Age-Dependent Serotype-Associated Case-Fatality Rate in Invasive Pneumococcal Disease in the Autonomous Community of Madrid between 2007 and 2020
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Htar, M.T.; Christopoulou, D.; Schmitt, H.-J. Pneumococcal serotype evolution in Western Europe. BMC Infect. Dis. 2015, 15, 419. [Google Scholar]
- Song, J.-H.; Dagan, R.; Klugman, K.P.; Fritzell, B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine 2012, 30, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, I.; Stevenson, A.; Hsu, K.K.; Pelton, S.I. Evolving picture of invasive pneumococcal disease in massachusetts children: A comparison of disease in 2007–2009 with earlier periods. Pediatr. Infect. Dis. J. 2012, 31, 1016–1021. [Google Scholar] [CrossRef]
- Lipsitch, M.; Siber, G.R. How Can Vaccines Contribute to Solving the Antimicrobial Resistance Problem? mBio 2016, 7, e00428-16. [Google Scholar] [CrossRef] [PubMed]
- Ricketson, L.J.; Conradi, N.G.; Vanderkooi, O.G.; Kellner, J.D. Changes in the Nature and Severity of Invasive Pneumococcal Disease in Children before and after the Seven-valent and Thirteen-valent Pneumococcal Conjugate Vaccine Programs in Calgary, Canada. Pediatr. Infect. Dis. J. 2018, 37, 22–27. [Google Scholar] [CrossRef] [PubMed]
- B.O.C.M. de 7 de Febrero de 2007. ORDEN 74/2007, de 22 de Enero, del Consejero de Sanidad y Consumo, por la que se Mo-Difica la Orden 9/1997, de 15 de enero, Para el Desarrollo del Decreto 184/1996, de 19 de Diciembre, en lo que se Refiere a las Enfermedades de declarAción Obligatoria, a las Situaciones Epidémicas y Brotes, y al Síndrome de Inmunodeficiencia Adqui-Rida (SIDA) e Infección por Virus de la Inmunodeficiencia Humana; B.O.C.M.: Madrid, Spain, 2007; Number 32; p. 4. [Google Scholar]
- Rückinger, S.; von Kries, R.; Siedler, A.; van der Linden, M. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr. Infect. Dis. J. 2009, 28, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Estadística. Comunidad de Madrid. Padrón Anual. Resultados Detallados. Available online: https://www.madrid.org/iestadis/fijas/estructu/demograficas/padron/estructupcrd.htm (accessed on 17 June 2021).
- Latasa, P.; Ordobás, M.; Garrido-Estepa, M.; Gil De Miguel, A.; Sanz, J.; Barranco, M.; Insúa, E.; García-Comas, L. Effectiveness of different vaccine schedules for heptavalent and 13-valent conjugate vaccines against pneumococcal disease in the Community of Madrid. Vaccine 2017, 35, 5381–5387. [Google Scholar] [CrossRef]
- Inverarity, D.; Lamb, K.; Diggle, M.; Robertson, C.; Greenhalgh, D.; Mitchell, T.J.; Smith, A.; Jefferies, J.M.; Clarke, S.C.; McMenamin, J.; et al. Death or survival from invasive pneumococcal disease in Scotland: Associations with serogroups and multilocus sequence types. J. Med. Microbiol. 2011, 60 Pt 6, 793–802. [Google Scholar] [CrossRef]
- Hughes, G.J.; Wright, L.B.; Chapman, K.E.; Wilson, D.; Gorton, R. Serotype-specific differences in short- and longer-term mortality following invasive pneumococcal disease. Epidemiol. Infect. 2016, 144, 2654–2669. [Google Scholar] [CrossRef]
- De Miguel, S.; Domenech, M.; González-Camacho, F.; Sempere, J.; Vicioso, D.; Sanz, J.C.; Comas, L.G.; Ardanuy, C.; Fenoll, A.; Yuste, J. Nationwide trends of invasive pneumococcal disease in Spain (2009–2019) in children and adults during the pneumococcal conjugate vaccine era. Clin. Infect. Dis. Publ. Infect. Dis. Soc. Am. 2020, ciaa1483. [Google Scholar] [CrossRef] [PubMed]
