Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Bacterial Strains and Preparation of Conditioned Media
2.3. Extraction, Purification, and Detection of EPS
2.4. Cell Viability Assay
2.5. Calcein-AM/PI Staining
2.6. MDC Staining
2.7. Lyso-Tracker Red Staining
2.8. Acridine Orange Staining
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Pretreatment with EPS Reduced the Cell Death Induced by EPEC
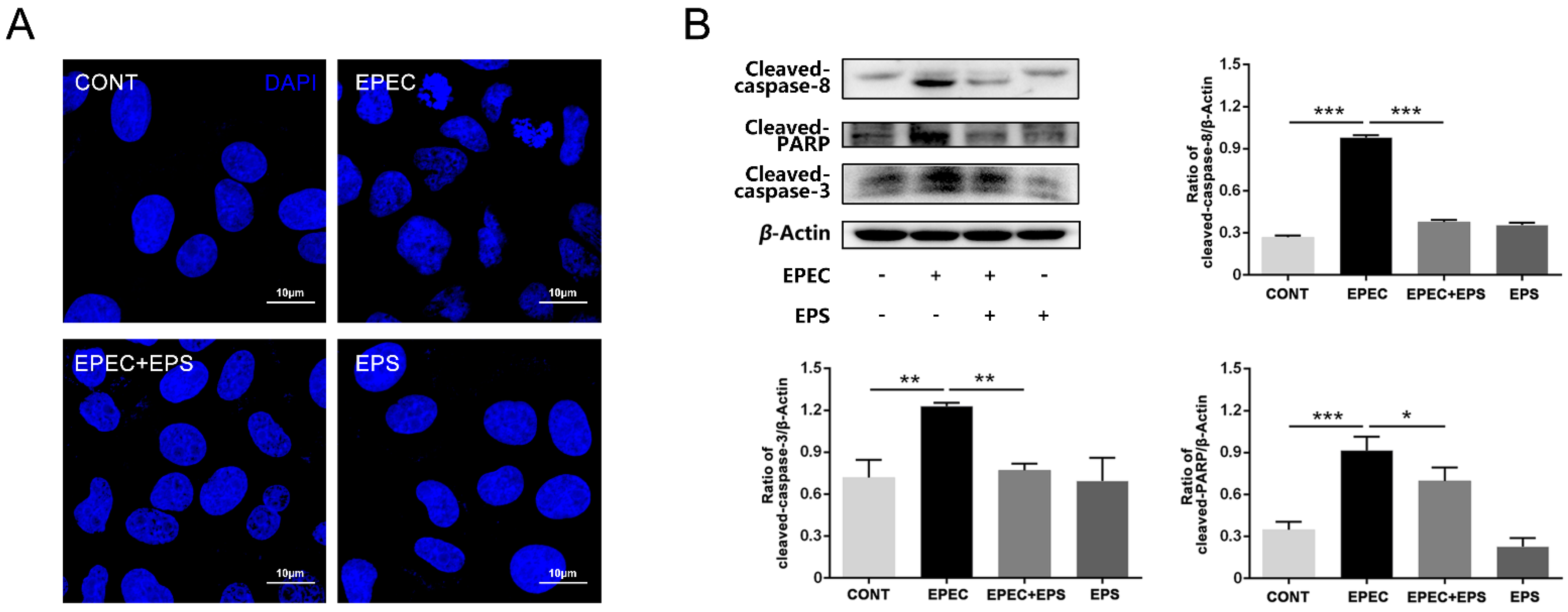
3.2. Pretreatment with EPS Alleviated EPEC-Induced Cell Apoptosis in IPEC-J2 cells
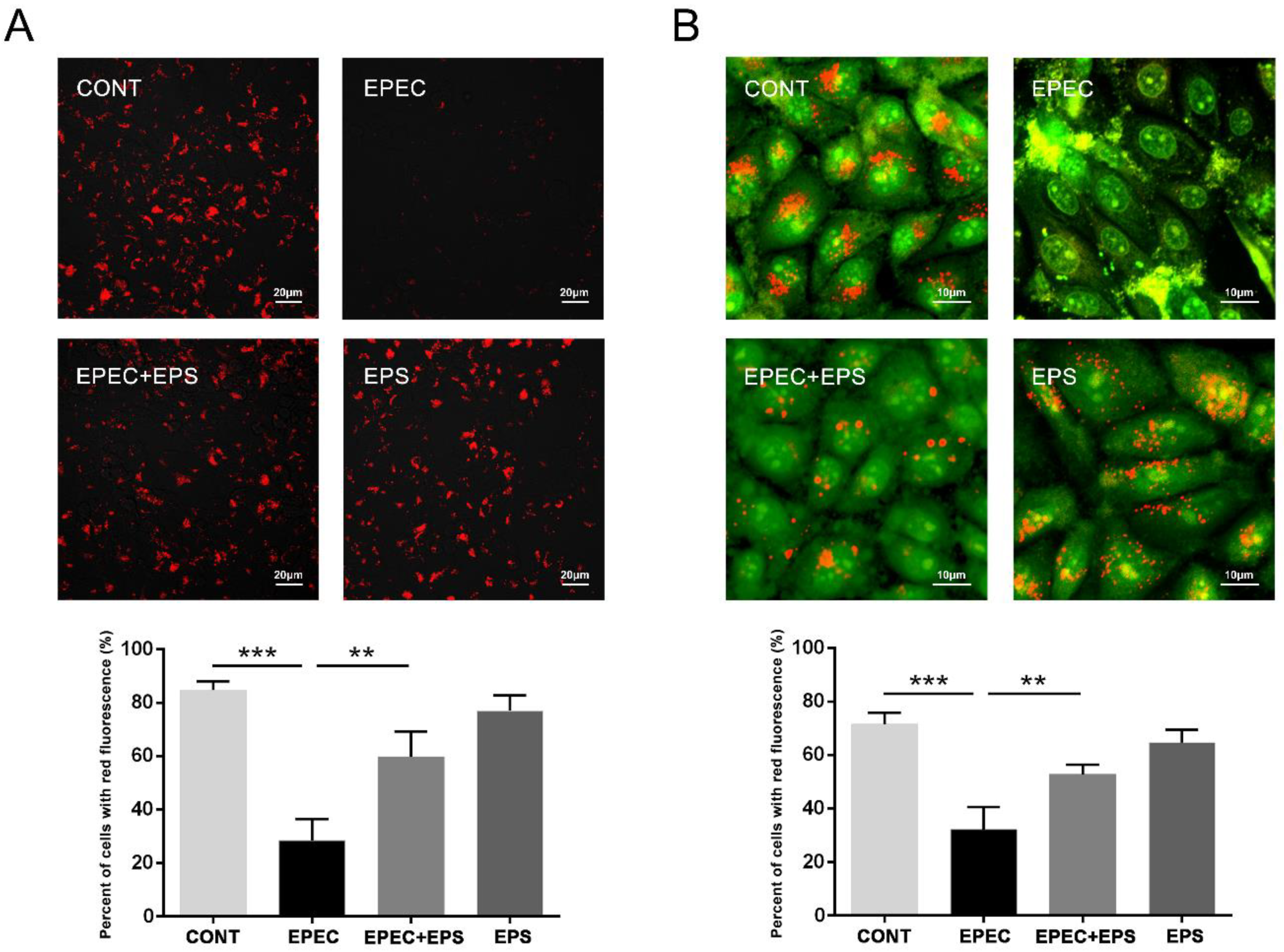
3.3. EPS restored the Autophagy Flux Inhibited by EPEC in IPEC-J2 Cells
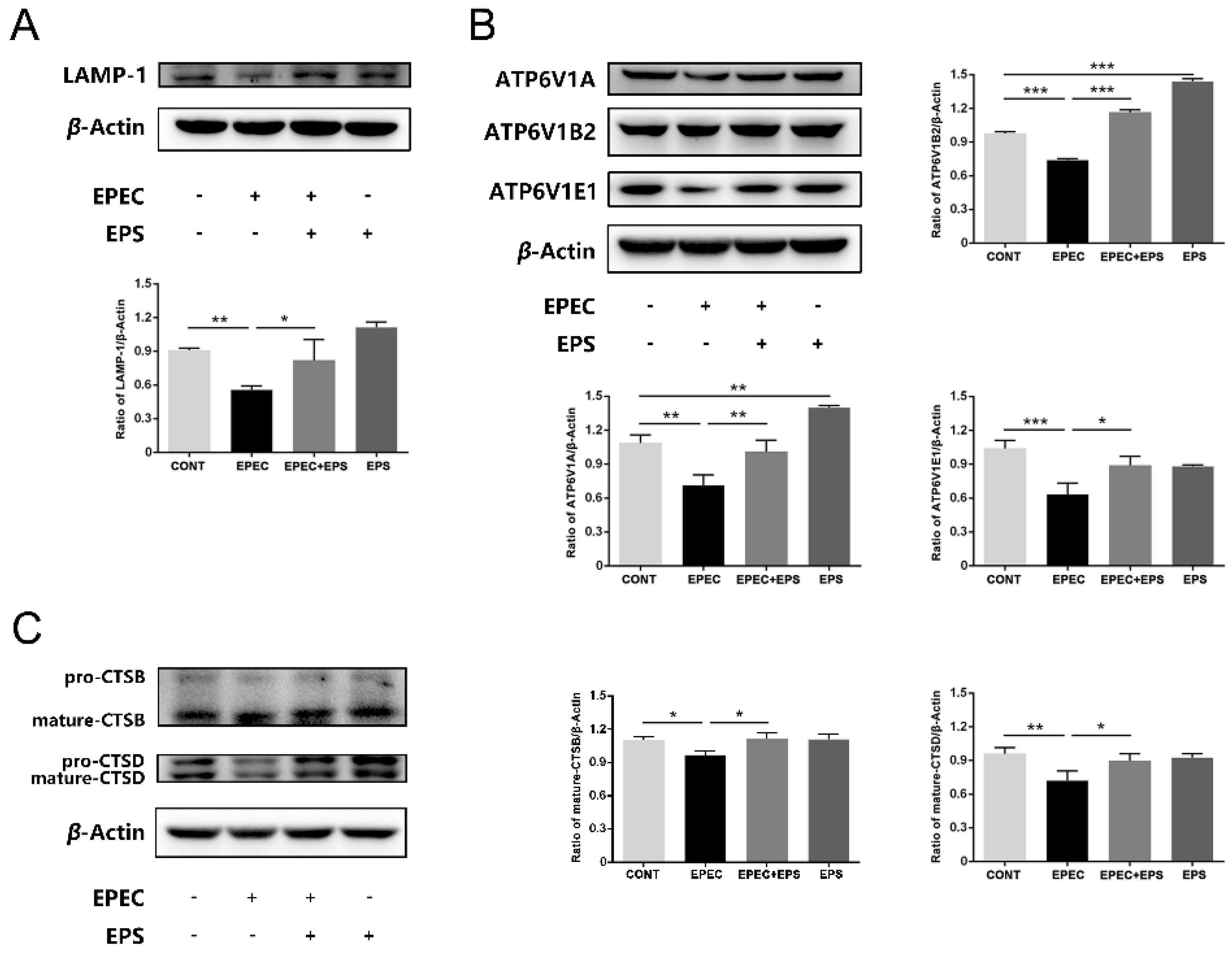
3.4. EPS Resisted Lysosomal Alkalization Caused by EPEC
3.5. EPS Alleviated Lysosomal Damage Caused by EPEC in IPEC-J2 Cells
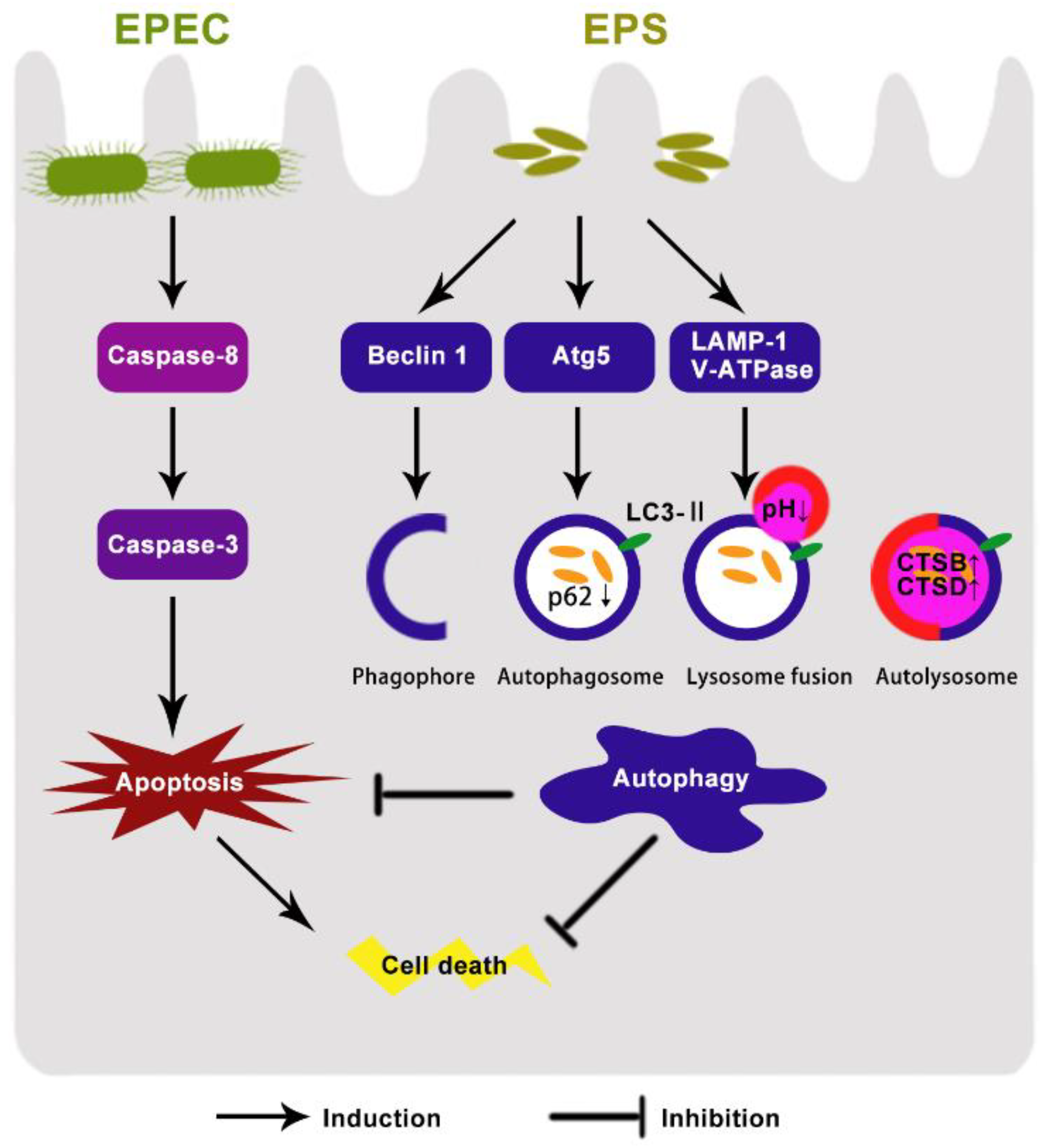
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bardiau, M.; Szalo, M.; Mainil, J.G. Initial adherence of EPEC, EHEC and VTEC to host cells. Vet. Res. 2010, 41, 57. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.P.; Spiegel, C.; Keren, Y.; Danieli, T.; Melamed-Book, N.; Pal, R.R.; Zlotkin-Rivkin, E.; Rosenshine, I.; Aroeti, B. Mitochondrial Targeting of the Enteropathogenic Escherichia coli Map Triggers Calcium Mobilization, ADAM10-MAP Kinase Signaling, and Host Cell Apoptosis. mBio 2020, 11, e01397-20. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, J.; Wang, S.; Su, J.; Wang, X.; Zhu, Y. Genome Characterization of mcr-1–Positive Escherichia coli Isolated from Pigs with Postweaning Diarrhea in China. Front. Vet. Sci. 2020, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Y.; Guo, L.; Su, J.-H.; Zhu, Y.-H.; Jiao, L.-G.; Wang, J.