Detection and Drug Susceptibility Testing of Neisseria gonorrhoeae Using Isothermal Microcalorimetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms
2.2. Detection in Various Medium
2.3. Drug Susceptibility Testing
2.4. Calorimetry Procedure
2.5. Statistical Analysis
3. Results
3.1. Effect of Medium and Supplements
3.2. Time to Detection
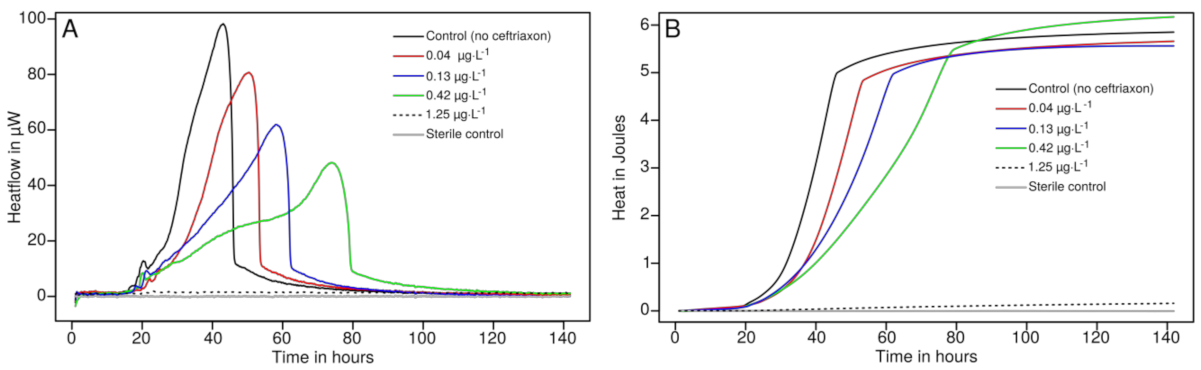
3.3. Drug Susceptibility Testing
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.; Hoorn, S.V.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548. [Google Scholar] [CrossRef]
- Rubin, D.H.F.; Ross, J.D.C.; Grad, Y.H. The frontiers of addressing antibiotic resistance in Neisseria gonorrhoeae. Transl. Res. 2020, 220, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Seifert, H.S.; Hook, E.W.; Hawkes, S.; Ndowa, F.; Dillon, J.-A.R. Gonorrhoea. Nat. Rev. Dis. Prim. 2019, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Hook, E.W.; Bernstein, K. Kissing, saliva exchange, and transmission of Neisseria gonorrhoeae. Lancet Infect. Dis. 2019, 19, e367–e369. [Google Scholar] [CrossRef]
- Elias, J.; Frosch, M.; Vogel, U. Neisseria. In Manual of Clinical Microbiology; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 635–651. [Google Scholar]
- Noble, R.C.; Cooper, R.M.; Miller, B.R. Pharyngeal colonisation by Neisseria gonorrhoeae and Neisseria meningitidis in black and white patients attending a venereal disease clinic. Sex. Transm. Infect. 1979, 55, 14–19. [Google Scholar] [CrossRef]
- Danby, C.S.; Cosentino, L.A.; Rabe, L.K.; Priest, C.L.; Damare, K.C.; Macio, I.S.; Meyn, L.A.; Wiesenfeld, H.C.; Hillier, S.L. Patterns of Extragenital Chlamydia and Gonorrhea in Women and Men Who Have Sex with Men Reporting a History of Receptive Anal Intercourse. Sex. Transm. Dis. 2016, 43, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hook, E.W.; Judson, F.N.; Handsfield, H.H.; Ehret, J.M.; Holmes, K.K.; Knapp, J.S. Auxotype/serovar diversity and antimicrobial resistance of neisseria gonorrhoeae in two mid-sized american cities. Sex. Transm. Dis. 1987, 14, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Gottlieb, S.L.; Paavonen, J. Pelvic Inflammatory Disease. N. Engl. J. Med. 2015, 372, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Pickard, R.; Bartoletti, R.; Cai, T.; Bruyere, F.; Geerlings, S.E.; Köves, B.; Wagenlehne, F.; Pilatz, A.; Pradere, B.; et al. EAU Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2019. [Google Scholar]
- Barbee, L.A.; Golden, M.R. Aztreonam for Neisseria gonorrhoeae: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2020, 75, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Buder, S. The laboratory diagnosis of neisseria gonorrhoeae: Current testing and future demands. Pathogens 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Bignell, C.; Unemo, M. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int. J. STD AIDS 2013, 24, 85–92. [Google Scholar] [CrossRef]
- Bachmann, L.H.; Johnson, R.E.; Cheng, H.; Markowitz, L.; Papp, J.R.; Palella, F.J.; Hook, E.W. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J. Clin. Microbiol. 2010, 48, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, L.H.; Johnson, R.E.; Cheng, H.; Markowitz, L.E.; Papp, J.R.; Edward, I.W.H. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J. Clin. Microbiol. 2009, 47, 902–907. [Google Scholar] [CrossRef]
- Palmer, H.M.; Mallinson, H.; Wood, R.L.; Herring, A.J. Evaluation of the specificities of five DNA amplification methods for the detection of Neisseria gonorrhoeae. J. Clin. Microbiol. 2003, 41, 835–837. [Google Scholar] [CrossRef]