- Houseman, C.; Chapman, K.E.; Manley, P.; Gorton, R.; Wilson, D.; Hughes, G.J. Decreasing case-fatality rate following invasive pneumococcal disease, North East England, 2006–2016. Epidemiol. Infect. 2019, 147, e175. [Google Scholar] [CrossRef]
- Aguinagalde, L.; Corsini, B.; Domenech, A.; Domenech, M.; Cámara, J.; Ardanuy, C.; García, E.; Liñares, J.; Fenoll, A.; Yuste, J. Emergence of amoxicilin-resistant variants of Spain 9V-ST156 Pneumococcal expressing serotype 11A correlates with their ability to evade the host inmune response. PLoS ONE 2015, 10, e0137565. [Google Scholar] [CrossRef] [PubMed]
- Savulescu, C.; Krizova, P.; Lepoutre, A.; Mereckiene, J.; Vestrheim, D.F.; Ciruela, P.; Ordobas, M.; Guevara, M.; McDonald, E.; Morfeldt, E.; et al. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: An observational multicentre study. Lancet Respir. Med. 2017, 5, 648–656. [Google Scholar] [CrossRef]
- Jansen, A.G.S.C.; Rodenburg, G.D.; Van Der Ende, A.; Van Alphen, L.; Veenhoven, R.H.; Spanjaard, L.; Sanders, E.A.M.; Hak, E. Invasive Pneumococcal Disease among Adults: Associations among Serotypes, Disease Characteristics, and Outcome. Clin. Infect. Dis. 2009, 49, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Grabenstein, J.D.; Musey, L.K. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine 2014, 32, 2399–2405. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Harboe, Z.B.; Sanders, E.A.M.; Ndiritu, M.; Klugman, K.P.; Rückinger, S.; Dagan, R.; Adegbola, R.A.; Cutts, F.; Johnson, H.L.; et al. Association of Serotype with Risk of Death Due to Pneumococcal Pneumonia: A Meta-Analysis. Clin. Infect. Dis. 2010, 51, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Harboe, Z.B.; Dalby, T.; Weinberger, D.M.; Benfield, T.; Mølbak, K.; Slotved, H.C.; Suppli, C.H.; Konradsen, H.B.; Valentiner-Branth, P. Impact of 13-Valent Pneumococcal Conjugate Vaccination in Invasive Pneumococcal Disease Incidence and Mortality. Clin. Infect. Dis. 2014, 59, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Policarpio, M.E.; Wong, K.; Gubbay, J.; Fediurek, J.; Deeks, S. The epidemiology of invasive pneumococcal disease in older adults from 2007 to 2014 in Ontario, Canada: A population-based study. CMAJ Open 2016, 4, E545–E550. [Google Scholar] [CrossRef]
- Selva, L.; Ciruela, P.; Esteva, C.; De Sevilla, M.F.; Codina, G.; Hernandez, S.; Moraga, F.; Garcia-Garcia, J.J.; Planes, A.; Coll, F.; et al. Serotype 3 is a common serotype causing invasive pneumococcal disease in children less than 5 years old, as identified by real-time PCR. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 1487–1495. [Google Scholar] [CrossRef]
- Brueggemann, A.B.; Peto, T.E.A.; Crook, D.W.; Butler, J.C.; Kristinsson, K.G.; Spratt, B.G. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 2004, 190, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.; Brotons, P.; Monsonis, M.; Triviño, M.; Iñigo, M.; Selva, L.; Sa-Leão, R.; Muóoz-Almagro, C. High invasiveness of pneumococcal serotypes included in the new generation of conjugate vaccines. Clin. Microbiol. Infect. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.C.; Hetrich, M.K.; Quesada, M.G.; Sinkevitch, J.N.; Knoll, M.D.; Feikin, D.R.; Zeger, S.L.; Kagucia, E.W.; Cohen, A.L.; Ampofo, K.; et al. Changes in Invasive Pneumococcal Disease Caused by Streptococcus pneumoniae Serotype 1 Following Introduction of PCV10 and PCV13: Findings from the PSERENADE Project. Microorganisms 2021, 9, 696. [Google Scholar] [CrossRef]