-F. Frequency of Diarrheagenic Virulence Genes and Characteristics in Escherichia coli Isolates from Pigs with Diarrhea in China. Microorganisms 2019, 7, 308. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Q.; Zhang, J.; Zhu, X. Occurrence and Characterization of Enteropathogenic Escherichia coli (EPEC) in Retail Ready-to-Eat Foods in China. Foodborne Pathog. Dis. 2016, 13, 49–55. [Google Scholar] [CrossRef]
- Hansen, L.; Nielsen, B.; Boll, E.; Skjøt-Rasmussen, L.; Wellejus, A.; Jørgensen, L.; Lauridsen, C.; Canibe, N. Functional in vitro screening of probiotic strains for inoculation of piglets as a prophylactic measure towards Enterotoxigenic Escherichia coli infection. J. Microbiol. Methods 2021, 180, 106126. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, H.-M.; Rasinkangas, P.; Lehtinen, M.J.; Mäkelä, S.M.; Airaksinen, K.; Anglenius, H.; Ouwehand, A.C.; Maukonen, J. Bifidobacterium animalis subsp. lactis 420 for Metabolic Health: Review of the Research. Nutrients 2020, 12, 892. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Castillejos, L.; Roll, V.; Cifuentes-Orjuela, G.; Muñoz, J.A.M.; Martín-Orúe, S.M. The Probiotic Combination of Bifidobacterium longum subsp. infantis CECT 7210 and Bifidobacterium animalis subsp. lactis BPL6 Reduces Pathogen Loads and Improves Gut Health of Weaned Piglets Orally Challenged with Salmonella Typhimurium. Front. Microbiol. 2017, 8, 1570. [Google Scholar] [CrossRef] [PubMed]
- Leccese, G.; Bibi, A.; Mazza, S.; Facciotti, F.; Caprioli, F.; Landini, P.; Paroni, M. Probiotic Lactobacillus and Bifidobacterium Strains Counteract Adherent-Invasive Escherichia coli (AIEC) Virulence and Hamper IL-23/Th17 Axis in Ulcerative Colitis, but Not in Crohn’s Disease. Cells 2020, 9, 1824. