDA; Polish Scientific AIDS Society. Statement on Risk of COVID-19 for People Living with HIV (PLWH). Available online: https://www.eacsociety.org (accessed on 15 November 2020).
- Vena, A.; Berruti, M.; Adessi, A.; Blumetti, P.; Brignole, M.; Colognato, R.; Gaggioli, G.; Giacobbe, D.R.; Bracci-Laudiero, L.B.; Magnasco, L.; et al. Prevalence of antibodies to SARS-CoV-2 in Italian adults and associated risk factors. J. Clin. Med. 2020, 9, 2780. [Google Scholar] [CrossRef] [PubMed]
| Patients Characteristics | Total n = 600 | COVID-19 Negative Patients n = 584 | COVID-19 Confirmed Cases n = 16 | p-Value |
|---|---|---|---|---|
| Age (years) | 49 ± 13 | 49 ± 13 | 49 ± 15 | 0.99 |
| Male gender (%) | 446 (74.3) | 432 (73.8) | 14(93.3) | 0.13 |
| Obesity (BMI ≥ 30 Kg/m2) | 104 (17.3) | 101 (17.3) | 3 (20) | 0.73 |
| Pregnancy | 1 (0.17) | 1 (0.17) | 0 (0) | 1.00 |
| Comorbidities | ||||
| Other factor of immunodepression | 16 (2.7) | 15 (2.6) | 1 (6.7) | 0.34 |
| Diabetes melitus | 44 (7.3) | 43 (7.4) | 1 (6.7) | 1.00 |
| Cardiovascular diseases | 162 (27.0) | 158 (27.0) | 4 (26.7) | 1.00 |
| Chronic hepatitis | 66 (11.0) | 64 (10.9) | 2 (13.3) | 0.62 |
| Chronic pulmonary disease | 62 (10.3) | 62 (10.6) | 0 (0.0) | 0.39 |
| Chronic kidney disease | 32 (5.3) | 32 (5.5) | 0 (0.0) | 1.00 |
| HIV infection | ||||
| CDC stage | 0.07 | |||
| A | 372 (62) | 361 (61.7) | 11 (73.3) | |
| B | 109 (18.2) | 108 (18.5) | 1 (6.7) | |
| C | 116 (19.3) | 114 (19.5) | 2 (13.3) | |
| Nadir of CD4 cell count (/mm3) | 265 ± 183 | 266 ± 183 | 243 ± 156 | 0.60 |
| CD4 cell count < 200/mm3 | 10 (1.7) | 9 (1.5) | 1 (7.1) | 0.39 |
| Current CD4 cell count | 707 ± 300 | 709 ± 302 | 630 ± 220 | 0.21 |
| Current viral load | 242 ± 2978 | 232 ± 2998 | 657 ± 1969 | 0.46 |
| Viral load < 20 copies/mL | 532 (89) | 521 (89) | 11 (79) | 0.20 |
| Duration of HIV (years) | 12.1 ± 8.1 | 12.1 ± 8.1 | 12.6 ± 10.3 | 0.84 |
| Current antiretroviral therapy | ||||
| TDF/TAF | 365 (61) | 354 (61) | 11 (73) | 0.46 |
| Lopinavir | 5 (0.8) | 5 (0.9) | 0 (0.0) | 1.00 |
| Characteristics | Total n = 600 | COVID-19 Negative Patients n = 584 | COVID-19 Confirmed Cases n = 16 | p-Value |
|---|---|---|---|---|
| Exposure factors: | ||||
| Travel abroad since January 2020 | 66 (11) | 63 (10.8) | 3 (20.0) | 0.22 |
| Contact with a COVID-19 case | 82 (13.7) | 76 (13.0) | 6 (40.0) | 0.01 |
| Healthcare worker | 24 (4) | 22(3.8) | 2 (13.3) | 0.12 |
| Symptoms: | 194 (32.3) | 184 (31.5) | 10 (66.7) | 0.02 |
| Fever | 76 (12.7) | 70 (12.0) | 6 (40) | 0.007 |
| Cough | 105 (17.5) | 98 (16.8) | 7 (46.7) | 0.008 |
| Myalgia | 112 (18.7) | 103(17.6) | 9 (60) | <0.001 |
| Fatigue | 110 (18.3) | 101 (17.3) | 9 (60) | <0.001 |
| Headache/dizziness | 70 (11.7) | 64 (10.9) | 6 (40) | 0.004 |
| Digestive disorders | 49 (8.2) | 48 (8.2) | 1 (6.7) | 1.00 |
| Dyspnea | 47 (7.8) | 43 (7.3) | 4 (26.7) | 0.02 |
| Anosmia | 15 (2.5) | 11 (1.9) | 4 (26.7) | <0.001 |
| Loss of taste | 17 (2.8) | 14 (2.4) | 3 (20.0) | 0.007 |
| SARS-CoV-2 PCR test: | 13 (2.2) | 10 (1.7) | 3 (20.0) | 0.03 |
| Positive | 3 (0.5) | 0 (0.0) | 3 (20.0) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meybeck, A.; Huleux, T.; Tétart, M.; Thill, P.; Derdour, V.; Bocket, L.; Alidjinou, E.K.; Patoz, P.; Robineau, O.; Ajana, F. History of COVID-19 Symptoms and Seroprevalence of SARS-CoV-2 Antibodies in HIV-Infected Patients in Northern France after the First Wave of the Pandemic. Microorganisms 2021, 9, 2491. https://doi.org/10.3390/microorganisms9122491
Meybeck A, Huleux T, Tétart M, Thill P, Derdour V, Bocket L, Alidjinou EK, Patoz P, Robineau O, Ajana F. History of COVID-19 Symptoms and Seroprevalence of SARS-CoV-2 Antibodies in HIV-Infected Patients in Northern France after the First Wave of the Pandemic. Microorganisms. 2021; 9(12):2491. https://doi.org/10.3390/microorganisms9122491
Chicago/Turabian StyleMeybeck, Agnès, Thomas Huleux, Macha Tétart, Pauline Thill, Vincent Derdour, Laurence Bocket, Enagnon Kazali Alidjinou, Pierre Patoz, Olivier Robineau, and Faiza Ajana. 2021. "History of COVID-19 Symptoms and Seroprevalence of SARS-CoV-2 Antibodies in HIV-Infected Patients in Northern France after the First Wave of the Pandemic" Microorganisms 9, no. 12: 2491. https://doi.org/10.3390/microorganisms9122491
APA StyleMeybeck, A., Huleux, T., Tétart, M., Thill, P., Derdour, V., Bocket, L., Alidjinou, E. K., Patoz, P., Robineau, O., & Ajana, F. (2021). History of COVID-19 Symptoms and Seroprevalence of SARS-CoV-2 Antibodies in HIV-Infected Patients in Northern France after the First Wave of the Pandemic. Microorganisms, 9(12), 2491. https://doi.org/10.3390/microorganisms9122491