- Kobayashi, M.; Misegades, L.; Fleming-Dutra, K.E.; Ahmed, S.; Gierke, R.; Nanduri, S.A.; Healy, J.M.; Nguyen, D.T.; Carvalho, M.D.G.; Pimenta, F.; et al. Pneumococcal Serotype 5 Colonization Prevalence Among Newly Arrived Unaccompanied Children 1 Year After an Outbreak—Texas, 2015. Pediatr. Infect. Dis. J. 2017, 36, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.A.G.; González, A.V.; Gavín, M.A.O.; Martínez, F.M.; Marín, N.G.; Blázquez, B.R.; Moreno, J.C.S. Invasive pneumococcal disease: Association between serotype, clinical presentation and lethality. Vaccine 2011, 29, 5740–5746. [Google Scholar] [CrossRef]
- Picazo, J.; Ruiz-Contreras, J.; Casado-Flores, J.; Negreira, S.; García-de-Miguel, M.-J.; Hernández-Sampelayo, T.; Otheo, E.; Méndez, C. Expansion of serotype coverage in the universal pediatric vaccination calendar: Short-term effects on age- and serotype-dependent incidence of invasive pneumococcal clinical presentations in Madrid, Spain. Clin. Vaccine Immunol. CVI. 2013, 20, 1524–1530. [Google Scholar] [CrossRef]
- Oligbu, G.; Collins, S.; Djennad, A.; Sheppard, C.L.; Fry, N.K.; Andrews, N.J.; Borrow, R.; Ramsay, M.E.; Ladhani, S.N. Effect of Pneumococcal Conjugate Vaccines on Pneumococcal Meningitis, England and Wales, July 1, 2000–June 30, 2016. Emerg. Infect. Dis. 2019, 25, 1708–1718. [Google Scholar] [CrossRef]
- Latasa, P.; Sanz, J.C.; Ordobás, M.; Barranco, M.D.; Insúa, E.; Gil, Á.; Fernández, A.C.; García-Comas, L. Trends of invasive pneumococcal disease and its serotypes in the Autonomous Community of Madrid. Enferm. Infecc. Micro-Biol. Clin. 2018, 36, 612–620. [Google Scholar]
- Sempere, J.; de Miguel, S.; González-Camacho, F.; Yuste, J.; Domenech, M. Clinical Relevance and Molecular Pathogenesis of the Emerging Serotypes 22F and 33F of Streptococcus pneumoniae in Spain. Front. Microbiol. 2020, 11, 309. [Google Scholar] [CrossRef]
- Yildirim, I.; Hanage, W.P.; Lipsitch, M.; Shea, K.M.; Stevenson, A.; Finkelstein, J.; Huang, S.S.; Lee, G.M.; Kleinman, K.; Pelton, S.I. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine 2010, 29, 283–288. [Google Scholar] [CrossRef]
- Backhaus, E.; Berg, S.; Andersson, R.; Ockborn, G.; Malmström, P.; Dahl, M.; Nasic, S.; Trollfors, B. Epidemiology of invasive pneumococcal infections: Manifestations, incidence and case-fatality rate correlated to age, gender and risk factors. BMC Infect. Dis. 2016, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Wagenvoort, G.H.J.; Knol, M.J.; de Melker, H.E.; Vlaminckx, B.J.; van der Ende, A.; Rozenbaum, M.H.; Sanders, E.A. Risk and outcomes of invasive pneumococcal disease in adults with underlying conditions in the post-PCV7 era, The Netherlands. Vaccine 2016, 34, 334–340. [Google Scholar] [CrossRef]
- Bechini, A.; Taddei, C.; Barchielli, A.; Levi, M.; Tiscione, E.; Santini, M.G.; Niccolini, F.; Mechi, M.T.; Panatto, D.; Amicizia, D.; et al. A retrospective analysis of hospital discharge records for S. pneumoniae diseases in the elderly population of Florence, Italy, 2010–2012. Hum. Vaccines Immunother. 2015, 11, 156–165. [Google Scholar] [CrossRef][Green Version]