- Tabrizi, S.N.; Unemo, M.; Limnios, A.E.; Hogan, T.R.; Hjelmevoll, S.O.; Garland, S.M.; Tapsall, J. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J. Clin. Microbiol. 2011, 49, 3610–3615. [Google Scholar] [CrossRef]
- Tabrizi, S.N.; Unemo, M.; Golparian, D.; Twin, J.; Limnios, A.E.; Lahra, M.; Guy, R. Analytical evaluation of GeneXpert CT/NG, the first genetic point-of-care assay for simultaneous detection of Neisseria gonorrhoeae and Chlamydia trachomatis. J. Clin. Microbiol. 2013, 51, 1945–1947. [Google Scholar] [CrossRef]
- Humbert, M.V.; Christodoulides, M. Atypical, yet not infrequent, infections with neisseria species. Pathogens 2020, 9, 10. [Google Scholar] [CrossRef]
- Cheng, A.; Kirby, J.E. Evaluation of the hologic gen-probe PANTHER, APTIMA combo 2 assay in a tertiary care teaching hospital. Am. J. Clin. Pathol. 2014, 141, 397–403. [Google Scholar] [CrossRef]
- Van Der Pol, B.; Williams, J.A.; Fuller, D.A.; Taylor, S.N.; Hook, E.W. Combined testing for chlamydia, gonorrhea, and trichomonas by use of the BD max CT/GC/TV assay with genitourinary specimen types. J. Clin. Microbiol. 2017, 55, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Serra-Pladevall, J.; Caballero, E.; Roig, G.; Juvé, R.; Barbera, M.J.; Andreu, A. Comparison between conventional culture and NAATs for the microbiological diagnosis in gonococcal infection. Diagn. Microbiol. Infect. Dis. 2015, 83, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Wirz, D.; Göpfert, B.; Daniels, A.U. Biomedical use of isothermal microcalorimeters. Sensors 2010, 10, 9369–9383. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Wirz, D.; Göpfert, B.; Daniels, A.U. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol. Lett. 2010, 303, 1–8. [Google Scholar] [CrossRef]
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell. Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef]
- Nykyri, J.; Herrmann, A.M.; Håkansson, S. Isothermal microcalorimetry for thermal viable count of microorganisms in pure cultures and stabilized formulations. BMC Microbiol. 2019, 19, 65. [Google Scholar] [CrossRef]
- Braissant, O.; Astasov-Frauenhoffer, M.; Waltimo, T.; Bonkat, G. A review of methods to determine viability, vitality, and metabolic rates in microbiology. Front. Microbiol 2020, 11, 547458. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Braissant, O.; Widmer, A.F.; Frei, R.; Rieken, M.; Wyler, S.; Gasser, T.C.; Wirz, D.; Daniels, A.U.; Bachmann, A. Rapid detection of urinary tract pathogens using microcalorimetry: Principle, technique and first results. BJU Int. 2012, 110, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Müller, G.; Egli, A.; Widmer, A.; Frei, R.; Halla, A.; Wirz, D.; Gasser, T.C.; Bachmann, A.; Wagenlehner, F.; et al. Seven hours to adequate antimicrobial therapy in urosepsis using isothermal microcalorimetry. J. Clin. Microbiol. 2014, 52, 624–626. [Google Scholar] [CrossRef]
- Baldoni, D.; Hermann, H.; Frei, R.; Trampuz, A.; Steinhuber, A. Performance of microcalorimetry for early detection of methicillin resistance in clinical isolates of Staphylococcus aureus. J. Clin. Microbiol. 2009, 47, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Furustrand Tafin, U.; Orasch, C.; Trampuz, A. Activity of antifungal combinations against Aspergillus species evaluated by isothermal microcalorimetry. Diagn. Microbiol. Infect. Dis. 2013, 77, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Boillat-Blanco, N.; Furustrand Tafin, U.; Jaton, K.; Trampuz, A. Susceptibility testing of Mycobacterium abscessus by isothermal microcalorimetry. Diagn. Microbiol. Infect. Dis. 2015, 83, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, U.; Wirz, D.; Daniels, A. Isothermal micro calorimetry a new method for MIC determinations: Results for 12 antibiotics and reference strains of E. coli and S. aureus. BMC Microbiol. 