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.-J.; Yang, X.-Y.; Liu, Y.; Wang, J.-Y.; Man, C.-X. Immunoregulatory effects on Caco-2 cells and mice of exopolysaccharides isolated from Lactobacillus acidophilus NCFM. Food Funct. 2014, 5, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Dia, V.P.; Qiao, Y.; Shi, B. Antibacterial potential of a novel Lactobacillus casei strain isolated from Chinese northeast sauerkraut and the antibiofilm activity of its exopolysaccharides. Food Funct. 2020, 11, 4697–4706. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Xu, R.; Li, P. Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr. Polym. 2013, 91, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bravo, N.; Hidalgo-Cantabrana, C.; Rodriguez-Carvajal, M.A.; Ruas-Madiedo, P.; Margolles, A. Gene Replacement and Fluorescent Labeling to Study the Functional Role of Exopolysaccharides in Bifidobacterium animalis subsp. lactis. Front. Microbiol. 2017, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Torres, A. Enteropathogenic Escherichia coli: Foe or innocent bystander? Clin. Microbiol. Infect. 2015, 21, 729–734. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, M.; Xu, Z.; Li, W.; Dong, X.; Chen, Y.; Lin, B.; Li, M. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol. Res. 2019, 52, 1–10. [Google Scholar] [CrossRef]
- Flynn, A.N.; Buret, A.G. Caspases-3, -8, and -9 are required for induction of epithelial cell apoptosis by enteropathogenic E. coli but are dispensable for increased paracellular permeability. Microb. Pathog. 2008, 44, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.R.; Harnisch, L.C.; Alcon-Giner, C.; Mitra, S.; Wright, C.J.; Ketskemety, J.; van Sinderen, D.; Watson, A.J.M.; Hall, L.J. Bifidobacterium breve reduces apoptotic epithelial cell shedding in an exopolysaccharide and MyD88-dependent manner. Open Biol. 2017, 7, 160155. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Ganguli, K.; Zhu, W.; Shi, H.N.; Walker, W.A. Conditioned medium from Bifidobacteria infantis protects against Cronobacter sakazakii-induced intestinal inflammation in newborn mice. Am. J. Physiol. Liver Physiol. 2014, 306, G779–G787. [Google Scholar] [CrossRef] [PubMed]
- Grizotte-Lake, M.; Vaishnava, S. Autophagy: Suicide Prevention Hotline for the Gut Epithelium. Cell Host Microbe 2018, 23, 147–148. [Google Scholar] [CrossRef]
- Riebisch, A.; Mühlen, S.; Beer, Y.; Schmitz, I. Autophagy—A Story of Bacteria Interfering with the Host Cell Degradation Machinery. Pathogens 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Du, M.; Sheng, H.; Hovde, C.J.; Zhu, M.-J. Escherichia coli O157:H7 suppresses host autophagy and promotes epithelial adhesion via Tir-mediated and cAMP-independent activation of protein kinase A. Cell Death Discov. 2017, 3, 17055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, R.; Jiang, Y.; Zhao, X.Y.; Guan, Y.; Qian, W.; Fu, X.C.; Ren, H.Y.; Hou, X.H. Four types of Bifidobacteria trigger autophagy response in intestinal epithelial cells. J. Dig. Dis. 2014, 15, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-T.; Huang, F.-C. Probiotics exert reciprocal effects on autophagy and interleukin-1β expression in Salmonella-infected intestinal epithelial cells via autophagy-related 16L1 protein. Benef. Microbes 2019, 10, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Yu, J.; He, T.; Liu, X.; Su, J.; Wang, M.; Wang, J.; Zhu, Y. Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB J. 2019, 34, 2821–2839. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J. Lactobacillus rhamnosus GR-1 prevents Escherichia coli-induced apoptosis through PINK1/Parkin-mediated mitophagy in bovine mastitis. Front. Immunol. 2021, 12, 715098. [Google Scholar] [CrossRef]