- Yildirim, I.; Shea, K.M.; Little, B.A.; Silverio, A.L.; Pelton, S.I.; Members of the Massachusetts Department of Public Health. Vaccination, underlying comorbidities, and risk of invasive pneumococcal disease. Pediatrics 2015, 135, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Shigayeva, A.; Rudnick, W.; Green, K.; Chen, D.K.; Demczuk, W.; Gold, W.L.; Johnstone, J.; Kitai, I.; Krajden, S.; Lovinsky, R.; et al. Invasive Pneumococcal Disease Among Immun-ocompromised Persons: Implications for Vaccination Programs. Clin. Infect. Dis. Publ. Infect. Dis. Soc. Am. 2016, 62, 139–147. [Google Scholar] [CrossRef]
- Ciruela, P.; Broner, S.; Izquierdo, C.; Pallarés, R.; Muñoz-Almagro, C.; Hernández, S.; Grau, I.; Domínguez, A.; Jané, M.; Esteva, C.; et al. Indirect effects of paediatric conjugate vaccines on invasive pneumococcal disease in older adults. Int. J. Infect. Dis. 2019, 86, 122–130. [Google Scholar] [CrossRef]
- Sanz, J.C.; de Miguel, S.; Ordobás, M.; Comas, L.G. Serotipos de Streptococcus pneumoniae con tropismo meníngeo en casos de enfermedad neumocócica invasora. Comunidad de Madrid, 2007–2018. Enferm. Infecc. Microbiol. Clínica 2020, 38, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Ahl, J.; Littorin, N.; Forsgren, A.; Odenholt, I.; Resman, F.; Riesbeck, K. High incidence of septic shock caused by Streptococcus pneumoniae serotype 3—A retrospective epidemiological study. BMC Infect. Dis. 2013, 13, 492. [Google Scholar] [CrossRef]
- Navarro-Torné, A.; Dias, J.G.; Hruba, F.; Lopalco, P.L.; Pastore-Celentano, L.; Gauci, A.J.A. Invasive Pneumococcal Disease Study Group Risk Factors for Death from Invasive Pneumococcal Disease, Europe, 2010. Emerg. Infect. Dis. 2015, 21, 417–425. [Google Scholar] [CrossRef]
- Liñares, J.; Ardanuy, C.; Pallares, R.; Fenoll, A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin. Microbiol. Infect. 2010, 16, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Nahm, M.; Moseley, M.A. Clinical Implications of Pneumococcal Serotypes: Invasive Disease Potential, Clinical Presentations, and Antibiotic Resistance. J. Korean Med. Sci. 2013, 28, 4–15. [Google Scholar] [CrossRef]
- Sá-Leão, R.; Pinto, F.; Aguiar, S.; Nunes, S.; Carriço, J.A.; Frazão, N.; Gonçalves-Sousa, N.; Melo-Cristino, J.; de Lencastre, H. and Ramirez, M. Analysis of invasiveness of pneumococcal serotypes and clones circulating in Portugal before widespread use of conjugate vaccines reveals heterogeneous behavior of clones ex-pressing the same serotype. J. Clin. Microbiol. 2011, 49, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, A.; Zucs, P.; Droz, S.; Mühlemann, K. Distribution and Invasiveness of Streptococcus pneumoniae Serotypes in Switzerland, a Country with Low Antibiotic Selection Pressure, from 2001 to 2004. J. Clin. Microbiol. 2006, 44, 2032–2038. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meulen, A.S.; Vesikari, T.; Malacaman, E.A.; Shapiro, S.A.; Dallas, M.J.; Hoover, P.A.; McFetridge, R.; Stek, J.E.; Marchese, R.D.; Hartzel, J.; et al. Safety, tolerability and immunogenicity of 15-valent pneumococcal conjugate vaccine in toddlers previously vaccinated with 7-valent pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 2015, 34, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.J.; Martin-Montalvo, L.P.J.; Isturiz, R.E.; Reinert, R.R. Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens, Kits Comprising the Same and Uses Thereof. WO2017013548A1, 2017. Available online: https://patents.google.com/patent/WO2017013548A1/en (accessed on 17 June 2021).