2009, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Wirz, D.; Daniels, A.U.; Braissant, O. Application of a microcalorimetric method for determining drug susceptibility in Mycobacterium species. J. Clin. Microbiol. 2012, 50, 16–20. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, D.; Daniels, A.U.; Urrusti, J.L.; Wirz, D.; Braissant, O. Evaluation of a low-cost calorimetric approach for rapid detection of tuberculosis and other mycobacteria in culture. J. Appl. Microbiol. 2011, 111, 1016–1024. [Google Scholar] [CrossRef]
- Battley, E.H. Absorbed heat and heat of formation of dried microbial biomassstudies on the thermodynamics of microbial growth. J. Therm. Anal. Calorim. 2003, 74, 709–721. [Google Scholar] [CrossRef]
- Braissant, O.; Bonkat, G.; Wirz, D.; Bachmann, A. Microbial growth and isothermal microcalorimetry: Growth models and their application to microcalorimetric data. Thermochim. Acta 2013, 555, 64–71. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Kahm, M.; Hasenbrink, G.; Lichtenberg-Fraté, H.; Ludwig, J.; Kschischo, M. grofit: Fitting Biological Growth Curves with R. J. Stat. Softw. 2010, 33. [Google Scholar] [CrossRef]
- Braissant, O.; Bachmann, A.; Bonkat, G. Microcalorimetric assays for measuring cell growth and metabolic activity: Methodology and applications. Methods 2015, 76, 27–34. [Google Scholar] [CrossRef]
- Braissant, O.; Bonkat, G.; Bachmann, A. Isothermal Microcalorimetry for the Investigation of Clinical Samples: Past and Present. In Biocalorimetry; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Maskow, T.; Paufler, S. What does calorimetry and thermodynamics of living cells tell us? Methods 2015, 76, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0429129033. [Google Scholar]
- Greiner, L.L.; Edwards, J.L.; Shao, J.; Rabinak, C.; Entz, D.; Apicella, M.A. Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 2005, 73, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Maskow, T.; Morais, F.M.; Rosa, L.F.M.; Qian, Y.G.; Harnisch, F. Insufficient oxygen diffusion leads to distortions of microbial growth parameters assessed by isothermal microcalorimetry. RSC Adv. 2014, 4, 32730–32737. [Google Scholar] [CrossRef]
- Kemp, R.B.; Guan, Y.H. The application of heat flux measurements to improve the growth of mammalian cells in culture. Thermochim. Acta 2000, 349, 23–30. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Schuler, M.M.; Hama, A.; Hughes, K.M.; Marison, I.W. Biocalorimetry as a process analytical technology process analyser; Robust in-line monitoring and control of aerobic fed-batch cultures of crabtree-negative yeast cells. J. Therm. Anal. Calorim. 2011, 104, 75–85. [Google Scholar] [CrossRef]
- Schuler, M.M.; Sivaprakasam, S.; Freeland, B.; Hama, A.; Hughes, K.M.; Marison, I.W. Investigation of the potential of biocalorimetry as a process analytical technology (PAT) tool for monitoring and control of Crabtree-negative yeast cultures. Appl. Microbiol. Biotechnol. 2012, 93, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Putnam, S.D.; Lavin, B.S.; Stone, J.R.; Oldfield, E.C.; Hooper, D.G. Evaluation of the standardized disk diffusion and agar dilution antibiotic susceptibility test methods by using strains of Neisseria gonorrhoeae from the United States and Southeast Asia. J. Clin. Microbiol. 1992, 30, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Sabour, S. Etest to detect drug-resistant neisseria gonorrhoeae to contemporary treatment; methodological issues concerning accuracy and reproducibility. J. Med. Microbiol. 2018, 67, 465. [Google Scholar] [CrossRef]