- Nie, N.; Bai, C.; Song, S.; Zhang, Y.; Wang, B.; Li, Z. Bifidobacterium plays a protective role in TNF-alpha-induced inflammatory response in Caco-2 cell through NF-kappaB and p38MAPK pathways. Mol. Cell. Biochem. 2019, 464, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, S.; Zhao, Y.; Chen, S.; Huang, R.; Zhu, G.; Yin, Y.; Ren, W.; Deng, J. GABA attenuates ETEC-induced intestinal epithelial cell apoptosis involving GABAAR signaling and the AMPK-autophagy pathway. Food Funct. 2019, 10, 7509–7522. [Google Scholar] [CrossRef]
- Khailova, L.; Patrick, S.K.M.; Arganbright, K.M.; Halpern, M.D.; Kinouchi, T.; Dvorak, B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am. J. Physiol. Liver Physiol. 2010, 299, G1118–G1127. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, B.; Xu, H.; Tang, L.; Li, Y.; Gong, L.; Wang, Y.; Li, W. Probiotic Bacillus Attenuates Oxidative Stress- Induced Intestinal Injury via p38-Mediated Autophagy. Front. Microbiol. 2019, 10, 2185. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ashida, H.; Ogawa, M.; Yoshikawa, Y.; Mimuro, H.; Sasakawa, C. Bacterial Interactions with the Host Epithelium. Cell Host Microbe 2010, 8, 20–35. [Google Scholar] [CrossRef]
- Fernández, Á.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.