| Cases | Deaths | ||||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | CI (95%) | OR | CI (95%) | |
| Age group | |||||||
| 0–14 | 1095 | 18.21 | 16 | 2.16 | (0.8–2.2) | 1 | |
| 15–34 | 329 | 5.47 | 8 | 1.08 | (0.8–4.1) | 1.68 | (0.7–4.0) |
| 35–50 | 984 | 16.36 | 70 | 9.45 | (5.5–8.7) | 5.16 | (3.0–9.0) |
| 50–64 | 1186 | 19.72 | 148 | 19.97 | (10.6–14.4) | 9.62 | (5.7–16.2) |
| 65–74 | 815 | 13.55 | 103 | 13.90 | (10.4–14.9) | 9.76 | (5.7–16.7) |
| 75–84 | 890 | 14.80 | 182 | 24.56 | (17.8–23.1) | 17.34 | (10.3–29.2) |
| ≥85 | 714 | 11.87 | 214 | 28.88 | (26.6–33.3) | 28.86 | (17.2–48.5) |
| Sex | |||||||
| Female | 2577 | 42.86 | 303 | 40.89 | (10.5–13.0) | 1 | |
| Male | 3436 | 57.14 | 438 | 59.11 | (11.6–13.9) | 1.10 | (0.9–1.3) |
| Clinical presentation | |||||||
| Pneumonia | 3239 | 53.87 | 256 | 34.55 | (7.0–8.8) | 1 | |
| Bacteraemia | 975 | 16.21 | 97 | 13.09 | (8.1–11.8) | 1.29 | (1.0–1.6) |
| Meningitis | 511 | 8.50 | 69 | 9.31 | (10.5–16.5) | 1.82 | (1.4–2.4) |
| Other * | 433 | 7.20 | 46 | 6.21 | (7.7–13.5) | 1.39 | (1.0–1.9) |
| Sepsis | 855 | 14.22 | 273 | 36.84 | (28.8–35.1) | 5.47 | (4.5–6.6) |
| Risk Factors ** | |||||||
| No | 2546 | 42.34 | 150 | 20.24 | (5.0–6.8) | 1 | |
| Yes | 3467 | 57.66 | 591 | 79.76 | (15.8–18.3) | 3.28 | (2.7–4.0) |
| Year | |||||||
| 2007 | 446 | 7.42 | 59 | 7.96 | (10.1–16.4) | 1 | |
| 2008 | 533 | 8.86 | 56 | 7.56 | (7.9–13.1) | 0.77 | (0.5–1.1) |
| 2009 | 574 | 9.55 | 48 | 6.48 | (6.1–10.6) | 0.60 | (0.4–0.9) |
| 2010 | 410 | 6.82 | 37 | 4.99 | (6.2–11.8) | 0.65 | (0.4–1.0) |
| 2011 | 422 | 7.02 | 54 | 7.29 | (9.6–16.0) | 0.96 | (0.6–1.4) |
| 2012 | 330 | 5.49 | 57 | 7.69 | (13.2–21.4) | 1.37 | (0.9–2.0) |
| 2013 | 297 | 4.94 | 49 | 6.61 | (12.3–20.7) | 1.30 | (0.9–2.0) |
| 2014 | 373 | 6.20 | 54 | 7.29 | (10.9–18.1) | 1.11 | (0.7–1.7) |
| 2015 | 419 | 6.97 | 62 | 8.37 | (11.4–18.2) | 1.14 | (0.8–1.7) |