- Chen, L.; Shin, D.J.; Zheng, S.; Melendez, J.H.; Gaydos, C.A.; Wang, T.H. Direct-qPCR Assay for Coupled Identification and Antimicrobial Susceptibility Testing of Neisseria gonorrhoeae. ACS Infect. Dis. 2018, 4, 1377–1384. [Google Scholar] [CrossRef] [PubMed]

| No Supplement | Urine | Urine + Hemoglobin (1% w/v) | GC Medium | GC Medium + Hemoglobin (1% w/v) |
|---|---|---|---|---|
| Isovitale X | 1% v/v | 1% v/v | 1% v/v | 1% v/v * |
| Sheep blood ** | 1% v/v | 1% v/v | 1% v/v | 1% v/v |
| Growth Medium | Growth Rate (h−1) | Lag Phase (h) | Total Heat (J) | TTP (h) |
|---|---|---|---|---|
| GC | 0.095 ± 0.010 | 39.4 ± 16.2 | 3.74 ± 1.30 | 67.5 ± 10.7 |
| GC + Isovitale X | 0.307 ± 0.015 | 28.8 ± 1.8 | 4.83 ± 0.38 | 40.8 ± 0.8 |
| GC + Isovitale X + Blood | 0.234 ± 0.025 | 19.6 ± 1.3 | 5.34 ± 0.14 | 39.6 ± 3.6 |
| GC + hemoglobin | 0.119 ± 0.016 | 16.7 ± 1.2 | 4.65 ± 0.17 | 36.2 ± 5.2 |
| GC + hemoglobin + Isovitale X | 0.322 ± 0.025 | 17.3 ± 0.7 | 5.26 ± 0.12 | 29.4 ± 1.0 |
| GC + hemoglobin + Isovitale X + blood | 0.305 ± 0.016 | 19.3 ± 1.0 | 5.47 ± 0.08 | 28.9 ± 2.4 |
| Sterile GC medium | 0.002 ± 0.001 | ND | 0.12 ± 0.05 | ND |
| Urine | 0.007 ± 0.001 | ND | 0.28 ± 0.08 | ND |
| Urine + Isovitale X | 0.012 ± 0.001 | ND | 0.59 ± 0.08 | ND |
| Urine + Isovitale X + blood | 0.011 ± 0.003 | ND | 0.47 ± 0.08 | ND |
| Urine + hemoglobin | 0.004 ± 0.003 | ND | 0.60 ± 0.71 | ND |
| Urine + hemoglobin + Isovitale X | 0.006 ± 0.003 | ND | 0.60 ± 0.51 | ND |
| Urine + hemoglobin + Isovitale X + blood | 0.004 ± 0.001 | ND | 0.36 ± 0.06 | ND |
| Sterile filtered urine | 0.004 ± 0.001 | ND | 0.62 ± 0.45 | ND |
| ATCC 19424 | ATCC 43069 | ||
|---|---|---|---|
| CFU·mL−1 | Time to Detection (h) | CFU·mL−1 | Time to Detection (h) |
| ~2400 | 12.0 ± 0.3 | ~460 | 18.9 ± 0.4 |
| ~240 | 14.6 ± 0.2 | ~46 | 26.0 ± 0.4 |
| ~24 | 16.9 ± 0.4 | ~5 | 33.6 ± 3.3 |
| ~2 | 21.9 ± 3.2 | ~0.5 ** | ND * |
| 0 | ND | 0 | ND |
| Sterile controls | ND | Sterile controls | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grütter, A.E.; Lafranca, T.; Sigg, A.P.; Mariotti, M.; Bonkat, G.; Braissant, O. Detection and Drug Susceptibility Testing of Neisseria gonorrhoeae Using Isothermal Microcalorimetry. Microorganisms 2021, 9, 2337. https://doi.org/10.3390/microorganisms9112337
Grütter AE, Lafranca T, Sigg AP, Mariotti M, Bonkat G, Braissant O. Detection and Drug Susceptibility Testing of Neisseria gonorrhoeae Using Isothermal Microcalorimetry. Microorganisms. 2021; 9(11):2337. https://doi.org/10.3390/microorganisms9112337
Chicago/Turabian StyleGrütter, Anabel E., Tecla Lafranca, Aurelia Pahnita Sigg, Max Mariotti, Gernot Bonkat, and Olivier Braissant. 2021. "Detection and Drug Susceptibility Testing of Neisseria gonorrhoeae Using Isothermal Microcalorimetry" Microorganisms 9, no. 11: 2337. https://doi.org/10.3390/microorganisms9112337
APA StyleGrütter, A. E., Lafranca, T., Sigg, A. P., Mariotti, M., Bonkat, G., & Braissant, O. (2021). Detection and Drug Susceptibility Testing of Neisseria gonorrhoeae Using Isothermal Microcalorimetry. Microorganisms, 9(11), 2337. https://doi.org/10.3390/microorganisms9112337