-C.; et al. Disruption of the beclin 1–BCL2 autophagy regulatory complex promotes longevity in mice. Nat. Cell Biol. 2018, 558, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Brdarić, E.; Đokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić-Vidojević, A. Yogurt Produced by Novel Natural Starter Cultures Improves Gut Epithelial Barrier In Vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal Biology and Function: Modern View of Cellular Debris Bin. Cells 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.-L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Labruna, G.; Nanayakkara, M.; Pagliuca, C.; Nunziato, M.; Iaffaldano, L.; D’Argenio, V.; Colicchio, R.; Budelli, A.; Nigro, R.; Salvatore, P.; et al. Celiac disease-associated Neisseria flavescens decreases mitochondrial respiration in CaCo-2 epithelial cells: Impact of Lactobacillus paracasei CBA L74 on bacterial-induced cellular imbalance. Cell. Microbiol. 2019, 21, e13035. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Chu, B.; Chen, S.; Li, Y.; Liu, N.; Zhu, Y.; Zhou, D. Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy. Microorganisms 2021, 9, 2363. https://doi.org/10.3390/microorganisms9112363
Yuan L, Chu B, Chen S, Li Y, Liu N, Zhu Y, Zhou D. Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy. Microorganisms. 2021; 9(11):2363. https://doi.org/10.3390/microorganisms9112363
Chicago/Turabian StyleYuan, Lanxin, Bingxin Chu, Shiyan Chen, Yanan Li, Ning Liu, Yaohong Zhu, and Dong Zhou. 2021. "Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy" Microorganisms 9, no. 11: 2363. https://doi.org/10.3390/microorganisms9112363
APA StyleYuan, L., Chu, B., Chen, S., Li, Y., Liu, N., Zhu, Y., & Zhou, D. (2021). Exopolysaccharides from Bifidobacterium animalis Ameliorate Escherichia coli-Induced IPEC-J2 Cell Damage via Inhibiting Apoptosis and Restoring Autophagy. Microorganisms, 9(11), 2363. https://doi.org/10.3390/microorganisms9112363