| 2016 | 447 | 7.43 | 64 | 8.64 | (11.1–17.6) | 1.10 | (0.7–1.6) |
| 2017 | 586 | 9.75 | 67 | 9.04 | (8.9–14.0) | 0.85 | (0.6–1.2) |
| 2018 | 515 | 8.56 | 61 | 8.23 | (9.1–14.6) | 0.88 | (0.6–1.3) |
| 2019 | 554 | 9.21 | 50 | 6.75 | (6.6–11.4) | 0.65 | (0.4–1.0) |
| 2020 | 107 | 1.78 | 23 | 3.10 | (13.7–29.3) | 1.80 | (1.1–3.1) |
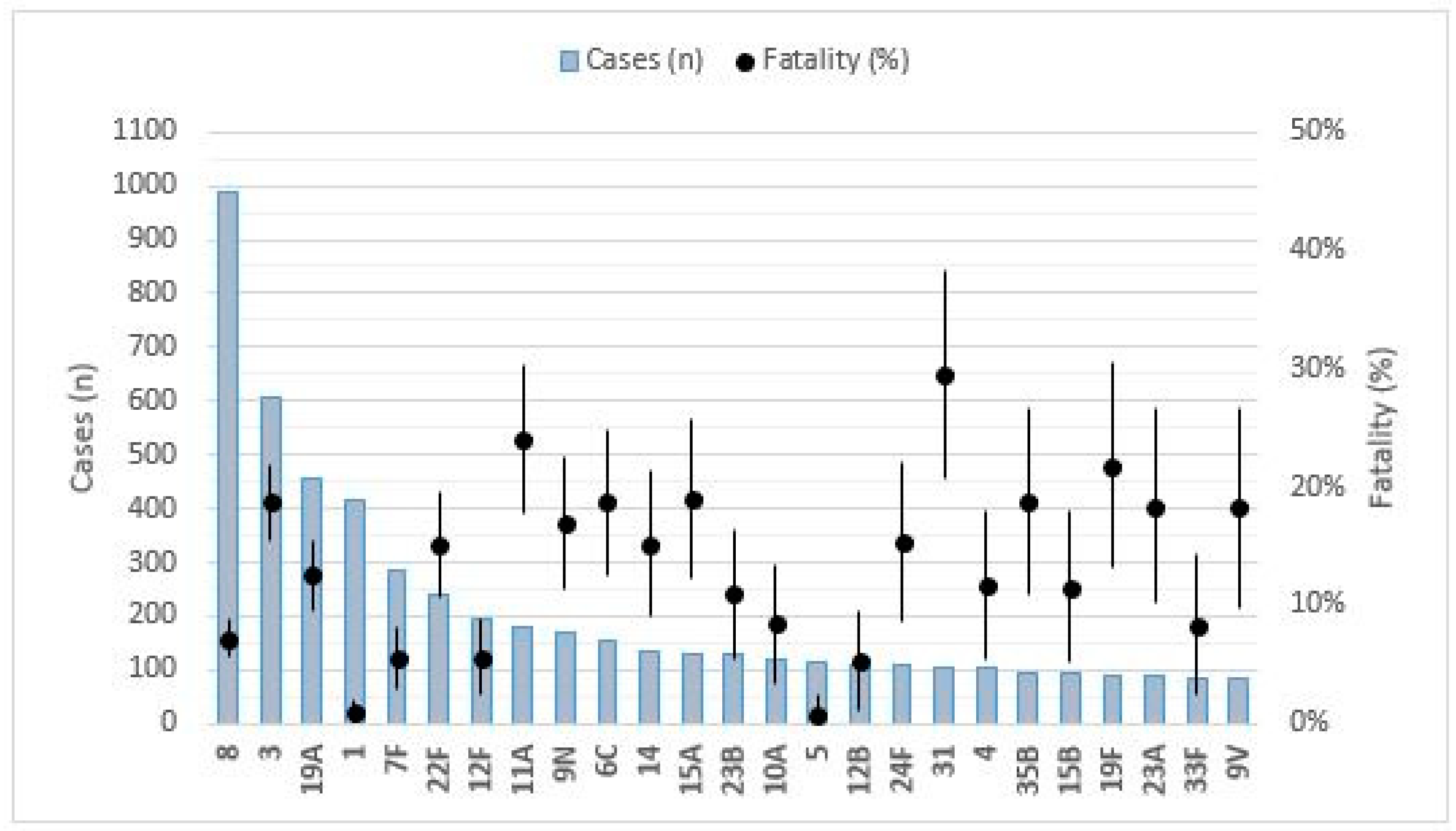
| SEROTYPE | CASES (N) | INCIDENCE + | DEATHS (N) | MORTALITY + | FATALITY (%) | ORC (CI 95%) | P-CRUDE | ORA (CI 95%) | P-ADJUSTED |
|---|---|---|---|---|---|---|---|---|---|
| 8 | 990 | 1.09 | 71 | 0.08 | 7.17 | 0.50 (0.4–0.6) | 0.00 * | 0.50 (0.4–0.6) | 0.00 * |
| 3 | 605 | 0.67 | 113 | 0.12 | 18.68 | 1.75 (1.4–2.2) | 0.00 * | 1.35 (1.1–1.7) | 0.01 * |
| 19A | 455 | 0.50 | 57 | 0.06 | 12.53 | 1.02 (0.8–1.4) | 0.89 | 1.20 (0.9–1.6) | 0.25 |
| 1 | 417 | 0.46 | 4 | 0.00 | 0.96 | 0.06 (0.0–0.2) | 0.00 * | 0.16 (0.1–0.4) | 0.00 * |
| 7F | 286 | 0.32 | 16 | 0.02 | 5.59 | 0.41 (0.2–0.7) | 0.00 * | 0.61 (0.4–1.0) | 0.07 |
| 22F | 238 | 0.26 | 36 | 0.04 | 15.13 | 1.28 (0.9–1.8) | 0.18 | 1.08 (0.7–1.6) | 0.68 |
| 12F | 195 | 0.22 | 11 | 0.01 | 5.64 | 0.42 (0.2–0.8) | 0.01 * | 0.49 (0.3–0.9) | 0.02 * |
| 11A | 179 | 0.20 | 43 | 0.05 | 24.02 | 2.33 (1.6–3.3) | 0.00 * | 1.93 (1.3–2.8) | 0.00 * |
| 9N | 172 | 0.19 | 29 | 0.03 | 16.86 | 1.46 (1.0–2.2) | 0.07 | 1.28 (0.8–2.0) | 0.25 |
| 6C | 155 | 0.17 | 29 | 0.03 | 18.71 | 1.66 (1.1–2.5) | 0.02 * | 1.12 (0.7–1.7) | 0.60 |
| 14 | 132 | 0.15 | 20 | 0.02 | 15.15 | 1.28 (0.8–2.1) | 0.32 | 1.01 (0.6–1.7) | 0.98 |
| 15A | 131 | 0.14 | 25 | 0.03 | 19.08 | 1.70 (1.1–2.6) | 0.02 * | 1.43 (0.9–2.3) | 0.13 |
| 23B | 128 | 0.14 | 14 | 0.02 | 10.94 | 0.87 (0.5–1.5) | 0.63 | 1.05 (0.6–1.9) | 0.87 |
| 10A | 118 | 0.13 | 10 | 0.01 | 8.47 | 0.65 (0.3–1.3) | 0.20 | 0.71 (0.4–1.4) | 0.32 |
| 5 | 116 | 0.13 | 1 | 0.00 | 0.86 | 0.06 (0.0–0.4) | 0.01 * | 0.16 (0.0–1.2) | 0.07 |
| 12B | 111 | 0.12 | 6 | 0.01 | 5.41 | 0.40 (0.2–0.9) | 0.03 * | 0.44 (0.2–1.0) | 0.06 |
| 24F | 111 | 0.12 | 17 | 0.02 | 15.32 | 1.29 (0.8–2.2) | 0.33 | 1.47 (0.8–2.6) | 0.17 |
| 31 | 105 | 0.12 | 31 | 0.03 | 29.52 | 3.07 (2.0–4.7) | 0.00 * | 1.81 (1.2–2.8) | 0.01 * |
| 4 | 103 | 0.11 | 12 | 0.01 | 11.65 | 0.94 (0.5–1.7) | 0.83 | 1.12 (0.6–2.1) | 0.72 |
| 35B | 96 | 0.11 | 18 | 0.02 | 18.75 | 1.66 (1.0–2.8) | 0.06 | 1.28 (0.7–2.2) | 0.38 |
| 15B | 95 | 0.10 | 11 | 0.01 | 11.58 | 0.93 (0.5–1.8) | 0.82 | 1.08 (0.6–2.1) | 0.83 |
| 19F | 87 | 0.10 | 19 | 0.02 | 21.84 | 2.01 (1.2–3.4) | 0.01 * | 2.17 (1.3–3.8) | 0.01 * |
| 23A | 87 | 0.10 | 16 | 0.02 | 18.39 | 1.62 (0.9–2.8) | 0.09 | 1.30 (0.7–2.3) | 0.36 |
| 33 | 84 | 0.09 | 7 | 0.01 | 8.33 | 0.64 (0.3–1.4) | 0.27 | 0.70 (0.3–1.6) | 0.39 |
| 9V | 82 | 0.09 | 15 | 0.02 | 18.29 | 1.61 (0.9–2.8) | 0.10 | 1.05 (0.6–1.9) | 0.88 |
| Serotype | Deaths | Cases | Mortality * | Fatality (%) |
|---|---|---|---|---|
| 11D | 1 | 1 | 0.01 | 100.00 |
| 24A | 1 | 2 | 0.01 | 50.00 |
| 23F | 2 | 5 | 0.03 | 40.00 |
| 6C | 11 | 36 | 0.16 | 30.56 |
| 37 | 1 | 4 | 0.01 | 25.00 |
| 23A | 5 | 22 | 0.07 | 22.73 |
| 24F | 5 | 22 | 0.07 | 22.73 |
| 11A | 8 | 38 | 0.12 | 21.05 |
| 20 | 3 | 15 | 0.04 | 20.00 |
| 31 | 4 | 20 | 0.06 | 20.00 |
| 11B | 1 | 5 | 0.01 | 20.00 |
| 15B | 3 | 15 | 0.04 | 20.00 |
| 19F | 3 | 15 | 0.04 | 20.00 |
| 35F | 3 | 15 | 0.04 | 20.00 |
| 13 | 1 | 6 | 0.01 | 16.67 |
| 12A | 1 | 6 | 0.01 | 16.67 |
| 15A | 6 | 37 | 0.09 | 16.22 |
| 19A | 4 | 28 | 0.06 | 14.29 |
| 25A | 2 | 14 | 0.03 | 14.29 |
| 35B | 2 | 14 | 0.03 | 14.29 |
| 9V | 2 | 14 | 0.03 | 14.29 |
| 3 | 18 | 130 | 0.27 | 13.85 |
| 22F | 6 | 45 | 0.09 | 13.33 |
| 9N | 5 | 41 | 0.07 | 12.20 |
| 16F | 3 | 28 | 0.04 | 10.71 |
| 17F | 1 | 10 | 0.01 | 10.00 |
| 24B | 1 | 11 | 0.01 | 9.09 |
| 8 | 23 | 340 | 0.34 | 6.76 |
| 14 | 1 | 16 | 0.01 | 6.25 |
| 4 | 1 | 17 | 0.01 | 5.88 |
| 12F | 1 | 20 | 0.01 | 5.00 |
| 12B | 2 | 47 | 0.03 | 4.26 |
| 33F | 1 | 25 | 0.01 | 4.00 |
| 10A | 1 | 29 | 0.01 | 3.45 |
| 1 | 0 | 1 | 0.00 | 0.00 |
| 21 | 0 | 5 | 0.00 | 0.00 |
| 15C | 0 | 10 | 0.00 | 0.00 |
| 15F | 0 | 1 | 0.00 | 0.00 |
| 18C | 0 | 4 | 0.00 | 0.00 |
| 18F | 0 | 1 | 0.00 | 0.00 |
| 23B | 0 | 24 | 0.00 | 0.00 |
| 35A | 0 | 2 | 0.00 | 0.00 |
| 6A | 0 | 2 | 0.00 | 0.00 |
| 6B | 0 | 1 | 0.00 | 0.00 |
| 7F | 0 | 7 | 0.00 | 0.00 |
| 9A | 0 | 1 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Miguel, S.; Latasa, P.; Yuste, J.; García, L.; Ordobás, M.; Ramos, B.; Pérez, M.; Ortiz, M.A.; Sanz, J.C. Age-Dependent Serotype-Associated Case-Fatality Rate in Invasive Pneumococcal Disease in the Autonomous Community of Madrid between 2007 and 2020. Microorganisms 2021, 9, 2286. https://doi.org/10.3390/microorganisms9112286
De Miguel S, Latasa P, Yuste J, García L, Ordobás M, Ramos B, Pérez M, Ortiz MA, Sanz JC. Age-Dependent Serotype-Associated Case-Fatality Rate in Invasive Pneumococcal Disease in the Autonomous Community of Madrid between 2007 and 2020. Microorganisms. 2021; 9(11):2286. https://doi.org/10.3390/microorganisms9112286
Chicago/Turabian StyleDe Miguel, Sara, Pello Latasa, José Yuste, Luis García, María Ordobás, Belén Ramos, Marta Pérez, Maira Alejandra Ortiz, and Juan Carlos Sanz. 2021. "Age-Dependent Serotype-Associated Case-Fatality Rate in Invasive Pneumococcal Disease in the Autonomous Community of Madrid between 2007 and 2020" Microorganisms 9, no. 11: 2286. https://doi.org/10.3390/microorganisms9112286
APA StyleDe Miguel, S., Latasa, P., Yuste, J., García, L., Ordobás, M., Ramos, B., Pérez, M., Ortiz, M. A., & Sanz, J. C. (2021). Age-Dependent Serotype-Associated Case-Fatality Rate in Invasive Pneumococcal Disease in the Autonomous Community of Madrid between 2007 and 2020. Microorganisms, 9(11), 2286. https://doi.org/10.3390/microorganisms9112286






